Effects of Three Different Bee Pollen on Digestion, Immunity, Antioxidant Capacity, and Gut Microbes in Apis mellifera
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honeybee Selection
2.2. Determination of Nutrient Composition in Three Pollen Species
2.3. Determination of Amino Acid Content in Three Pollen Species
2.4. Pollen Protein Digestibility
2.5. Effect of Pollen on Enzyme Activity
2.6. Real-Time Quantitative Polymerase Chain Reaction Analysis
2.7. Gut Microbial Analysis
2.8. Statistical Analysis
3. Results
3.1. Determination of Nutrient Content of Pollen
3.2. Determination of Amino Acid Content of Pollen
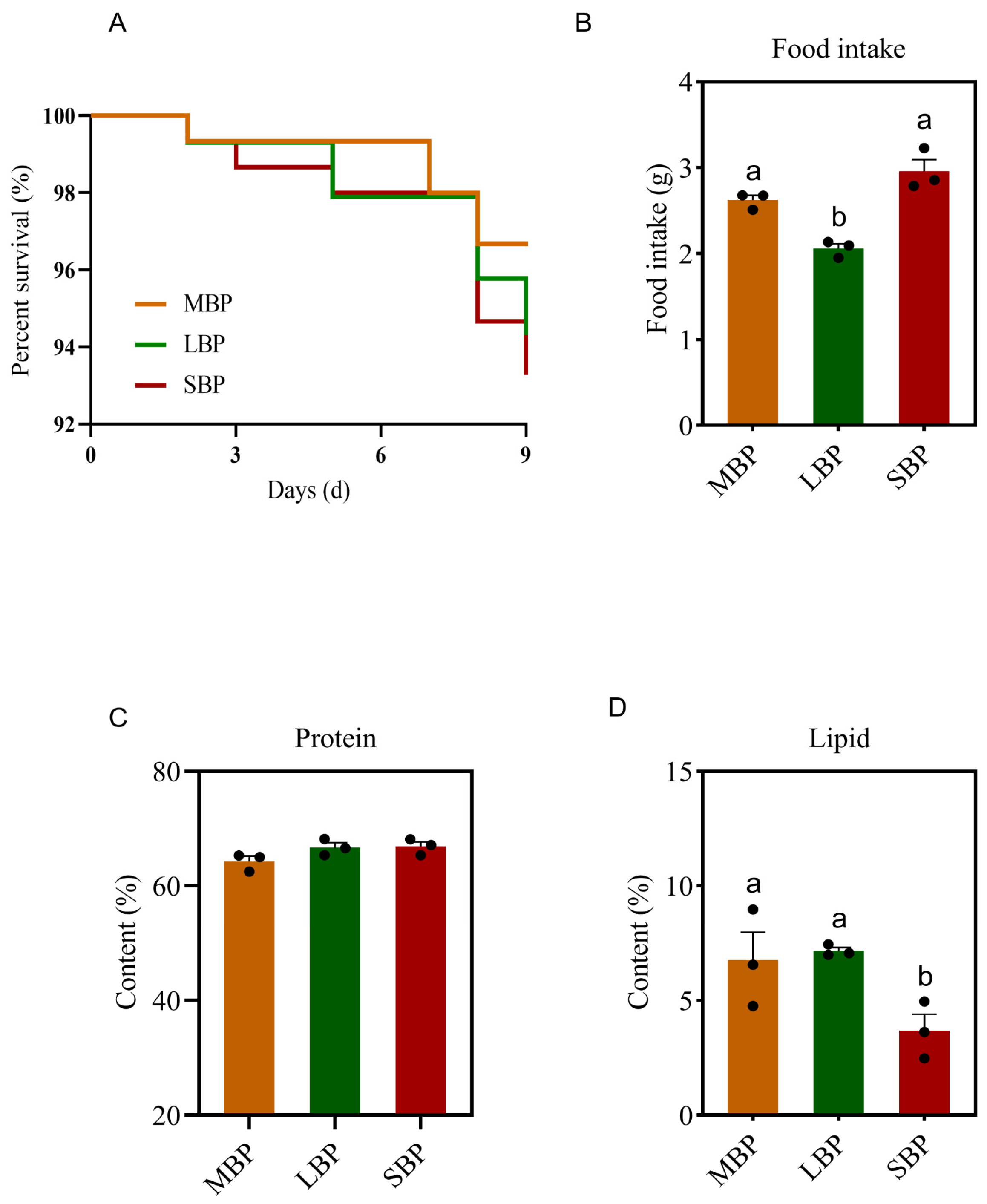
3.3. Feed Intake, Nutrient Deposition, and Survival Rate of Honeybees Fed Different Pollen Types
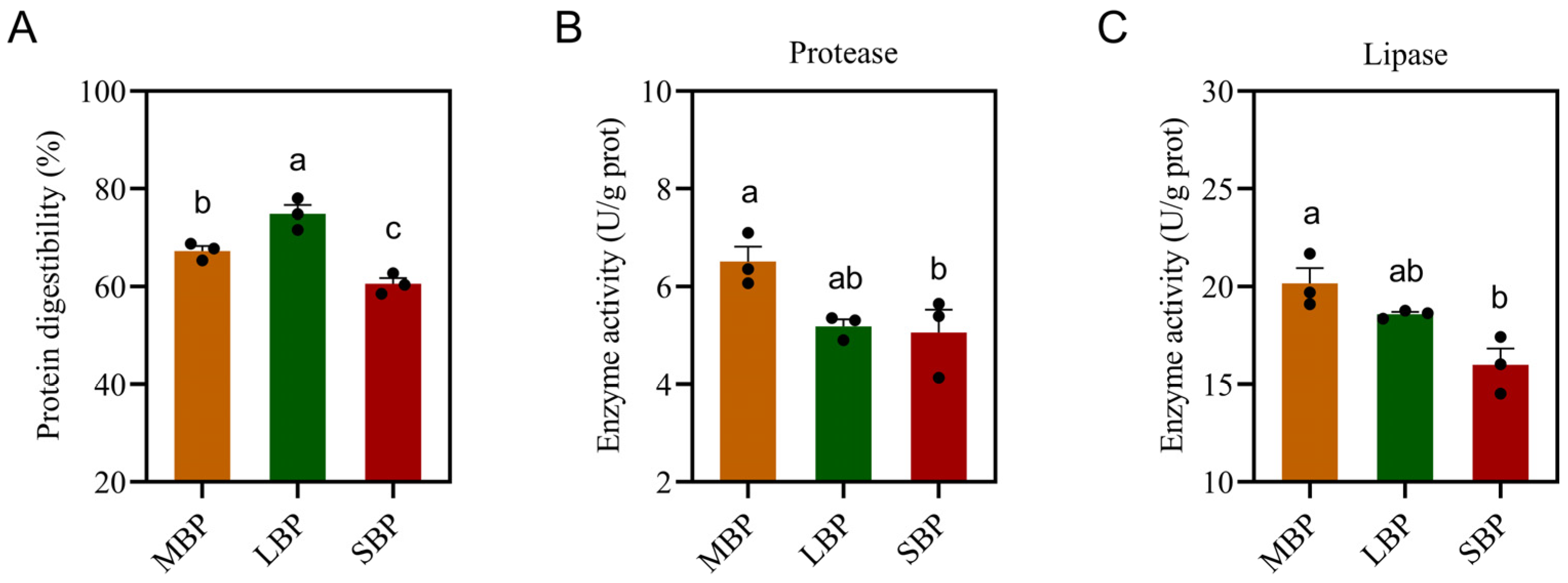
3.4. Protein Digestibility and Digestive Enzyme Activity of Honeybees Fed Different Pollen Types
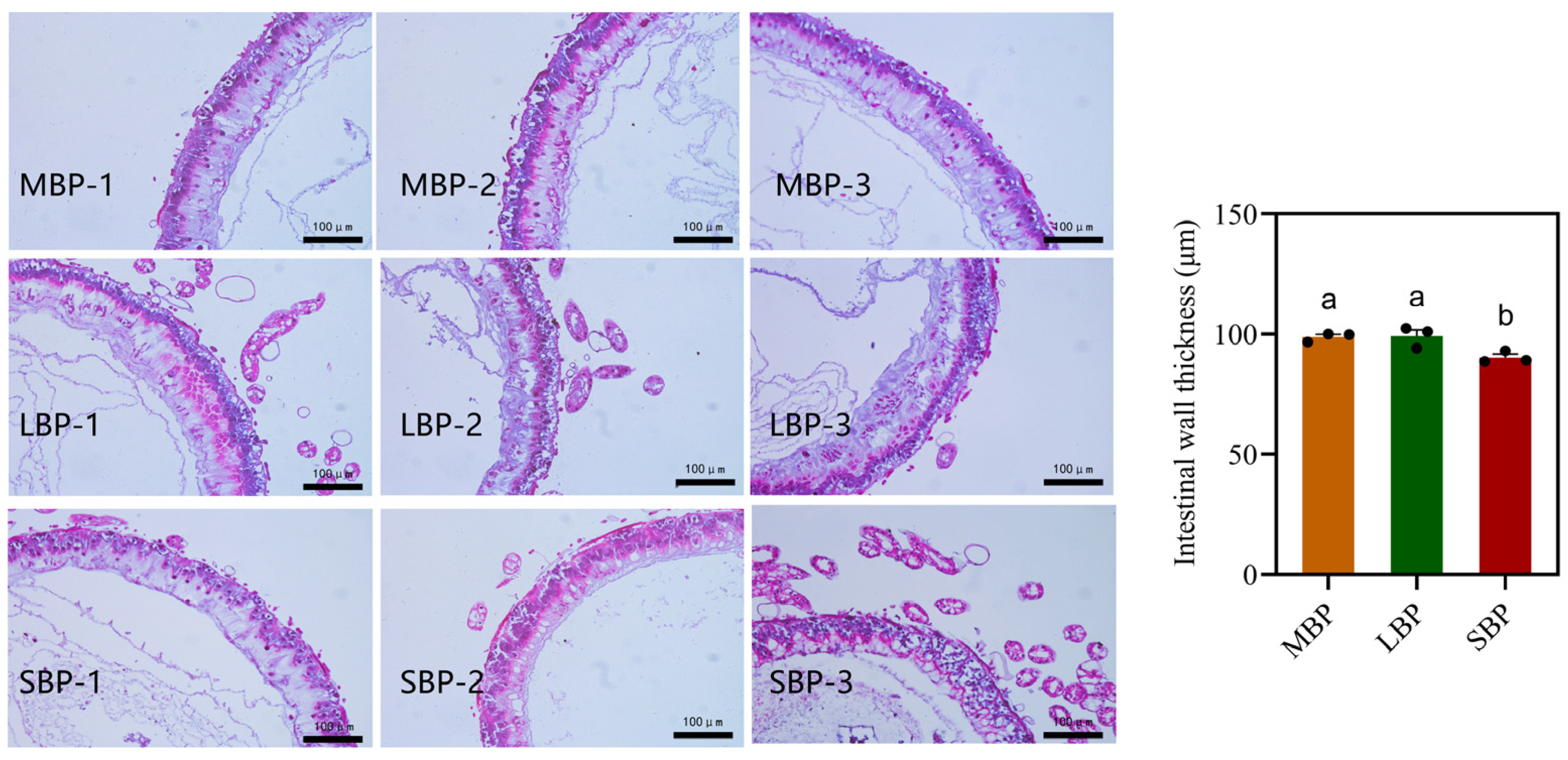
3.5. Effects of Different Pollens on Midgut Morphology in Honeybees
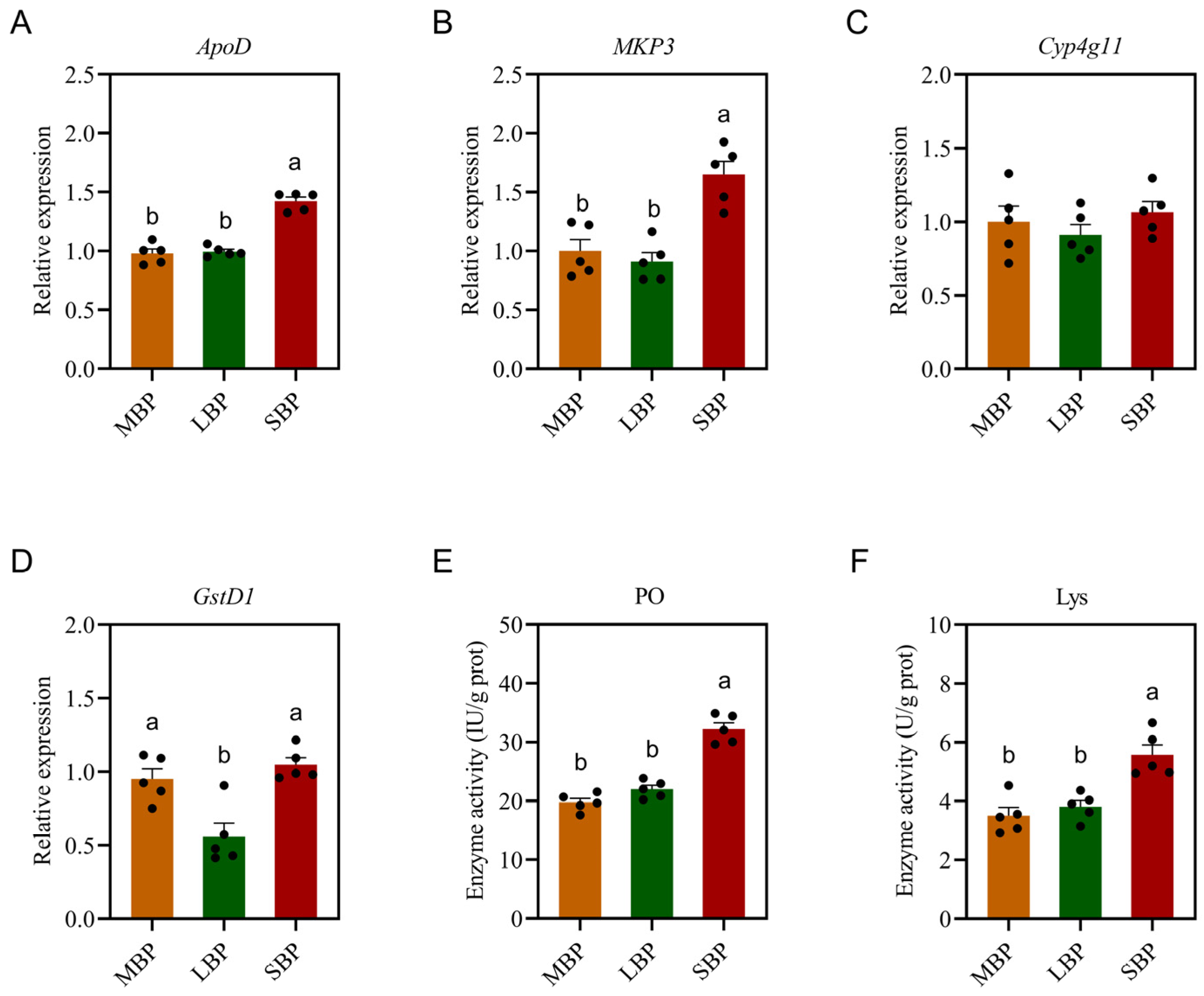
3.6. Effects of Different Pollens on Immune Enzyme Activities and Immune Gene Expression in Honeybees
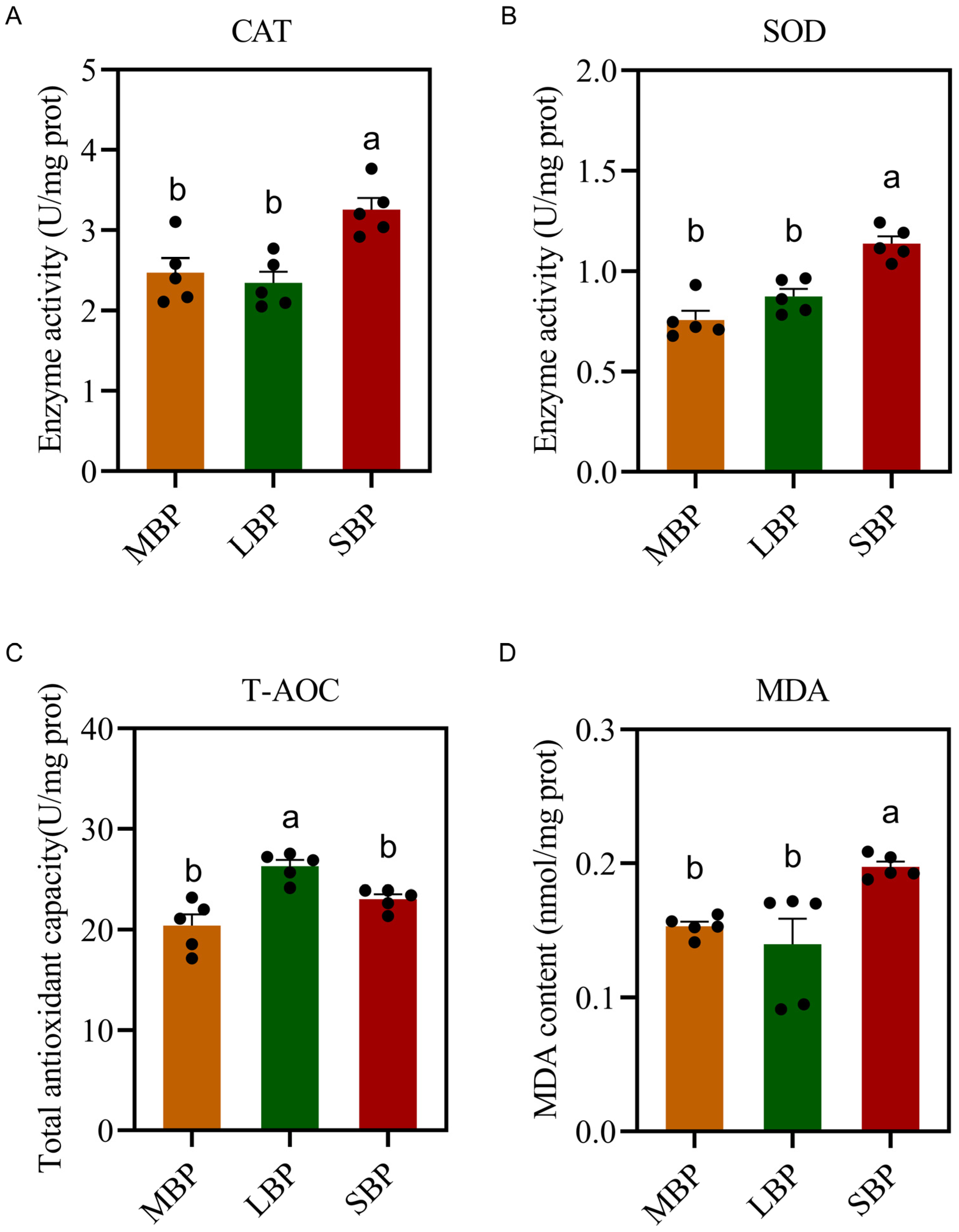
3.7. Effects of Different Pollens on Antioxidant Enzyme Activity in A. mellifera
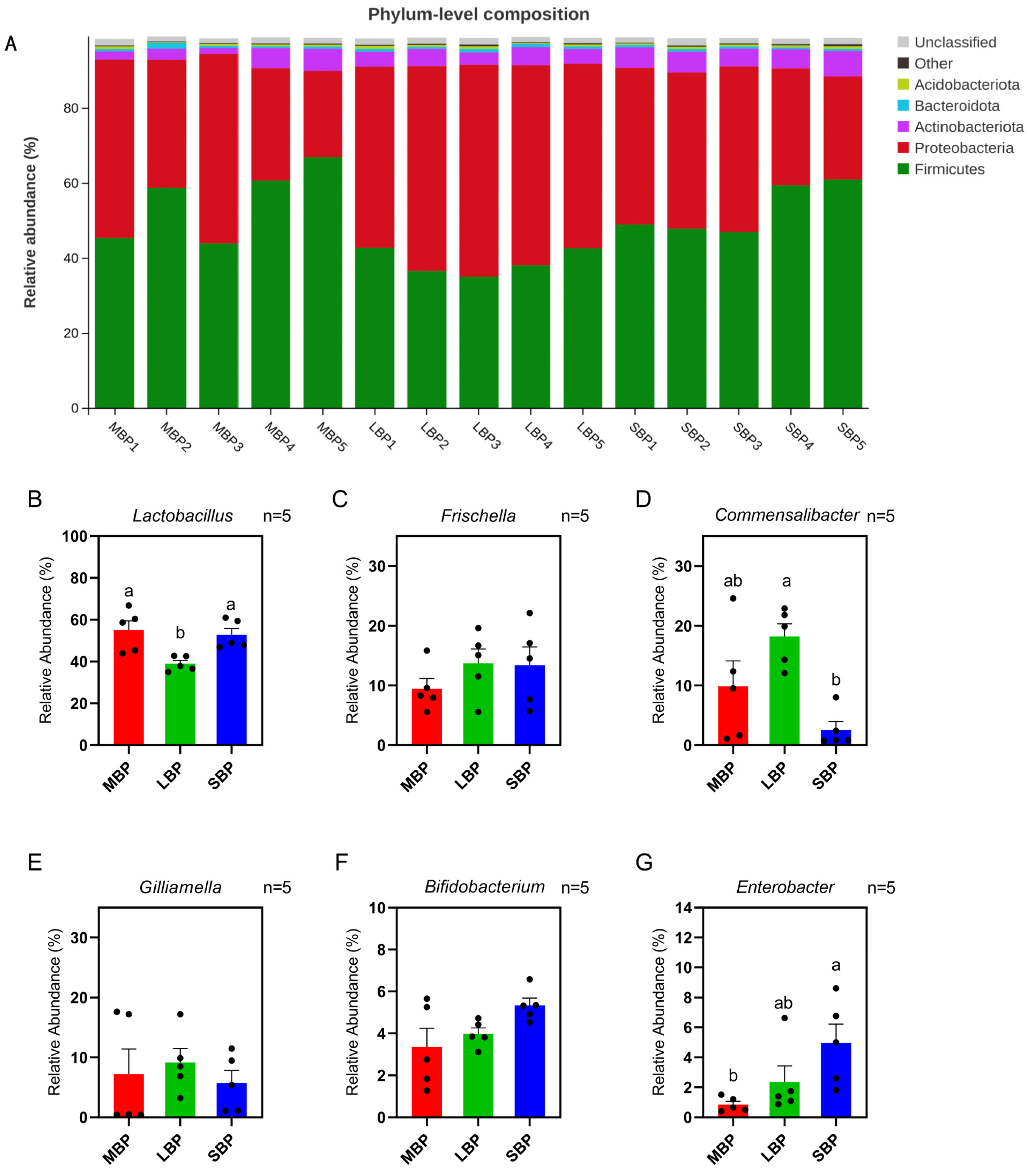
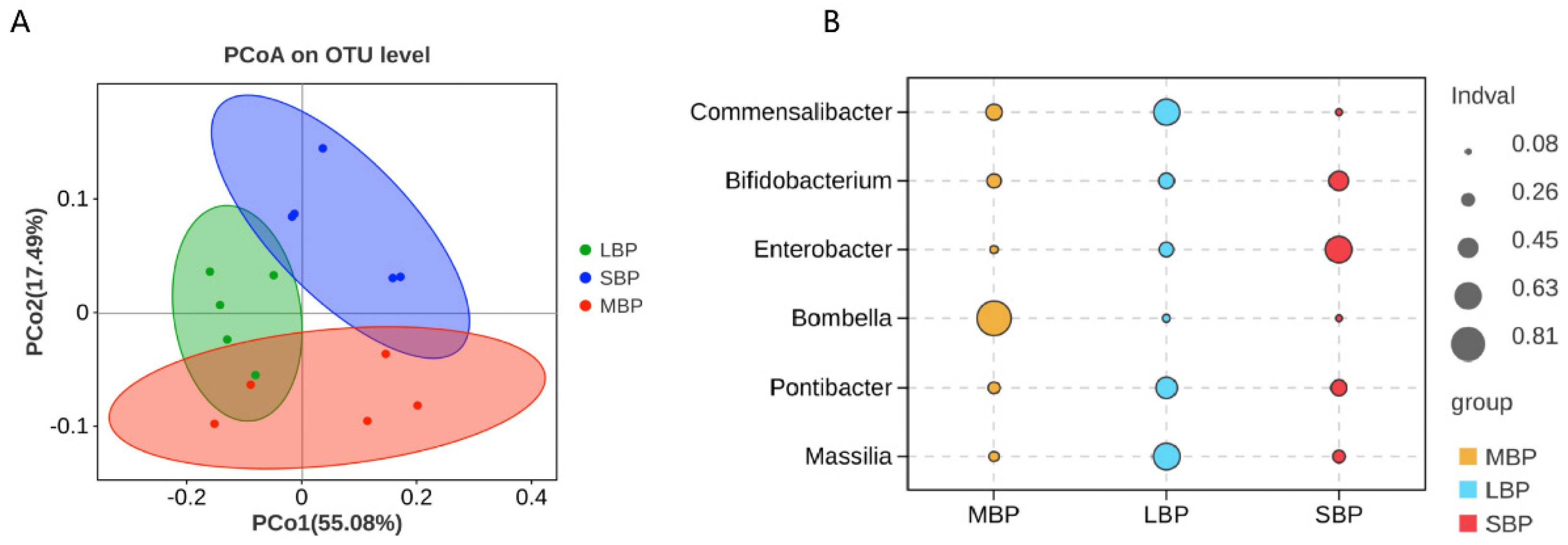
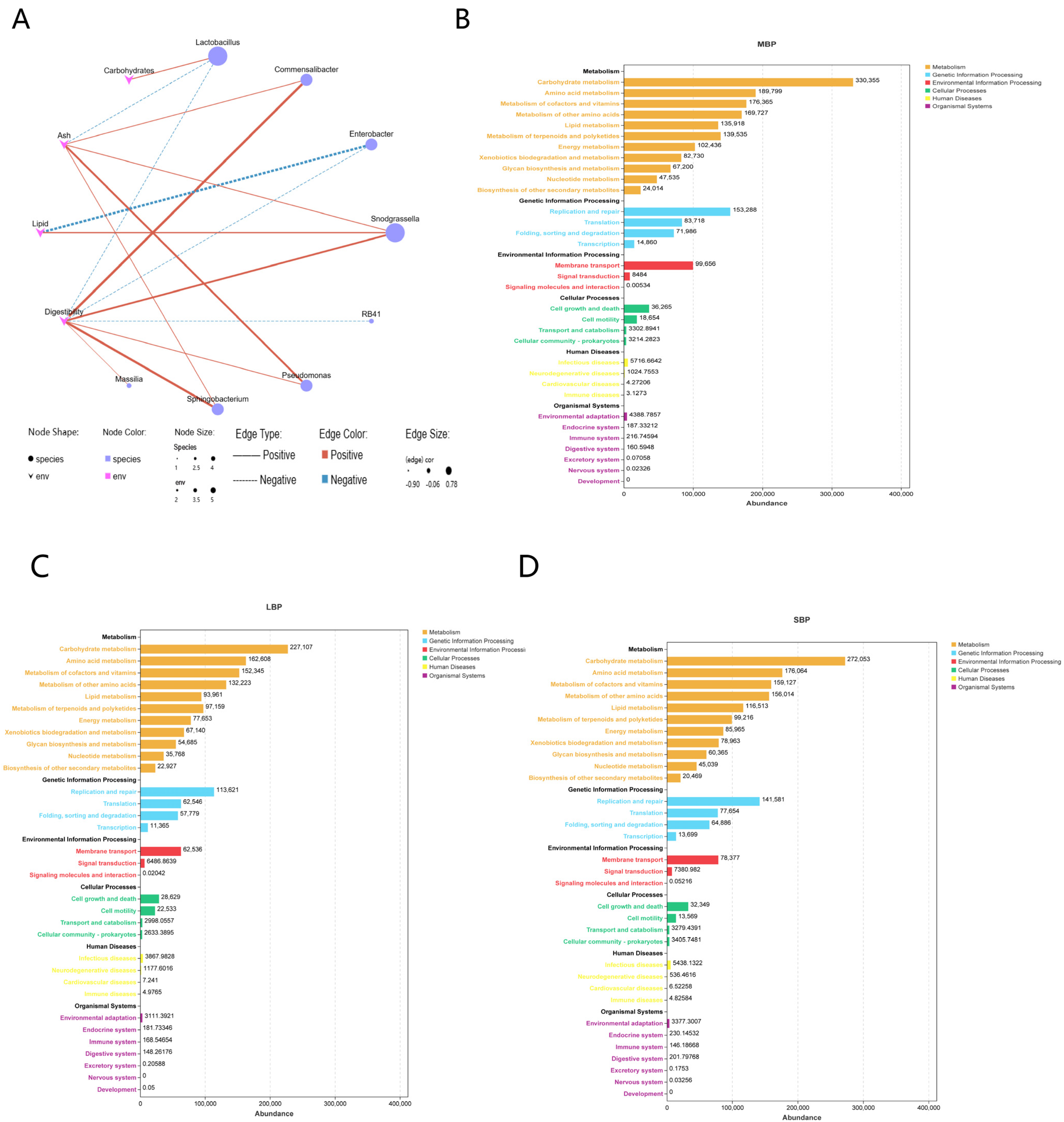
3.8. Effect of Pollen on Gut Microbial Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Bryś, M.S.; Skowronek, P.; Strachecka, A. Pollen Diet-Properties and Impact on a Bee Colony. Insects 2021, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional Physiology and Ecology of Honey Bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef]
- Pacini, E.; Hesse, M. Pollenkitt—Its composition, forms and functions. Flora Morphol. Distrib. Funct. Ecol. Plants 2005, 200, 399–415. [Google Scholar] [CrossRef]
- Herbert, E.W., Jr.; Shimanuki, H. Mineral Requirements for Brood-Rearing by Honeybees Fed a Synthetic Diet. J. Apic. Res. 1978, 17, 118–122. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.-F.; Wu, L.-M.; Hu, F.-L. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Azzouz-Olden, F.; Hunt, A.; DeGrandi-Hoffman, G. Transcriptional response of honey bee (Apis mellifera) to differential nutritional status and Nosema infection. BMC Genom. 2018, 19, 628. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, S.; Deng, Z.; Li, G.; Li, H. Impact of particle size on the nutrition release and antioxidant activity of rape, buckwheat and rose bee pollens. Food Funct. 2023, 14, 1897–1908. [Google Scholar] [CrossRef]
- Zeil, J.; Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e722016. [Google Scholar] [CrossRef]
- Naug, D. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 2009, 142, 2369–2372. [Google Scholar] [CrossRef]
- Keller, I.; Fluri, P.; Imdorf, A. Pollen nutrition and colony development in honey bees: Part 1. Bee World 2005, 86, 3–10. [Google Scholar] [CrossRef]
- Zheng, H.; Perreau, J.; Powell, J.E.; Han, B.; Zhang, Z.; Kwong, W.K.; Tringe, S.G.; Moran, N.A. Division of labor in honey bee gut microbiota for plant polysaccharide digestion. Proc. Natl. Acad. Sci. USA 2019, 116, 25909–25916. [Google Scholar] [CrossRef] [PubMed]
- Martinson, V.G.; Danforth, B.N.; Minckley, R.L.; Rueppell, O.; Tingek, S.; Moran, N.A. A simple and distinctive microbiota associated with honey bees and bumble bees. Mol. Ecol. 2011, 20, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.K.; Mancenido, A.L.; Moran, N.A. Genome Sequences of Lactobacillus sp. Strains wkB8 and wkB10, Members of the Firm-5 Clade, from Honey Bee Guts. Genome Announc. 2014, 2, e2003467. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.K.; Moran, N.A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016, 14, 374–384. [Google Scholar] [CrossRef]
- Bidhendi, A.J.; Chebli, Y.; Geitmann, A. Fluorescence visualization of cellulose and pectin in the primary plant cell wall. J. Microsc. 2020, 278, 164–181. [Google Scholar] [CrossRef]
- Kešnerová, L.; Mars, R.A.T.; Ellegaard, K.M.; Troilo, M.; Sauer, U.; Engel, P. Disentangling metabolic functions of bacteria in the honey bee gut. PLoS Biol. 2017, 15, e2003467. [Google Scholar] [CrossRef]
- Wei, X.; Yu, L.; Zhang, C.; Ni, Y.; Zhang, H.; Zhai, Q.; Tian, F. Genetic-Phenotype Analysis of Bifidobacterium bifidum and Its Glycoside Hydrolase Gene Distribution at Different Age Groups. Foods 2023, 12, 922. [Google Scholar] [CrossRef]
- Tang, J.; Zuo, W.; Guo, L.; Han, Z.; Yang, C.; Han, B.; Dai, L.; Zhang, X.; Zhou, X. Synergistic pectin deconstruction is a prerequisite for mutualistic interactions between honeybee gut bacteria. Nat. Commun. 2024, 15, 6937. [Google Scholar] [CrossRef]
- Engel, P.; Bartlett, K.D.; Moran, N.A. The Bacterium Frischella perrara Causes Scab Formation in the Gut of its Honeybee Host. mBio 2015, 6, 8. [Google Scholar] [CrossRef]
- Sadeghi, M.; Haghshenas, B.; Nami, Y. Bifidobacterium exopolysaccharides: New insights into engineering strategies, physicochemical functions, and immunomodulatory effects on host health. Front. Microbiol. 2024, 15, 1396308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Ma, L.; Li, G.; Han, K.; Liu, Z.; Wang, H.; Xu, B. The Native Dietary Habits of the Two Sympatric Bee Species and Their Effects on Shaping Midgut Microorganisms. Front. Microbiol. 2021, 12, 738226. [Google Scholar] [CrossRef]
- Disayathanoowat, T.; Li, H.; Supapimon, N.; Suwannarach, N.; Lumyong, S.; Chantawannakul, P.; Guo, J. Different Dynamics of Bacterial and Fungal Communities in Hive-Stored Bee Bread and Their Possible Roles: A Case Study from Two Commercial Honey Bees in China. Microorganisms 2020, 8, 264. [Google Scholar] [CrossRef]
- Kwong, W.K.; Moran, N.A. Evolution of host specialization in gut microbes: The bee gut as a model. Gut Microbes 2015, 6, 214–220. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.-T.; Hang, X.-B.; Yang, W.-R.; Liu, F.; Xu, B.-H. Digestion of protein of two pollen types in China by the honeybee (Apis mellifera L.). Apidologie 2014, 45, 590–600. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International; Oxford University Press: Oxford, UK, 2023.
- Serra Bonvehí, J.; Escolà Jordà, R. Nutrient Composition and Microbiological Quality of Honeybee-Collected Pollen in Spain. J. Agric. Food Chem. 1997, 45, 725–732. [Google Scholar] [CrossRef]
- Applegate, E.A.; Baumgartner, B.; Clarkson, P.M.; Grivetti, L.E.; Haislett, N.; McDonald, R.B.; Mills, B.; Oerter, A. Gold medal roundtable: Athlete presentations, audience questions, and summary statements. J. Nutr. 1997, 127, 886S. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Studies on chemical constituents and bioactivity of Rosa micrantha: An alternative antioxidants source for food, pharmaceutical, or cosmetic applications. J. Agric. Food Chem. 2010, 58, 6277–6284. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Knauer, A.C.; Alaux, C.; Barascou, L.; Barraud, A.; Dievart, V.; Ghazoul, J.; Michez, D.; Albrecht, M. Diverse pollen nutrition can improve the development of solitary bees but does not mitigate negative pesticide impacts. Sci. Total Environ. 2024, 912, 169494. [Google Scholar] [CrossRef]
- Du, G.; Xu, J.; Gao, C.; Lu, J.; Li, Q.; Du, J.; Lv, M.; Sun, X. Effect of low storage temperature on pollen viability of fifteen herbaceous peonies. Biotechnol. Rep. 2019, 21, e00309. [Google Scholar] [CrossRef] [PubMed]
- Basualdo, M.; Barragán, S.; Vanagas, L.; García, C.; Solana, H.; Rodríguez, E.; Bedascarrasbure, E. Conversion of High and Low Pollen Protein Diets Into Protein in Worker Honey Bees (Hymenoptera: Apidae). J. Econ. Entomol. 2013, 106, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.C.; Broeckling, C.; H.S. (Seshadri), A. Pollen foraging preferences in honey bees and the nutrient profiles of the pollen. Sci. Rep. 2024, 14, 15028. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of Chemical Composition of Bee Pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.E. Assessment of midgut enteroendocrine peptide complement in the honey bee, Apis mellifera. Insect Biochem. Mol. Biol. 2020, 116, 103257. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, S.; Deng, Z.; Li, G.; Zhang, J.; Li, H. Effect of wall-disruption on nutrient composition and in vitro digestion of camellia and lotus bee pollens. Food Sci. Human. Wellness 2024, 13, 1567–1577. [Google Scholar] [CrossRef]
- Klungness, L.M.; Peng, Y.-S. A histochemical study of pollen digestion in the alimentary canal of honeybees (Apis mellifera L.). J. Insect Physiol. 1984, 30, 511–521. [Google Scholar] [CrossRef]
- Schmidt, J.O.; Buchmann, S.L. Pollen digestion and nitrogen utilization by Apis mellifera L. (Hymenoptera: Apidae). Comp. Biochem. Physiol. Part. A Physiol. 1985, 82, 499–503. [Google Scholar] [CrossRef]
- Mohammad, S.M.; Mahmud-Ab-Rashid, N.-K.; Zawawi, N. Botanical Origin and Nutritional Values of Bee Bread of Stingless Bee (Heterotrigona itama) from Malaysia. J. Food Qual. 2020, 2020, 2845757. [Google Scholar] [CrossRef]
- Villalba, A.; Cecchetto, F.; Vazquez, N.D.; Amarilla, L.; Ramirez, C.L.; Galetto, L.; Maggi, M.; Miglioranza, K.S.B. Contaminant dynamics in honey bees and hive products of apiaries from environmentally contrasting Argentinean regions. Environ. Res. 2024, 249, 118306. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, D.; Gabriele, M.; Summa, M.; Colosimo, R.; Leonardi, D.; Domenici, V.; Pucci, L. Antioxidant, Nutraceutical Properties, and Fluorescence Spectral Profiles of Bee Pollen Samples from Different Botanical Origins. Antioxidants 2020, 9, 1001. [Google Scholar] [CrossRef]
- Şahin, S.; Karkar, B. The antioxidant properties of the chestnut bee pollen extract and its preventive action against oxidatively induced damage in DNA bases. J. Food Biochem. 2019, 43, e12888. [Google Scholar] [CrossRef] [PubMed]
- İspir, Ü.; Mişe Yonar, S.; Yonar, M.E.; Silici, S. Dietary chestnut bee pollen as an immunostimulant for rainbow trout (Oncorhynchus mykiss): Effects on the growth, haematological values, immune response, oxidant/antioxidant status, and survival against Aeromonas salmonicida subsp. achromogenes. Cell Mol. Biol. 2023, 69, 179–188. [Google Scholar] [CrossRef]
- Bryś, M.S.; Olszewski, K.; Bartoń, M.; Strachecka, A. Changes in the Activities of Antioxidant Enzymes in the Fat Body and Hemolymph of Apis mellifera L. Due to Pollen Monodiets. Antioxidants 2025, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Shamjana, U.; Vasu, D.A.; Hembrom, P.S.; Nayak, K.; Grace, T. The role of insect gut microbiota in host fitness, detoxification and nutrient supplementation. Antonie Van Leeuwenhoek 2024, 117, 71. [Google Scholar] [CrossRef]
- Maes, P.W.; Rodrigues, P.A.P.; Oliver, R.; Mott, B.M.; Anderson, K.E. Diet-related gut bacterial dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honeybee (Apis mellifera). Mol. Ecol. 2016, 25, 5439–5450. [Google Scholar] [CrossRef]
- Khan, K.A.; Al-Ghamdi, A.A.; Ghramh, H.A.; Ansari, M.J.; Ali, H.; Alamri, S.A.; Al-Kahtani, S.N.; Adgaba, N.; Qasim, M.; Hafeez, M. Structural diversity and functional variability of gut microbial communities associated with honey bees. Microb. Pathog. 2020, 138, 103793. [Google Scholar] [CrossRef]
- Hou, T.; Dai, H.; Wang, Q.; Hou, Y.; Zhang, X.; Lin, H.; Wang, S.; Li, M.; Zhao, Z.; Lu, J.; et al. Dissecting the causal effect between gut microbiota, DHA, and urate metabolism: A large-scale bidirectional Mendelian randomization. Front. Immunol. 2023, 14, 1148591. [Google Scholar] [CrossRef]
- Figueroa, L.L.; Fowler, A.; Lopez, S.; Amaral, V.E.; Koch, H.; Stevenson, P.C.; Irwin, R.E.; Adler, L.S. Sunflower spines and beyond: Mechanisms and breadth of pollen that reduce gut pathogen infection in the common eastern bumble bee. Funct. Ecol. 2023, 37, 1757–1769. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Quinn, A.; El Chazli, Y.; Escrig, S.; Daraspe, J.; Neuschwander, N.; McNally, A.; Genoud, C.; Meibom, A.; Engel, P. Host-derived organic acids enable gut colonization of the honey bee symbiont Snodgrassella alvi. Nat. Microbiol. 2024, 9, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, Z.; Chen, X.; Hong, Z.; Lin, W.; Mu, X.; Hu, X.; Zheng, H. High-Fat Diets with Differential Fatty Acids Induce Obesity and Perturb Gut Microbiota in Honey Bee. Int. J. Mol. Sci. 2021, 22, 834. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Heerman, M.C.; Evans, J.D.; Rose, R.; Li, W.; Rodríguez-García, C.; DeGrandi-Hoffman, G.; Zhao, Y.; Huang, S.; Li, Z.; et al. Pollen reverses decreased lifespan, altered nutritional metabolism and suppressed immunity in honey bees (Apis mellifera) treated with antibiotics. J. Exp. Biol. 2019, 222, 77. [Google Scholar] [CrossRef]
- Steele, M.I.; Motta, E.V.S.; Gattu, T.; Martinez, D.; Moran, N.A. The Gut Microbiota Protects Bees from Invasion by a Bacterial Pathogen. Microbiol. Spectr. 2021, 9, e0039421. [Google Scholar] [CrossRef]
- Powell, J.E.; Carver, Z.; Leonard, S.P.; Moran, N.A. Field-Realistic Tylosin Exposure Impacts Honey Bee Microbiota and Pathogen Susceptibility, Which Is Ameliorated by Native Gut Probiotics. Microbiol. Spectr. 2021, 9, e0010321. [Google Scholar] [CrossRef]
| Target Gene | Primer Sequences (5′–3′) | Description | GenBank Number |
|---|---|---|---|
| β-Actin | F: CCGTGATTTGACTGACTACCT | Standard control primer, forward | NM_001185145 |
| R: AGTTGCCATTTCCTGTTC | Standard control primer, forward | ||
| ApoD | F: GAAGCGTGCGAGATTCTGTTCC | Standard control primer, forward | XM_392555.7 |
| R: CAGGTGATATTGCCGACGAGTTG | Standard control primer, forward | ||
| MKP3 | F: ATATCCCGAGTGGTGTGAAGGTAG | Standard control primer, forward | XM_006564514 |
| R: CTGGCTCCAATGATCGCTTATAGG | Standard control primer, forward | ||
| Cyp4g11 | F: CCGCCTGCCTTGCCGTTG | Standard control primer, forward | XM_006559340.3 |
| R: AGAAGACCATCGCCAAGCCATG | Standard control primer, forward | ||
| GstD1 | F: AAACCCGCAACACACGATACCC | Standard control primer, forward | NM_001178028.1 |
| R: CGATAGCGCCGTGCCGATG | Standard control primer, forward |
| Content | Types of Bee Pollen | ||
|---|---|---|---|
| MBP | LBP | SBP | |
| Protein (%) | 15.14 ± 1.52 | 15.37 ± 1.39 | 16.69 ± 1.45 |
| Lipid (%) | 6.87 ± 0.19 a | 6.27 ± 0.51 a | 4.15 ± 0.32 b |
| Carbohydrate (%) | 75.44 ± 1.48 | 72.79 ± 1.72 | 76.86 ± 1.88 |
| Crude ash (%) | 2.54 ± 0.04 b | 5.57 ± 0.15 a | 2.30 ± 0.37 b |
| Energy (Kcal/100 g) | 424.19 ± 0.87 a | 409.08 ± 2.85 b | 411.57 ± 1.95 b |
| Amino Acid (g/100 g) | Types of Bee Pollen | ||
|---|---|---|---|
| MBP | LBP | SBP | |
| * Histidine | 0.29 ± 0.03 c | 0.39 ± 0.02 b | 0.54 ± 0.05 a |
| * Methionine | 0.10 ± 0.02 | 0.08 ± 0.03 | 0.10 ± 0.05 |
| * Threonine | 0.64 ± 0.02 a | 0.64 ± 0.01 a | 0.52 ± 0.03 b |
| * Valine | 0.77 ± 0.03 a | 0.79 ± 0.01 a | 0.62 ± 0.03 b |
| * Tyrosine | 0.33 ± 0.02 a b | 0.36 ± 0.03 a | 0.31 ± 0.02 b |
| * Lysine | 0.84 ± 0.02 b | 0.94 ± 0.03 a | 0.63 ± 0.04 c |
| * Isoleucine | 0.61 ± 0.02 b | 0.69 ± 0.02 a | 0.55 ± 0.03 c |
| * Leucine | 0.93 ± 0.03 b | 1.06 ± 0.02 a | 0.88 ± 0.05 b |
| * Phenylalanine | 0.55 ± 0.02 b | 0.66 ± 0.02 a | 0.52 ± 0.04 b |
| * Arginine | 0.63 ± 0.02 b | 0.71 ± 0.01 a | 0.50 ± 0.02 c |
| Serine | 0.38 ± 0.01 a | 0.39 ± 0.01 a | 0.27 ± 0.02 b |
| Glycine | 0.68 ± 0.02 | 0.67 ± 0.01 | 0.67 ± 0.05 |
| Aspartic acid | 0.93 ± 0.01 b | 1.25 ± 0.03 a | 0.86 ± 0.09 b |
| Glutamic acid | 1.32 ± 0.02 b | 1.69 ± 0.02 a | 1.20 ± 0.08 c |
| Alanine | 0.92 ± 0.03 a | 0.81 ± 0.01 b | 0.67 ± 0.05 c |
| Proline | 2.15 ± 0.06 a | 0.59 ± 0.01 c | 0.81 ± 0.05 b |
| Cystine | 0.10 ± 0.01 | 0.10 ± 0.02 | 0.14 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-M.; Wang, Y.; Lei, L.; Zhang, G.; Xu, B.-H. Effects of Three Different Bee Pollen on Digestion, Immunity, Antioxidant Capacity, and Gut Microbes in Apis mellifera. Insects 2025, 16, 505. https://doi.org/10.3390/insects16050505
Li X-M, Wang Y, Lei L, Zhang G, Xu B-H. Effects of Three Different Bee Pollen on Digestion, Immunity, Antioxidant Capacity, and Gut Microbes in Apis mellifera. Insects. 2025; 16(5):505. https://doi.org/10.3390/insects16050505
Chicago/Turabian StyleLi, Xin-Meng, Ying Wang, Li Lei, Ge Zhang, and Bao-Hua Xu. 2025. "Effects of Three Different Bee Pollen on Digestion, Immunity, Antioxidant Capacity, and Gut Microbes in Apis mellifera" Insects 16, no. 5: 505. https://doi.org/10.3390/insects16050505
APA StyleLi, X.-M., Wang, Y., Lei, L., Zhang, G., & Xu, B.-H. (2025). Effects of Three Different Bee Pollen on Digestion, Immunity, Antioxidant Capacity, and Gut Microbes in Apis mellifera. Insects, 16(5), 505. https://doi.org/10.3390/insects16050505






