Survey of ‘Candidatus Liberibacter solanacearum’ and Its Potential Psyllid Vectors in Northwestern Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites, Dates, and Collection Methods of Psyllids and Plants
2.2. CLso Detection
2.3. CLso Characterization
3. Results
3.1. Psyllid Survey
3.2. CLso Detection
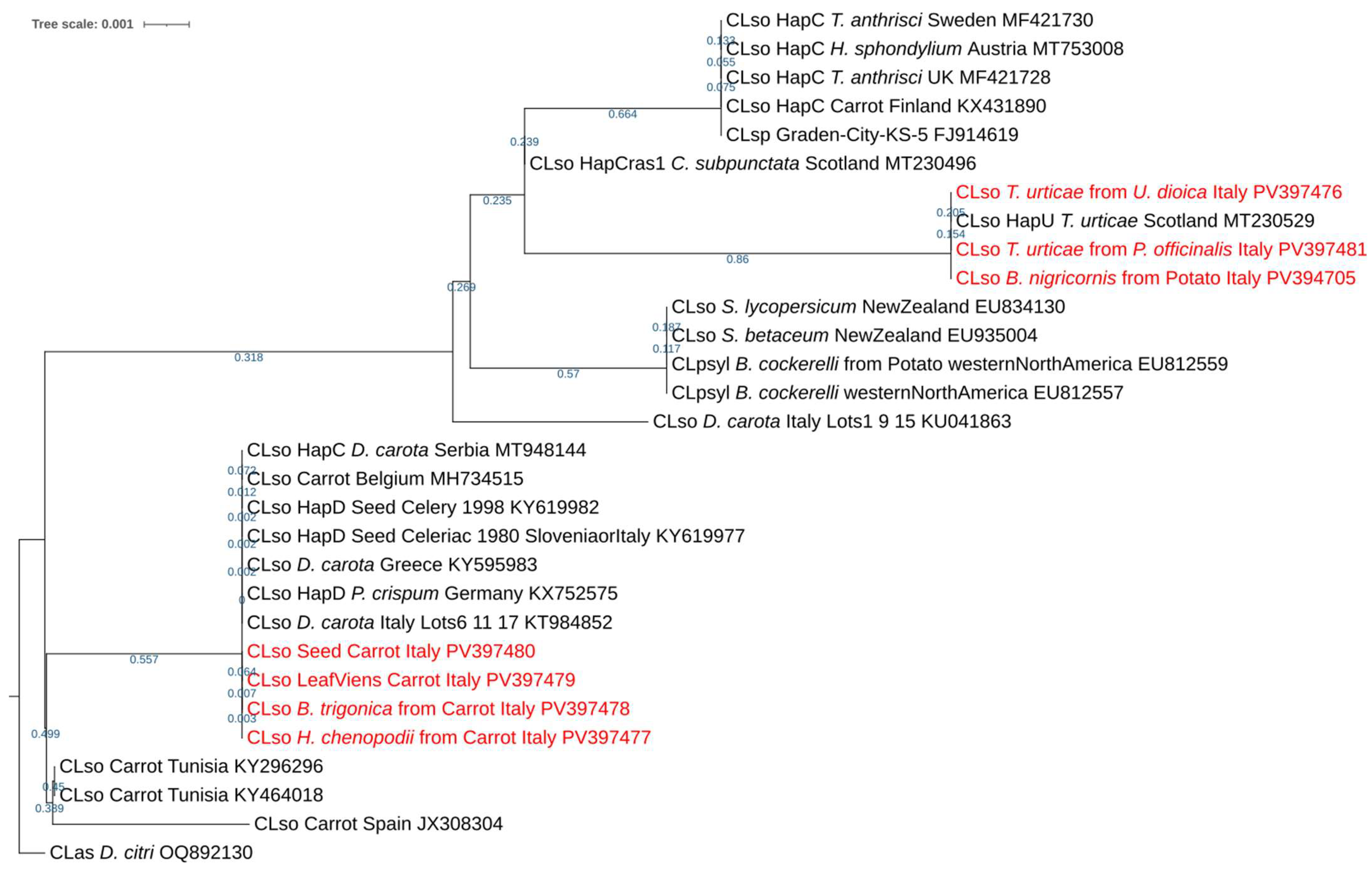
3.3. CLso Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, K.; Tamborindeguy, C.; Scheuring, D.C.; Herrera, A.M.; Silva, A.; Badillo-Vargas, I.E.; Miller, J.C.; Levy, J.G. Differences in zebra chip severity between ‘Candidatus Liberibacter solanacearum’haplotypes in Texas. Am. J. Potato Res. 2019, 96, 86–93. [Google Scholar] [CrossRef]
- Munyaneza, J. Candidatus Liberibacter Solanacearum (Zebra Chip). CABI Compendium. 2014. Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.109434 (accessed on 5 May 2025).
- Hansen, A.; Trumble, J.; Stouthamer, R.; Paine, T. A new huanglongbing species, “Candidatus Liberibacter psyllaurous,” found to infect tomato and potato, is vectored by the psyllid Bactericera cockerelli (Sulc). Appl. Environ. Microbiol. 2008, 74, 5862–5865. [Google Scholar] [CrossRef] [PubMed]
- Pitman, A.R.; Drayton, G.M.; Kraberger, S.J.; Genet, R.A.; Scott, I.A. Tuber transmission of ‘Candidatus Liberibacter solanacearum’ and its association with zebra chip on potato in New Zealand. Eur. J. Plant Pathol. 2011, 129, 389–398. [Google Scholar] [CrossRef]
- Mirmajlessi, M.; Sjolund, M.J.; Mand, M.; Loiseau, M.; Ilardi, V.; Haesaert, G.; Karise, R.; Gottsberger, R.A.; Sumner-Kalkun, J.; Bertaccini, A. PCR-based diagnostic methods for ’Candidatus Liberibacter solanacearum’. Plant Prot. Sci. 2019, 55, 229–242. [Google Scholar] [CrossRef]
- Tahzima, R.; Maes, M.; Achbani, E.; Swisher, K.; Munyaneza, J.; De Jonghe, K. First report of ‘Candidatus Liberibacter solanacearum’ on carrot in Africa. Plant Dis. 2014, 98, 1426. [Google Scholar] [CrossRef]
- Loiseau, M.; Garnier, S.; Boirin, V.; Merieau, M.; Leguay, A.; Renaudin, I.; Renvoisé, J.-P.; Gentit, P. First report of ‘Candidatus Liberibacter solanacearum’ in carrot in France. Plant Dis. 2014, 98, 839. [Google Scholar] [CrossRef]
- Alfaro-Fernández, A.; Siverio, F.; Cebrián, M.; Villaescusa, F.; Font, M. ‘Candidatus Liberibacter solanacearum’ associated with Bactericera trigonica-affected carrots in the Canary Islands. Plant Dis. 2012, 96, 581. [Google Scholar] [CrossRef]
- Nissinen, A.; Haapalainen, M.; Jauhiainen, L.; Lindman, M.; Pirhonen, M. Different symptoms in carrots caused by male and female carrot psyllid feeding and infection by ‘Candidatus Liberibacter solanacearum’. Plant Pathol. 2014, 63, 812–820. [Google Scholar] [CrossRef]
- Karahan, A.; Altundag, S.; Saracoglu, M.; Duman, K.; Ozdemir, I.; Ozdem, A.; Umar, S.; Ozden, E.D. First report of ‘Candidatus Liberibacter solanacearum’ on carrot and parsley in Turkey. New Dis. Rep 2022, 45, e12095. [Google Scholar] [CrossRef]
- Sumner-Kalkun, J.C.; Highet, F.; Arnsdorf, Y.M.; Back, E.; Carnegie, M.; Madden, S.; Carboni, S.; Billaud, W.; Lawrence, Z.; Kenyon, D. ‘Candidatus Liberibacter solanacearum’ distribution and diversity in Scotland and the characterisation of novel haplotypes from Craspedolepta spp.(Psylloidea: Aphalaridae). Sci. Rep. 2020, 10, 16567. [Google Scholar] [CrossRef]
- Nelson, W.R.; Fisher, T.W.; Munyaneza, J.E. Haplotypes of “Candidatus Liberibacter solanacearum” suggest long-standing separation. Eur. J. Plant Pathol. 2011, 130, 5–12. [Google Scholar] [CrossRef]
- Alfaro-Fernández, A.; Hernández-Llopis, D.; Font, M.I. Haplotypes of ‘Candidatus Liberibacter solanacearum’ identified in Umbeliferous crops in Spain. Eur. J. Plant Pathol. 2017, 149, 127–131. [Google Scholar] [CrossRef]
- French-Monar, R.; Patton III, A.; Douglas, J.; Abad, J.; Schuster, G.; Wallace, R.; Wheeler, T. First report of “Candidatus Liberibacter solanacearum” on field tomatoes in the United States. Plant Dis. 2010, 94, 481. [Google Scholar] [CrossRef] [PubMed]
- Vereijssen, J.; Taylor, N.; Barnes, A.; Thompson, S.; Logan, D.; Butler, R.; Yen, A.; Finlay, K. First report of ‘Candidatus Liberibacter solanacearum’ in Jerusalem cherry (Solanum pseudocapsicum) and thorn-apple (Datura stramonium) in New Zealand. New Dis. Rep. 2015, 32, 1. [Google Scholar] [CrossRef]
- Hajri, A.; Loiseau, M.; Cousseau-Suhard, P.; Renaudin, I.; Gentit, P. Genetic characterization of ‘Candidatus Liberibacter solanacearum’ haplotypes associated with apiaceous crops in France. Plant Dis. 2017, 101, 1383–1390. [Google Scholar] [CrossRef]
- Haapalainen, M.; Wang, J.; Latvala, S.; Lehtonen, M.T.; Pirhonen, M.; Nissinen, A. Genetic variation of ‘Candidatus Liberibacter solanacearum’ haplotype C and identification of a novel haplotype from Trioza urticae and stinging nettle. Phytopathology 2018, 108, 925–934. [Google Scholar] [CrossRef]
- Haapalainen, M.; Latvala, S.; Wickström, A.; Wang, J.; Pirhonen, M.; Nissinen, A.I. A novel haplotype of ‘Candidatus Liberibacter solanacearum’ found in Apiaceae and Polygonaceae family plants. Eur. J. Plant Pathol. 2020, 156, 413–423. [Google Scholar] [CrossRef]
- Wang, J.; Haapalainen, M.; Schott, T.; Thompson, S.M.; Smith, G.R.; Nissinen, A.I.; Pirhonen, M. Genomic sequence of ’Candidatus Liberibacter solanacearum’ haplotype C and its comparison with haplotype A and B genomes. PLoS ONE 2017, 12, e0171531. [Google Scholar] [CrossRef]
- Trkulja, V.; Tomić, A.; Matić, S.; Trkulja, N.; Iličić, R.; Popović Milovanović, T. An Overview of the Emergence of Plant Pathogen ‘Candidatus Liberibacter solanacearum’ in Europe. Microorganisms 2023, 11, 1699. [Google Scholar] [CrossRef]
- Munyaneza, J.E.; Fisher, T.W.; Sengoda, V.G.; Garczynski, S.F.; Nissinen, A.; Lemmetty, A. Association of “Candidatus Liberibacter solanacearum” with the psyllid, Trioza apicalis (Hemiptera: Triozidae) in Europe. J. Econ. Entomol. 2010, 103, 1060–1070. [Google Scholar] [CrossRef]
- Monger, W.A.; Jeffries, C.J. A survey of ‘Candidatus Liberibacter solanacearum’ in historical seed from collections of carrot and related Apiaceae species. Eur. J. Plant Pathol. 2018, 150, 803–815. [Google Scholar] [CrossRef]
- Haapalainen, M.; Latvala, S.; Rastas, M.; Wang, J.; Hannukkala, A.; Pirhonen, M.; Nissinen, A.I. Carrot pathogen ‘Candidatus Liberibacter solanacearum’ haplotype C detected in symptomless potato plants in Finland. Potato Res. 2018, 61, 31–50. [Google Scholar] [CrossRef]
- Monger, W.; Jeffries, C. First report of ’Candidatus Liberibacter solanacearum’ in parsley (Petroselinum crispum) seed. New Dis. Rep. 2016, 34, 31. [Google Scholar] [CrossRef]
- Teresani, G.; Hernández-Suárez, E.; Bertolini, E.; Siverio, F.; Moreno, A.; Fereres, A.; Cambra, M. Transmission of ‘Candidatus Liberibacter solanacearum’ by Bactericera trigonica Hodkinson to vegetable hosts. Span. J. Agric. Res. 2017, 15, e1011. [Google Scholar] [CrossRef]
- Bertinelli, G.; Tizzani, L.; Mosconi, F.; Ilardi, V.; Bertin, S. First Report of the Association of the Psyllid Vector Bactericera trigonica (Hemiptera: Triozidae) with ‘Candidatus Liberibacter Solanacearum’ in Italy. Insects 2024, 15, 117. [Google Scholar] [CrossRef]
- Ilardi, V.; Di Nicola, E.; Tavazza, M. First report of ’Candidatus Liberibacter solanacearum’ in commercial carrot seeds in Italy. J. Plant Pathol. 2016, 98. [Google Scholar] [CrossRef]
- Antolínez, C.A.; Moreno, A.; Ontiveros, I.; Pla, S.; Plaza, M.; Sanjuan, S.; Palomo, J.L.; Sjölund, M.J.; Sumner-Kalkun, J.C.; Arnsdorf, Y.M. Seasonal abundance of psyllid species on carrots and potato crops in Spain. Insects 2019, 10, 287. [Google Scholar] [CrossRef]
- Swisher Grimm, K.; Garczynski, S. Identification of a new haplotype of ‘Candidatus Liberibacter solanacearum’ in Solanum tuberosum. Plant Dis. 2019, 103, 468–474. [Google Scholar] [CrossRef]
- Mauck, K.E.; Sun, P.; Meduri, V.R.; Hansen, A.K. New Ca. Liberibacter psyllaurous haplotype resurrected from a 49-year-old specimen of Solanum umbelliferum: A native host of the psyllid vector. Sci. Rep. 2019, 9, 9530. [Google Scholar]
- Torres, G.L.; Cooper, W.R.; Horton, D.R.; Swisher, K.D.; Garczynski, S.F.; Munyaneza, J.E.; Barcenas, N.M. Horizontal transmission of “Candidatus Liberibacter solanacearum” by Bactericera cockerelli (Hemiptera: Triozidae) on Convolvulus and Ipomoea (Solanales: Convolvulaceae). PLoS ONE 2015, 10, e0142734. [Google Scholar] [CrossRef]
- Contreras-Rendón, A.; Sánchez-Pale, J.R.; Fuentes-Aragón, D.; Alanís-Martínez, I.; Silva-Rojas, H.V. Conventional and qPCR reveals the presence of ‘Candidatus Liberibacter solanacearum’ haplotypes A, and B in Physalis philadelphica plant, seed, and Βactericera cockerelli psyllids, with the assignment of a new haplotype H in Convolvulaceae. Antonie Van Leeuwenhoek 2020, 113, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Grimm, K.D.S.; Horton, D.R.; Lewis, T.M.; Garczynski, S.F.; Jensen, A.S.; Charlton, B.A. Identification of three new ‘Candidatus Liberibacter solanacearum’ haplotypes in four psyllid species (Hemiptera: Psylloidea). Sci. Rep. 2022, 12, 20618. [Google Scholar] [CrossRef] [PubMed]
- Trkulja, V.; Mitrović, P.; Mihić Salapura, J.; Iličić, R.; Ćurković, B.; Đalović, I.; Popović, T. First report of ‘Candidatus Liberibacter solanacearum’ on carrot in Serbia. Plant Dis. 2021, 105, 1188. [Google Scholar] [CrossRef] [PubMed]
- Ben Othmen, S.; Morán, F.E.; Navarro, I.; Barbé, S.; Martínez, C.; Marco-Noales, E.; Chermiti, B.; López, M.M. ‘Candidatus Liberibacter solanacearum’ haplotypes D and E in carrot plants and seeds in Tunisia. J. Plant Pathol. 2018, 100, 197–207. [Google Scholar] [CrossRef]
- Teresani, G.; Hernández-Suárez, E.; Bertolini, E.; Siverio, F.; Marroquín, C.; Molina, J.; Hermoso-De-Mendoza, A.; Cambra, M. Search for potential vectors of ’Candidatus Liberibacter solanacearum’: Population dynamics in host crops. Span. J. Agric. Res. 2015, 13, e1002. [Google Scholar] [CrossRef]
- Antolinez, C.; Fereres, A.; Moreno, A. Risk assessment of ‘Candidatus Liberibacter solanacearum’ transmission by the psyllids Bactericera trigonica and B. tremblayi from Apiaceae crops to potato. Sci. Rep. 2017, 7, 45534. [Google Scholar] [CrossRef]
- Sjolund, M.J.; Arnsdorf, Y.M.; Carnegie, M.; Fornefeld, E.; Will, T. ‘Candidatus Liberibacter solanacearum’ detected in Trioza urticae using suction trap-based monitoring of psyllids in Germany. J. Plant Dis. Prot. 2019, 126, 89–92. [Google Scholar] [CrossRef]
- EPPO. New Finding of ‘Candidatus Liberibacter Solanacearum’ in Estonia. EPPO Reporting Service no. 10 - 2020., Num. Article 2020/226. Available online: https://gd.eppo.int/reporting/article-6904 (accessed on 5 May 2025).
- Sjolund, M.; Clark, M.; Carnegie, M.; Greenslade, A.; Ouvrard, D.; Highet, F.; Sigvald, J.; Bell, J.; Arnsdorf, Y.; Cairns, R. First report of ’Candidatus Liberibacter solanacearum’ in the United Kingdom in the psyllid Trioza anthrisci. New Dis. Rep. 2017, 36, 4. [Google Scholar] [CrossRef]
- Hodkinson, I.; White, I. Homoptera Psylloidea. In Handbooks for the Identification of British Insects; Watson, A., Ed.; Royal Entomological Society of London: St Albans, UK, 1979; Volume 2. [Google Scholar]
- Ossiannilsson, F. The Psylloidea (Homoptera) of Fennoscandia and Denmark; Brill: Leiden, The Netherlands, 1992; Volume 26. [Google Scholar]
- Pignatti, S. Flora d'Italia; Edagricole, Ed.: Bologna, Italy, 1982; ISBN 88-506-2449-2. [Google Scholar]
- Casquet, J.; Thebaud, C.; Gillespie, R.G. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Mol. Ecol. Resour. 2012, 12, 136–141. [Google Scholar] [CrossRef]
- Quintana, M.; De-León, L.; Cubero, J.; Siverio, F. Assessment of Psyllid Handling and DNA Extraction Methods in the Detection of ‘Candidatus Liberibacter Solanacearum’ by qPCR. Microorganisms 2022, 10, 1104. [Google Scholar] [CrossRef]
- Kannan, M.; Suganya, T.; Arunprasanna, V.; Rameshkumar, N.; Krishnan, M. An efficient method for extraction of genomic DNA from insect gut bacteria-culture dependent. Curr. Res. Microbiol. Biotechnol. 2015, 3, 550–556. [Google Scholar]
- EPPO. ‘Candidatus Liberibacter solanacearum’ PM, 7/143. EPPO Bull. 2020, 50, 49–68. [Google Scholar] [CrossRef]
- Ravindran, A.; Levy, J.; Pierson, E.; Gross, D.C. Development of primers for improved PCR detection of the potato zebra chip pathogen, ‘Candidatus Liberibacter solanacearum’. Plant Dis. 2011, 95, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, 78–82. [Google Scholar] [CrossRef]
- Catara, V.; Licciardello, G.; Linguaglossa, M.; Salonia, F.; Rapisarda, C.; La Rosa, R.; Cocuzza Massimino, G. First report of ‘Candidatus Liberibacter solanacearum’ in carrot in Italy. Phytopathol. Mediterr 2017, 56, 296. [Google Scholar]
- Tizzani, L.; Bertinelli, G.; Bertin, S.; Ilardi, V. First report of ‘Candidatus Liberibacter solanacearum’ in carrot plants in mainland Italy. J. Plant Pathol. 2024, 106, 1859–1860. [Google Scholar] [CrossRef]
- Mifsud, D. The jumping plant-lice (Hemiptera: Psylloidea) of the Maltese Islands. Bull. Entomol. Soc. Malta 2020, 11, 103–117. [Google Scholar]
- Soliman, M.; Malash, A.; El-Hawagry, M. Seasonal abundance of Heterotrioza chenopodii and distribution of the known psylloid species (Hemiptera: Psylloidea) in Egypt. Afr. Entomol. 2021, 29, 201–211. [Google Scholar] [CrossRef]
- Butler, C.D.; Trumble, J.T. The potato psyllid, Bactericera cockerelli (Sulc)(Hemiptera: Triozidae): Life history, relationship to plant diseases, and management strategies. Terr. Arthropod Rev. 2012, 5, 87–111. [Google Scholar] [CrossRef]
- Caldwell, J.S. Some North American relatives of Aphalara calthae Linnaeus (Homoptera: Chermidae). Ann. Entomol. Soc. Am. 1937, 30, 563–571. [Google Scholar] [CrossRef]
- Klimaszewski, S.M. The jumping plant lice or psyllids (Homoptera, Psyllodea) of the Palaearctic. An annotated check-list. Annal. Zool. 1973, 30, 155–286. [Google Scholar]
- Asensio-S.-Manzanera, M.C.; Santiago-Calvo, Y.; Palomo-Gómez, J.L.; Marquínez-Ramírez, R.; Bastin, S.; García-Méndez, E.M.; Hernández-Suárez, E.; Siverio-de-la-Rosa, F. Survey of Candidatus Liberibacter Solanacearum and Its Associated Vectors in Potato Crop in Spain. Insects 2022, 13, 964. [Google Scholar] [CrossRef]
- Burckhardt, D.; Freuler, J. Jumping plant-lice (Hemiptera, Psylloidea) from sticky traps in carrot fields in Valais, Switzerland. Mitt. Schweiz. Entomol. Ges. 2000, 73, 191–209. [Google Scholar]
- Hodkinson, I. Status and taxonomy of the Trioza (Bactericera) nigricornis Förster complex (Hemiptera: Triozidae). Bull. Entomol. Res. 1981, 71, 671–679. [Google Scholar] [CrossRef]
- Conci, C.; Rapisarda, C.; Tamanini, L. Annotated catalogue of the Italian Psylloidea: Second part: (Insecta Homoptera). Atti Dell’accademia Roveretana Degli Agiati. B Cl. Di Sci. Mat. Fis. E Nat. 1995, 5, 5–207. [Google Scholar]
- Taylor, N.; Butler, R.; Vereijssen, J.; Davidson, M. Trap colour, size, and borders alter catches of Bactericera cockerelli in a potato crop. Entomol. Exp. Et Appl. 2014, 150, 226–231. [Google Scholar] [CrossRef]
- Horton, D.R. Diurnal patterns in yellow trap catch of pear psylla (Homoptera: Psyllidae): Differences between sexes and morphotypes. Can. Entomol. 1993, 125, 761–767. [Google Scholar] [CrossRef]
- Arismendi, N.; Carrillo, R.; Andrade, N.; Riegel, R.; Rojas, E. Evaluation of trap color and position on the capture of cicadellids in Gaultheria phillyreifolia (Ericaceae) affected by phytoplasmas. Neotrop. Entomol. 2009, 38, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.R.; Horton, D.R.; Swisher-Grimm, K.; Krey, K.; Wildung, M.R. Bacterial endosymbionts of Bactericera maculipennis and three mitochondrial haplotypes of B. cockerelli (Hemiptera: Psylloidea: Triozidae). Environ. Entomol. 2022, 51, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Ouvrard, D. The World Psylloidea Database (from Psyl’list) [Data Set Resource]. Natural History Museum 2015. Available online: https://data.nhm.ac.uk/dataset/psyl-list/resource/8746ceec-4846-4899-b607-9ba603002033 (accessed on 5 May 2025).
- Pizzinat, A.; Tedeschi, R.; Alma, A. Cacopsylla melanoneura (Foerster): Aestivation and overwintering habitats in Northwest Italy. Bull. Insectology 2011, 64, S135–S136. [Google Scholar]
- Jaraush, B.; Tedeschi, R.; Sauvion, N.; Gross, J.; Jarausc, W. Psyllid Vectors. In Phytoplasmas: Plant Pathogenic Bacteria—II: Transmission and Management of Phytoplasma-Associated Diseases, 1st ed.; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 53–78. [Google Scholar]
- Keshet-Sitton, A.; Piasezky, A.; Assoline, N.; Dror, O.; Bahar, O. Effect of plant age, temperature, and vector load on ‘Candidatus Liberibacter solanacearum’ in planta titer and shoot proliferation symptoms in carrot. Phytopathol® 2022, 112, 154–162. [Google Scholar] [CrossRef]
- Sauer, J.; Dewert, A.; Fornefeld, E.; Götz, M. Influence of ‘Candidatus Liberibacter solanacearum’ infection on carrot root weight in Germany. Eur. J. Plant Pathol. 2024, 169, 219–232. [Google Scholar] [CrossRef]
- Holeva, M.; Glynos, P.; Karafla, C. First report of ‘Candidatus Liberibacter solanacearum’ on carrot in Greece. Plant Dis. 2017, 101, 1819. [Google Scholar] [CrossRef]
- Jonghe, K.D.; Roo, I.D.; Goedefroit, T. A survey in carrot reveals a widespread aster yellows infection, and a first case of ‘Candidatus Liberibacter solanacearum’ in Belgium. Phytopathogenic Mollicutes 2019, 9, 139–140. [Google Scholar] [CrossRef]
- Nelson, W.R.; Sengoda, V.G.; Alfaro-Fernandez, A.O.; Font, M.I.; Crosslin, J.M.; Munyaneza, J.E. A new haplotype of “Candidatus Liberibacter solanacearum” identified in the Mediterranean region. Eur. J. Plant Pathol. 2013, 135, 633–639. [Google Scholar] [CrossRef]
- Vereijssen, J.; Smith, G.R.; Weintraub, P.G. Bactericera cockerelli (Hemiptera: Triozidae) and Candidatus Liberibacter solanacearum in potatoes in New Zealand: Biology, transmission, and implications for management. J. Integr. Pest Manag. 2018, 9, 13. [Google Scholar] [CrossRef]
- Wallis, C.; Rashed, A.; Chen, J.; Paetzold, L.; Workneh, F.; Rush, C. Effects of potato-psyllid-vectored ‘Candidatus Liberibacter solanacearum’ infection on potato leaf and stem physiology. Phytopathology 2015, 105, 189–198. [Google Scholar] [CrossRef]
- Swisher Grimm, K.D.; Mustafa, T.; Rodney Cooper, W.; Munyaneza, J.E. Role of ‘Candidatus Liberibacter solanacearum’ and Bactericera cockerelli haplotypes in zebra chip incidence and symptom severity. Am. J. Potato Res. 2018, 95, 709–719. [Google Scholar] [CrossRef]
- Thompson, S.M.; Johnson, C.P.; Lu, A.Y.; Frampton, R.A.; Sullivan, K.L.; Fiers, M.W.; Crowhurst, R.N.; Pitman, A.R.; Scott, I.A.; Wen, A. Genomes of ‘Candidatus Liberibacter solanacearum’ haplotype A from New Zealand and the United States suggest significant genome plasticity in the species. Phytopathology 2015, 105, 863–871. [Google Scholar] [CrossRef]
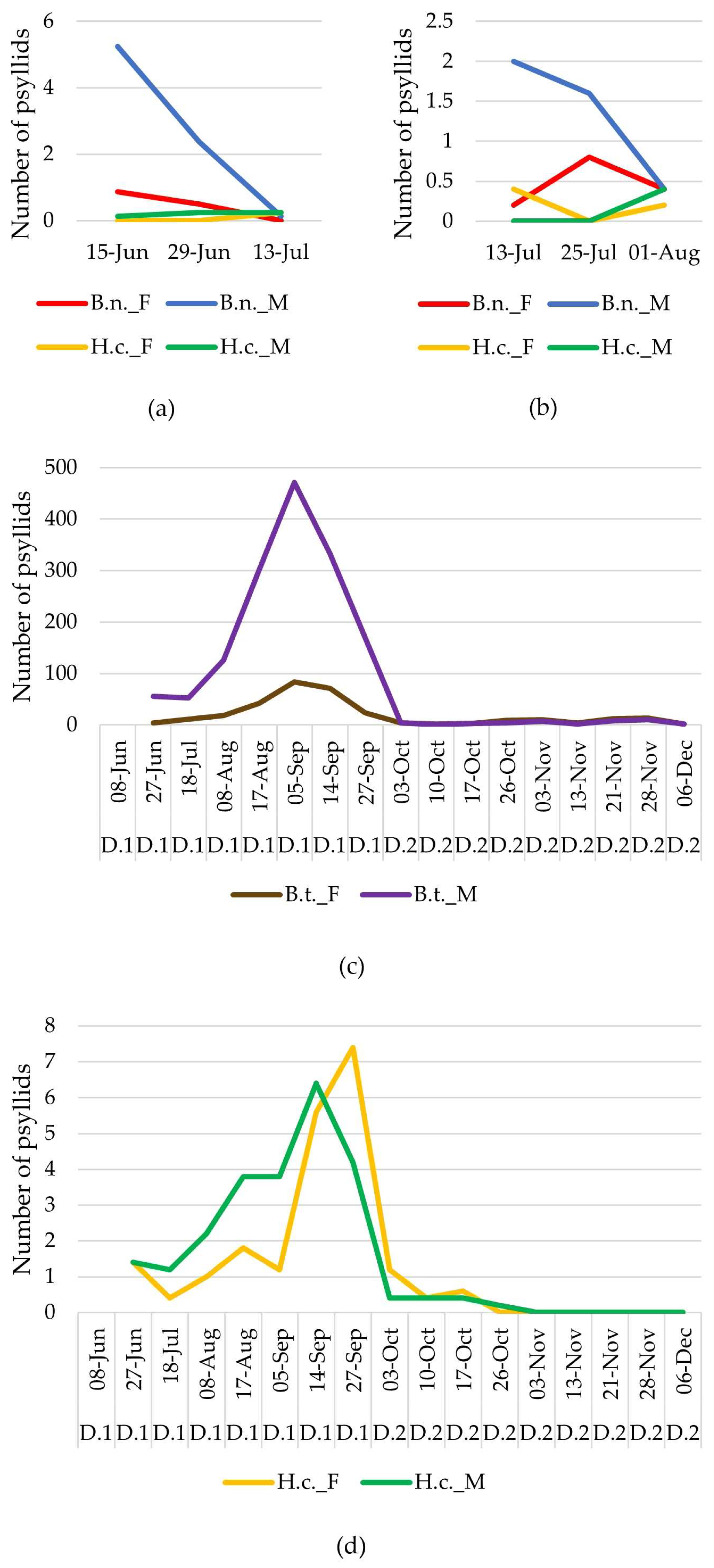

| Year | Site | Host Plant | Sampling Method | Sampling Date/Period | Number of Visits |
|---|---|---|---|---|---|
| 2022 | Govone (CN) (44°47′40.3″ N 8°06′51.8″ E) | Apium graveolens | beat tray | 31/05 07/06 | 2 |
| Grugliasco (TO) (45°04′12.8″ N 7°35′38.5″ E) | Solanum nigrum Daucus carota (wild) | beat tray | 28/07 | 1 | |
| Giaglione (TO) (45°08′35.6″ N 7°01′07.1″ E) | Fraxinus sp. Urtica dioica Urtica urens | beat tray sweep net | 08/08 | 1 | |
| Borgone Susa (TO) (45°07′09.7″ N 7°13′30.7″ E) | Chenopodium album | beat tray sweep net | 08/08 | 1 | |
| Chiusa di San Michele (TO) (45°06′02.2″ N 7°19′43.5″ E) | Convolvulus arvensis | beat tray sweep net | 08/08 | 1 | |
| Sant’Ambrogio di Torino (TO) (45°06′14.8″ N 7°20′49.1″ E) | Urtica dioica Artemisia vulgaris Parietaria officinalis Chenopodium album | beat tray sweep net | 08/08 24/08 | 2 | |
| Rivalta di Torino (TO) (45°01′40.9″ N 7°30′18.7″ E) | Laurus nobilis Ficus carica | beat tray | 25/08 | 1 | |
| Grugliasco (TO) (45°03′59.3″ N 7°35′32.5″ E) | Ficus carica | beat tray | 07/09 | 1 | |
| Benna (BI) (45°31′06.5″ N 8°07′16.8″ E) | Ficus carica | beat tray | 02/10 | 1 | |
| Lagnasco (CN) (44°37′26.3″ N 7°34′46.8″ E) | Chenopodium album | beat tray | 01/10 | 1 | |
| Benna (BI) (45°30′31.5″ N 8°07′54.1″ E) | Urtica dioica Chenopodium album | beat tray | 02/10 | 1 | |
| 2023 | Magnano 1 (BI) (45°27′56.4″ N 7°59′50.7″ E) | Solanum tuberosum | beat tray sweep net sticky traps | 30/05–13/07 | 4 |
| Magnano 2 (BI) (45°27′57.3″ N 7°59′49.5″ E) | Solanum tuberosum | beat tray sweep net sticky traps | 30/05–13/07 | 4 | |
| Pontecurone (AL) (44°56′00.7″ N 8°56′19.9″ E) | Solanum tuberosum Convolvolus arvensis Solanum physalifolium Amaranthus sp. Daucus sp. Galinsoga sp. Heliotropium sp. | beat tray sweep net sticky traps | 08/06–27/07 | 4 | |
| Avigliana (TO) (45°04′40.1″ N 7°24′24.5″ E) | Solanum tuberosum Amaranthus retroflexus Convolvolus arvensis Galinsoga parviflora | beat tray sweep net sticky traps | 26/06–01/08 | 4 | |
| Pontecurone (AL) (44°55′53.8″ N 8°56′06.6″ E) | Solanum lycopersicum | beat tray sweep net | 27/07 | 1 | |
| Dalmazzo 1 (CN) (44°21′11.2″ N 7°30′54.4″ E) | Daucus carrota Chenopodium album Trifolium sp. | beat tray sweep net sticky traps | 08/06–27/09 | 8 | |
| Dalmazzo 2 (CN) (44°21′13.2″ N 7°31′08.5″ E) | Daucus carrota Amaranthus blitoides Amaranthus hybridus Solanum nigrum Senecio squalidus Galinsoga parviflora | beat tray sweep net sticky traps | 27/09–06/12 | 10 |
| Location | Method | B. trigonica | B. nigricornis | H. chenopodii | T. urticae | T. alacris | A. freji | H. ficus | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | F | M | ||
| Grugliasco | BT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Giaglione | BT/SN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| B. Susa | BT/SN | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. Ambrogio | BT/SN | 0 | 0 | 0 | 0 | 22 | 13 | 8 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rivalta | BT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3 |
| Benna | BT | 0 | 0 | 0 | 0 | 43 | 42 | 1 | 0 | 0 | 0 | 0 | 0 | 23 | 18 |
| Lagnasco | BT | 0 | 0 | 0 | 0 | 18 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Magnano 1 | ST | 0 | 0 | 5 | 8 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Magnano 2 | ST | 0 | 0 | 6 | 54 | 2 | 3 | 0 | 1 | 0 | 0 | 1 | 12 | 0 | 0 |
| Avigliana | ST | 0 | 0 | 7 | 20 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dalmazzo 1 | BT/SN | 564 | 423 | 0 | 0 | 71 | 84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ST | 1270 | 7547 | 0 | 0 | 94 | 115 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dalmazzo 2 | BT/SN | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ST | 288 | 200 | 0 | 0 | 11 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Psyllid Species | Locality | Host Plant | CLso Positive/Tested |
|---|---|---|---|
| Heterotrioza chenopodii | Benna (BI) | Chenopodium album | 0/70 |
| Urtica dioica | 0/2 | ||
| Borgone Susa (TO) | Chenopodium album | 0/3 | |
| Lagnasco (CN) | Chenopodium album | 0/14 | |
| S. Ambrogio (TO) | Chenopodium album | 0/18 | |
| Homotoma ficus | Benna (BI) | Ficus carica | 0/3 |
| Grugliasco (TO) | Ficus carica | 0/5 | |
| Trioza alacris | Rivalta di Torino (TO) | Laurus nobilis | 0/4 |
| Trioza urticae | Benna (BI) | Urtica dioica | 1/1 |
| S. Ambrogio (TO) | Parietaria officinalis | 1/4 | |
| Urtica dioica | 0/11 |
| Host Plant | Location | Psyllid | CLso Positive/Tested | % CLso Positives | ||
|---|---|---|---|---|---|---|
| F | M | Total | ||||
| Solanum tuberosum | Avigliana | Bactericera nigricornis | 0/7 | 0/14 | 0/21 | 0 |
| Heterotrioza chenopodii | 0/2 | 0/2 | 0/4 | 0 | ||
| Magnano 1 | Bactericera nigricornis | 0/5 | 0/8 | 0/13 | 0 | |
| Heterotrioza chenopodii | 0/0 | 0/2 | 0/2 | 0 | ||
| Magnano 2 | Bactericera nigricornis | 0/5 | 1/31 | 1/36 | 2.8 | |
| Heterotrioza chenopodii | 0/2 | 0/3 | 0/5 | 0 | ||
| Aphalara freji | 0/1 | 0/7 | 0/8 | 0 | ||
| Daucus carota | Dalmazzo 1 | Bactericera trigonica | 6/156 | 3/124 | 9/280 | 3.2 |
| Heterotrioza chenopodii | 0/34 | 0/28 | 0/62 | 0 | ||
| Dalmazzo 2 | Bactericera trigonica | 0/78 | 2/139 | 2/217 | 0.9 | |
| Heterotrioza chenopodii | 2/11 | 1/6 | 3/17 | 17.6 | ||
| Chenopodium album | Dalmazzo 1 | Heterotrioza chenopodii | 0/9 | 0/6 | 0/15 | 0 |
| Locality | Plant | L | LS | Ro | V | Total | % CLso Positives |
|---|---|---|---|---|---|---|---|
| Avigliana | Solanum tuberosum | 0/1 | 0/1 | - | - | 0/2 | 0 |
| A. retroflexus | 0/2 | - | - | - | 0/2 | 0 | |
| C. arvensis | 0/1 | - | - | - | 0/1 | 0 | |
| G. parviflora | 0/1 | - | - | - | 0/1 | 0 | |
| Pontecurone | Solanum tuberosum | - | 0/14 | - | - | 0/14 | 0 |
| S. physalifolium | - | 0/3 | - | - | 0/3 | 0 | |
| C. arvensis | - | 0/1 | - | - | 0/1 | 0 | |
| D. carota (wild) | - | 0/1 | - | - | 0/1 | 0 | |
| Dalmazzo 1 | D. carota | - | 0/23 | 0/24 | 0/36 | 0/83 | 0 |
| C. album | - | - | - | 0/9 | 0/9 | 0 | |
| Dalmazzo 2 | D. carota | - | - | - | 3/39 | 3/39 | 3.39 |
| S. nigrum | - | - | - | 0/15 | 0/15 | 0 | |
| S. squalidus | - | - | - | 0/5 | 0/5 | 0 | |
| A. blitoides | - | - | - | 0/4 | 0/4 | 0 | |
| A. hybridus | - | - | - | 0/2 | 0/2 | 0 | |
| G. parviflora | - | - | - | 0/2 | 0/2 | 0 |
| Location | Plant | Asymptomatic | Symptomatic |
|---|---|---|---|
| Avigliana | Solanum tuberosum | (0/2) | - |
| Pontecurone | Solanum tuberosum | (0/9) | (0/5) |
| Dalmazzo 1 | Daucus carota | (0/17) | (0/66) |
| Dalmazzo 2 | Daucus carota | (1/25) | (2/14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oraby, A.Y.S.; Candian, V.; Tedeschi, R. Survey of ‘Candidatus Liberibacter solanacearum’ and Its Potential Psyllid Vectors in Northwestern Italy. Insects 2025, 16, 499. https://doi.org/10.3390/insects16050499
Oraby AYS, Candian V, Tedeschi R. Survey of ‘Candidatus Liberibacter solanacearum’ and Its Potential Psyllid Vectors in Northwestern Italy. Insects. 2025; 16(5):499. https://doi.org/10.3390/insects16050499
Chicago/Turabian StyleOraby, Ahmed Y. S., Valentina Candian, and Rosemarie Tedeschi. 2025. "Survey of ‘Candidatus Liberibacter solanacearum’ and Its Potential Psyllid Vectors in Northwestern Italy" Insects 16, no. 5: 499. https://doi.org/10.3390/insects16050499
APA StyleOraby, A. Y. S., Candian, V., & Tedeschi, R. (2025). Survey of ‘Candidatus Liberibacter solanacearum’ and Its Potential Psyllid Vectors in Northwestern Italy. Insects, 16(5), 499. https://doi.org/10.3390/insects16050499









