Effect of Spinetoram Stress on Midgut Detoxification Enzyme and Gene Expression of Apis cerana cerana Fabricius
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Samples
2.2. Exposure to SPI (Ethyl Polymyxin)
2.3. Enzyme Activity Assay
2.4. RNA Extraction and Sequencing
2.5. Data Quality Control, Filtering, and Analysis
3. Results
3.1. The Identification of Differentially Expressed Genes in the Pupae of Apis cerana cerana Fabricius in Response to SPI
3.2. Identification of Unique Genes Affected in SPI-Exposed Honey Bee Pupae
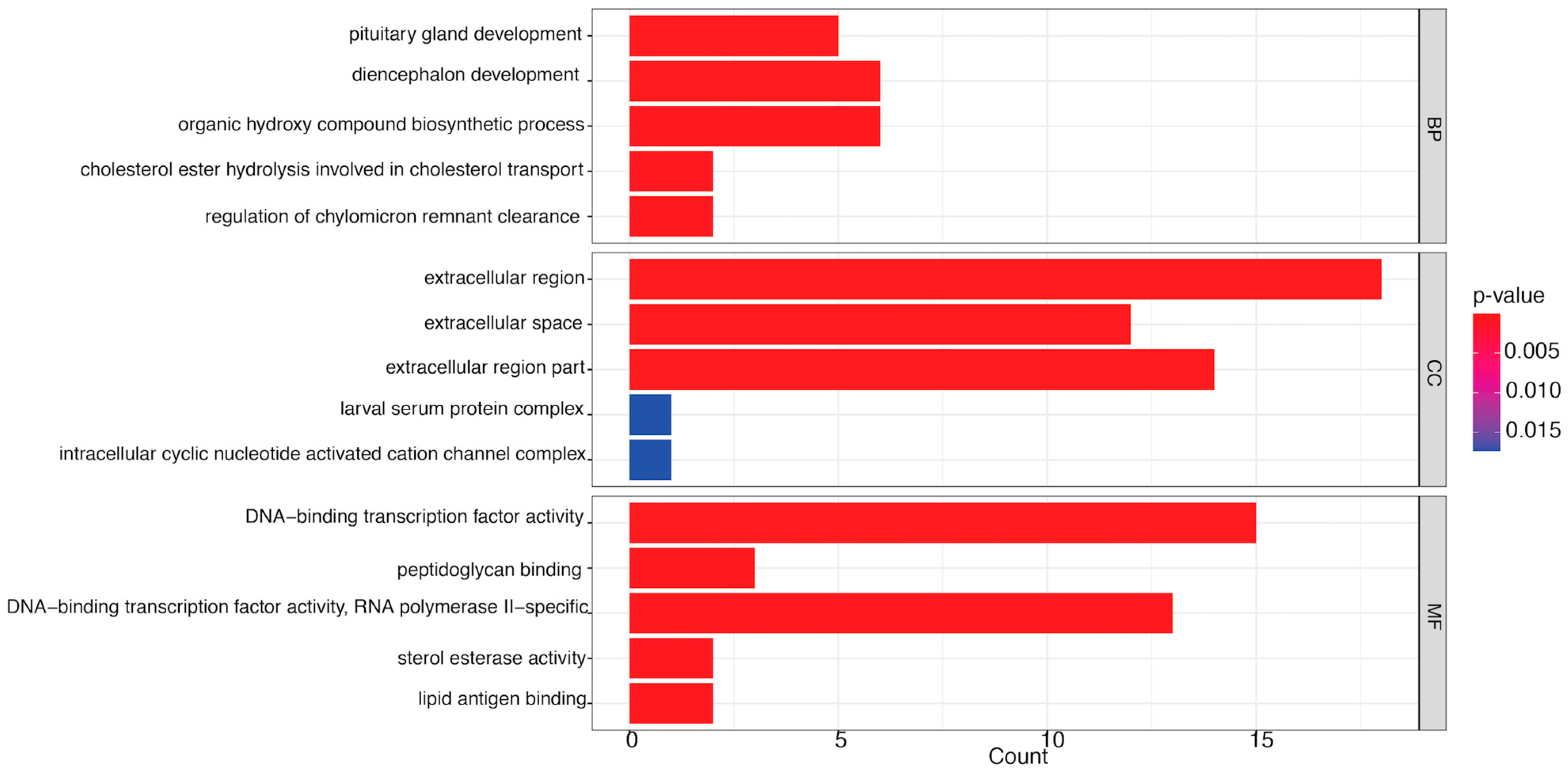
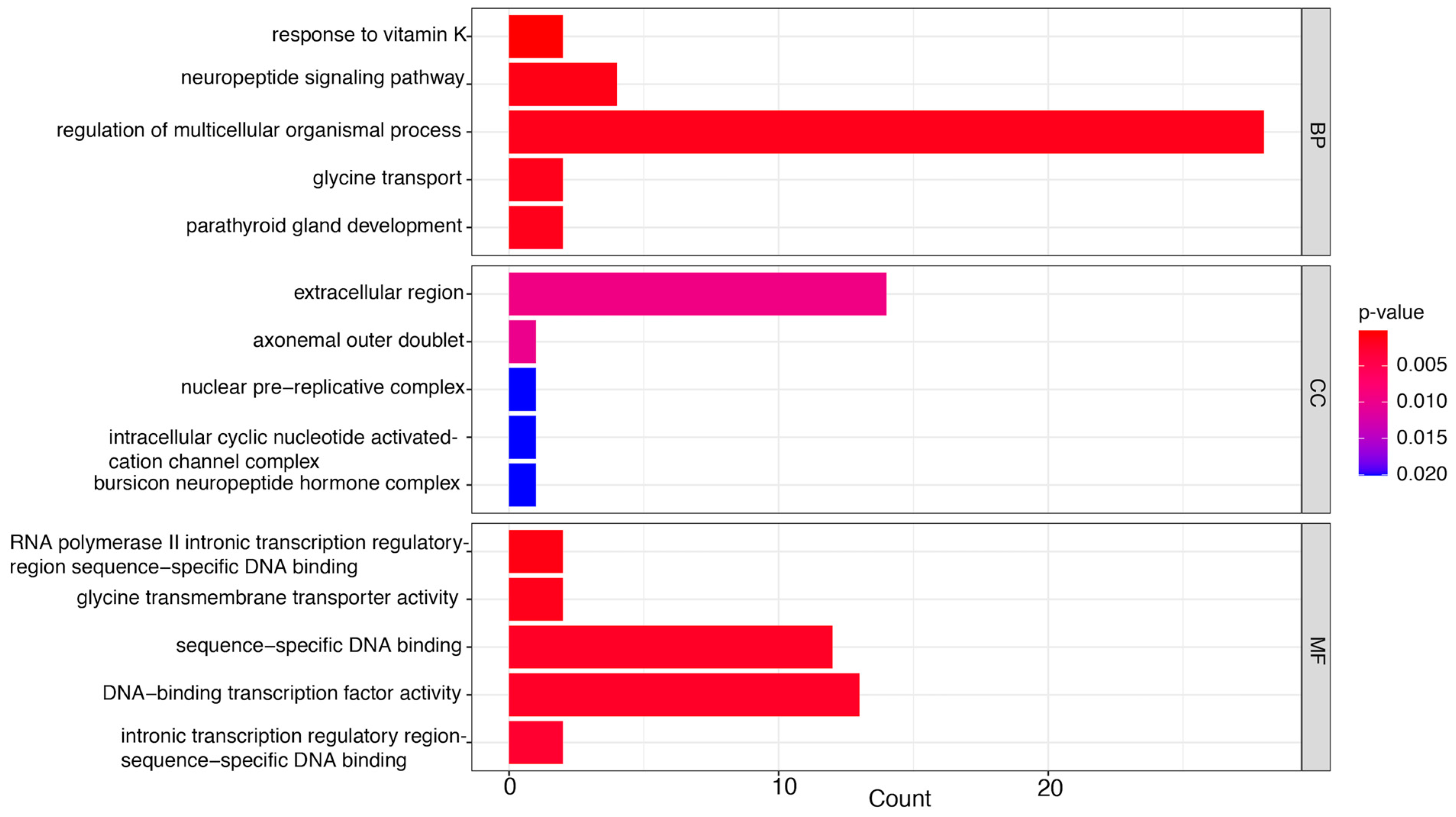
3.3. GO Enrichment Analysis of Significant DEGs
3.3.1. LC20 vs. CK
3.3.2. LC50 vs. CK
3.3.3. LC50 vs. LC20
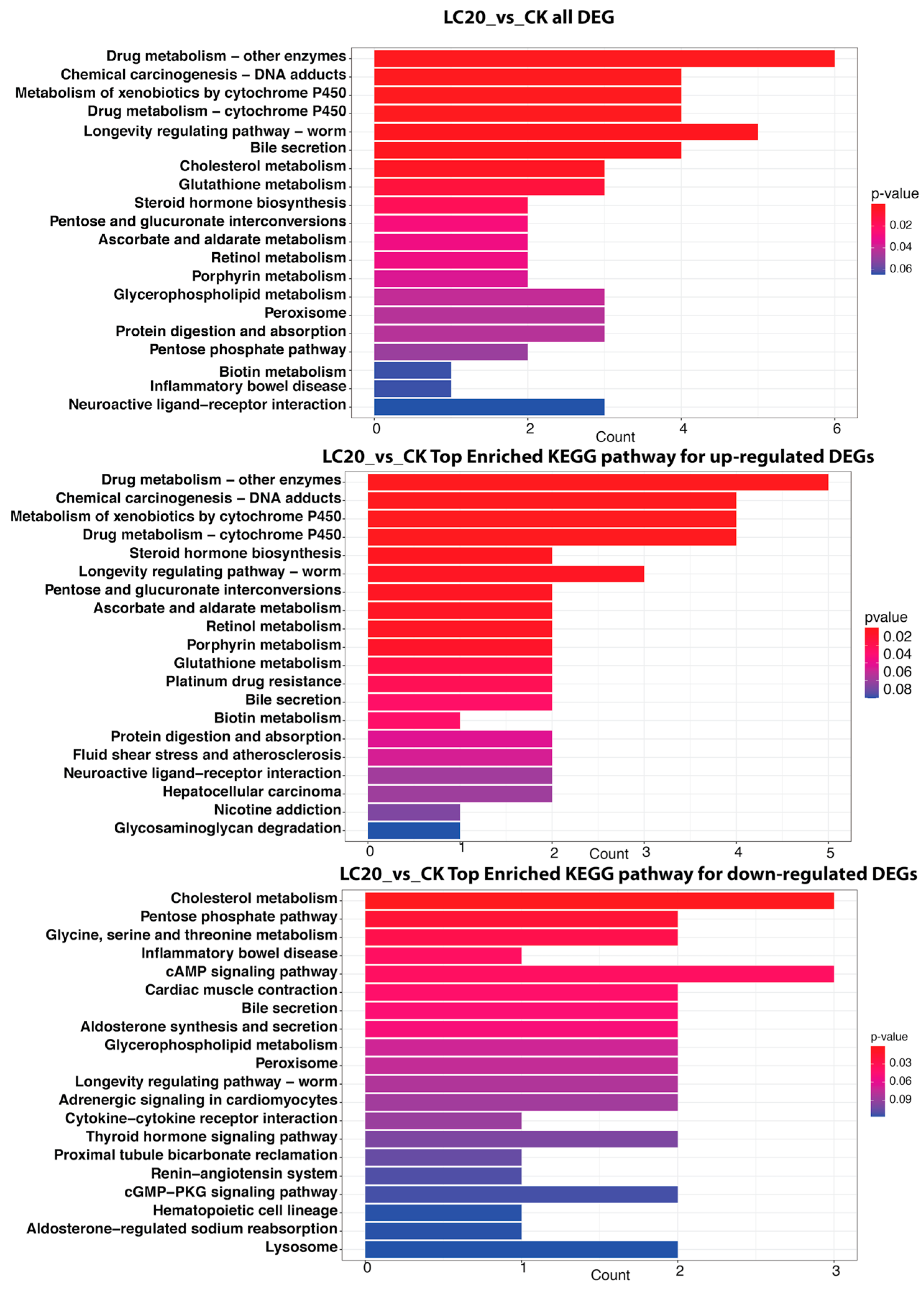
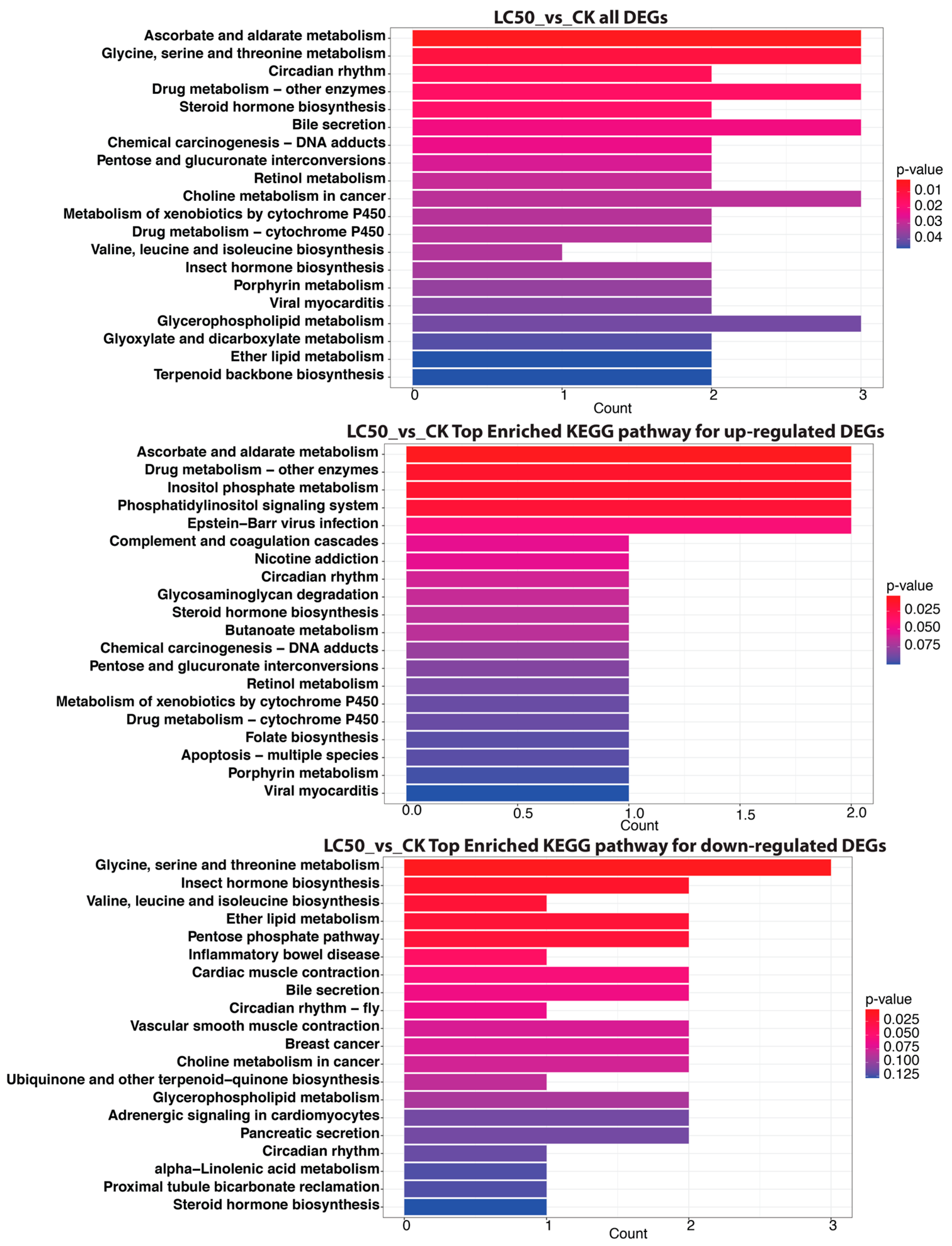
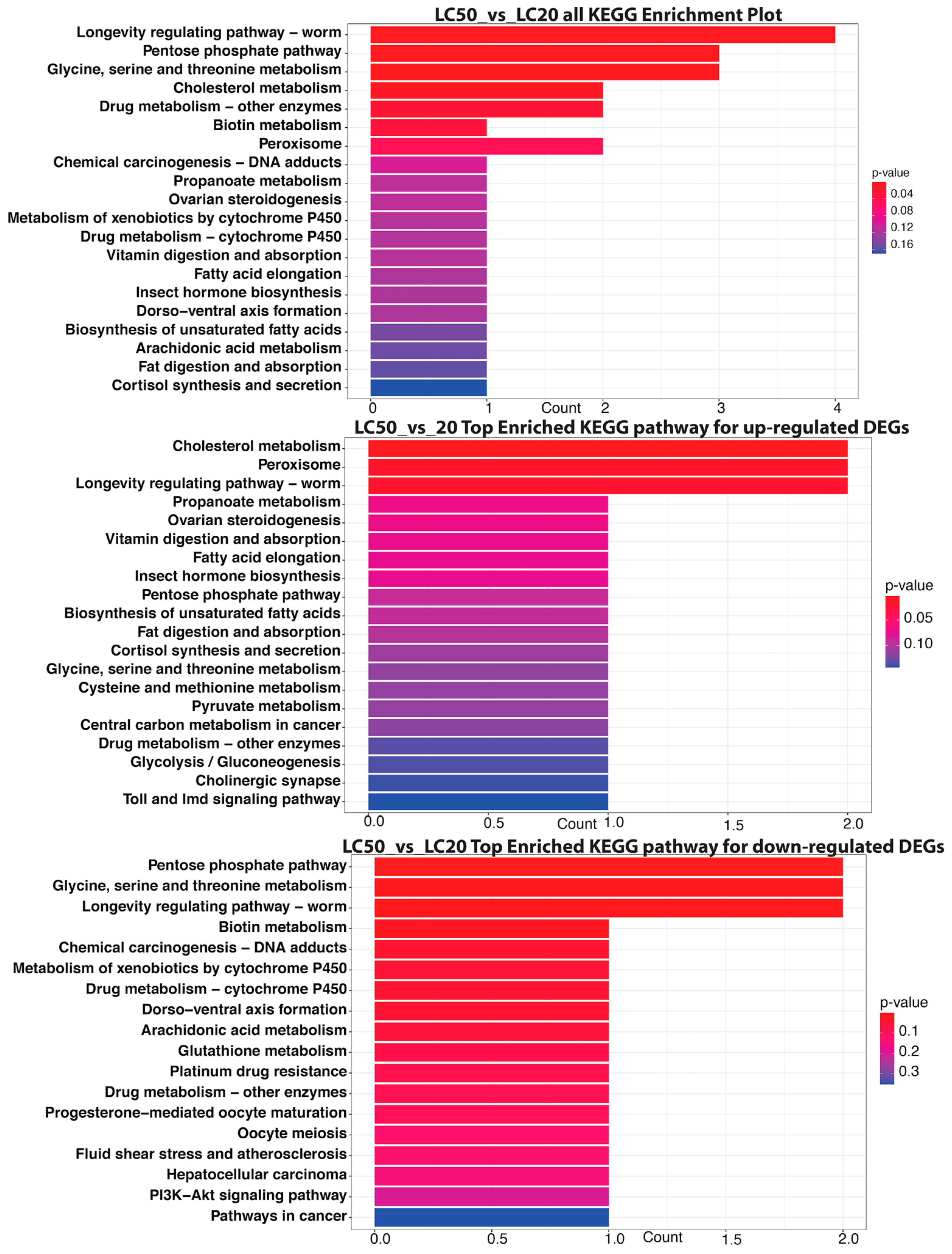
3.4. KEGG Pathway Enrichment Analysis of Significant DEGs
3.4.1. LC20 vs. CK
3.4.2. LC50 vs. CK
3.4.3. LC50 vs. LC20
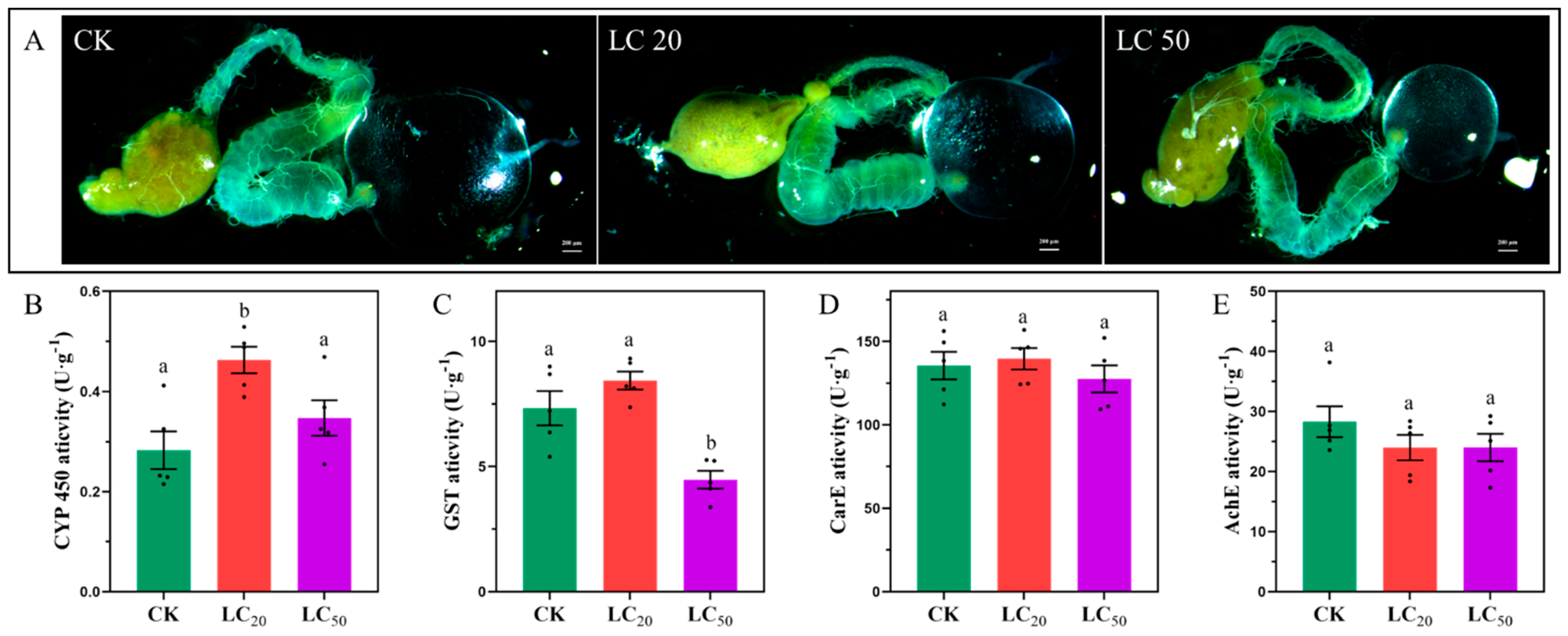
3.5. Morphological Changes in Midgut of Apis cerana cerana Fabricius
3.6. Enzyme Activity Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulhanek, K.; Steinhauer, N.; Rennich, K.; Caron, D.M.; Sagili, R.R.; Pettis, J.S.; Ellis, J.D.; Wilson, M.E.; Wilkes, J.T.; Tarpy, D.R.; et al. Encuesta nacional 2015–2016 sobre pérdidas anuales de colonias de la abeja de la miel manejada en los EE.UU. J. Apic. Res. 2017, 56, 328–340. [Google Scholar] [CrossRef]
- Christen, V. Different effects of pesticides on transcripts of the endocrine regulation and energy metabolism in honey bee foragers from different colonies. Sci. Rep. 2023, 13, 1985. [Google Scholar] [CrossRef]
- Choi, J.Y.; Chon, K.; Kim, J.; Vasamsetti, B.M.K.; Kim, B.S.; Yoon, C.Y.; Hwang, S.; Park, K.H.; Lee, J.H. Assessment of Lambda-Cyhalothrin and SPI Toxicity and Their Effects on the Activities of Antioxidant Enzymes and Acetylcholinesterase in honey bee (Apis mellifera) Larvae. Insects 2024, 15, 587. [Google Scholar] [CrossRef]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors associated with honey bee colony losses: A mini-review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goka, K. Pesticide residues and bees—A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; He, Q.; Liu, Q.; Wang, Z.; Yin, F.; Chai, Y.; Yang, Q.; Jiang, X.; Liao, M.; Yu, L.; et al. Analysis of honey bee exposure to multiple pesticide residues in the hive environment. Sci. Total Environ. 2022, 805, 150292. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Kim, J.; Chon, K.; Kim, B.S.; Yoon, C.Y.; Hwang, S.; Park, K.H. Molecular Impact of Sublethal SPI Exposure on honey bee (Apis mellifera) Larval and Adult Transcriptomes. Int. J. Mol. Sci. 2024, 25, 11923. [Google Scholar] [CrossRef]
- Li, B.; Ke, L.; Li, A.R.; Diao, Q.Y.; Wang, Q.; Liu, Y.J. Exposure of Larvae to Sublethal Thiacloprid Delays Bee Development and Affects Transcriptional Responses of Newly Emerged honey bees. Front. Insect Sci. 2022, 2, 844957. [Google Scholar] [CrossRef]
- Grella, T.C.; Soares-Lima, H.M.; Malaspina, O.; Nocelli, R.C.F. Semi-quantitative analysis of morphological changes in bee tissues: A toxicological approach. Chemosphere 2019, 236, 124255. [Google Scholar] [CrossRef]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 2011, 6, e14720. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, M.; Evison, S.E.F.; Gill, R.J.; Graystock, P. The threat of pesticide and disease co-exposure to managed and wild bee larvae. Int. J. Parasitol. Parasites Wildl. 2022, 17, 319–326. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crouse, G.D.; Benko, Z.; Demeter, D.; Giampietro, N.C.; Lambert, W.; Brown, A.V. The Spinosyns, Spinosad, SPI, and Synthetic Spinosyn Mimics—Discovery, Exploration, and Evolution of a Natural Product Chemistry and the Impact of Computational Tools; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Campos, M.R.; Silva, T.B.M.; Silva, W.M.; Silva, J.E.; Siqueira, H.A.A. Spinosyn resistance in the tomato borer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Pest Sci. 2015, 88, 405–412. [Google Scholar] [CrossRef]
- Jiang, M.; Qian, X.; Zhou, Z.; Liu, Y.; Zhang, M.; Yang, Y. Impacts of Sublethal Doses of SPI on the Biological Traits and Detoxifying Enzymes of the Tomato Leaf Miner, Tuta absoluta (Lepidoptera: Gelechiidae). Insects 2024, 15, 990. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, D.A.; Huseth, A.S.; Kennedy, G.G. Evaluation of alternative mode of action insecticides in managing neonicotinoid-resistant Frankliniella fusca in cotton. Crop Prot. 2018, 113, 56–63. [Google Scholar] [CrossRef]
- Galm, U.; Sparks, T.C. Natural product derived insecticides: Discovery and development of SPI. J. Ind. Microbiol. Biotechnol. 2016, 43, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.D.; Dutton, R.; Sparks, T.C. Spinosad—A case study: An example from a natural products discovery programme. Pest. Manag. Sci. Former. Pestic. Sci. 2000, 56, 696–702. [Google Scholar] [CrossRef]
- Martelli, F.; Ravenscroft, T.A.; Hutchison, W.; Batterham, P. Tissue-specific transcriptome analyses in Drosophila provide novel insights into the mode of action of the insecticide spinosad and the function of its target, nAChRα6. Pest Manag. Sci. 2023, 79, 3913–3925. [Google Scholar] [CrossRef]
- Martelli, F.; Hernandes, N.H.; Zuo, Z.; Wang, J.; Wong, C.O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Robin, C.; Venkatachalam, K.; et al. Low doses of the organic insecticide spinosad trigger lysosomal defects, elevated ROS, lipid dysregulation, and neurodegeneration in flies. Elife 2022, 11, e73812. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Miles, M.J.; Alix, A.; Bourgouin, C.; Schmitzer, S. Effects of spinosad on honey bees (Apis mellifera): Findings from over ten years of testing and commercial use. Jul.-Kühn-Archiv. 2012, 437, 107. [Google Scholar] [CrossRef]
- Epa, U.O.; Pesticide Programs. US EPA—Pesticides—Fact Sheet for SPI. 2009. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_G-4674_01-Oct-09.pdf (accessed on 25 January 2025).
- Zhang, Y.; Guo, W.; Chen, H.; Gao, J.; Xu, Z.; Tao, L.; Li, Z.; Xu, W. SPI confers its cytotoxic effects by inducing AMPK/mTOR-mediated autophagy and oxidative DNA damage. Ecotoxicol. Environ. Saf. 2019, 183, 109480. [Google Scholar] [CrossRef] [PubMed]
- Salgado, V.L. Studies on the Mode of Action of Spinosad: Insect Symptoms and Physiological Correlates. Pestic. Biochem. Physiol. 1998, 60, 91–1024. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, H.; Jia, K.; Li, E.; Zhang, S.; Yu, H.; Cao, Z.; Xiong, G.; Hu, C.; Lu, H. Effects of SPI on the developmental toxicity and immunotoxicity of zebrafish. Fish Shellfish. Immunol. 2020, 96, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Murawska, A.; Migdał, P.; Roman, A. Effects of plant protection products on biochemical markers in honey bees. Agriculture 2021, 11, 648. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Belzunces, L.P.; Carvalho, G.A.; Brunet, J.L.; Badiou-Beneteau, A. Enzymatic biomarkers as tools to assess environmental quality: A case study of exposure of the honey bee Apis mellifera to insecticides. Environ. Toxicol. Chem. 2013, 32, 2117–2124. [Google Scholar] [CrossRef]
- Qi, S.; Zhu, L.; Wang, D.; Wang, C.; Chen, X.; Xue, X.; Wu, L. Flumethrin at honey-relevant levels induces physiological stresses to honey bee larvae (Apis mellifera L.) in vitro. Ecotoxicol. Environ. Saf. 2020, 190, 110101. [Google Scholar] [CrossRef]
- Tavares, D.A.; Dussaubat, C.; Kretzschmar, A.; Carvalho, S.M.; Silva-Zacarin, E.C.; Malaspina, O.; Bérail, G.; Brunet, J.L.; Belzunces, L.P. Exposure of larvae to thiamethoxam affects the survival and physiology of the honey bee at post-embryonic stages. Environ. Pollut. 2017, 229, 386–393. [Google Scholar] [CrossRef]
- Peng, Y.S.C.; Mussen, E.; Fong, A.; Montague, M.A.; Tyler, T. Effects of Chlortetracycline on honey bee worker larvae reared in vitro. J. Invertebr. Pathol. 1992, 60, 127–133. [Google Scholar] [CrossRef]
- Rahman, A.; Muktadir, M.G. SPSS: An Imperative Quantitative Data Analysis Tool for Social Science Research. Int. J. Res. Innov. Soc. Sci. 2021, 5, 300–302. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; ScienceOpen, Inc.: Lexington, MA, USA, 2010. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, M.C.; Konaté, M.M.; Chen, L.; Das, B.; Karlovich, C.; Williams, P.M.; Evrard, Y.A.; Doroshow, J.H.; McShane, L.M. TPM, FPKM, or Normalized Counts? A Comparative Study of Quantification Measures for the Analysis of RNA-seq Data from the NCI Patient-Derived Models Repository. J. Transl. Med. 2021, 19, 269. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. Gene ontology consortium: Going forward. Nucleic Acids Res. 2015, 43, D1049–D1056. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2014, 42, D199–D205. [Google Scholar] [CrossRef]
- Kadonaga, J.T. Review Regulation of RNA Polymerase II Transcription by Sequence-Specific DNA Binding Factors. Cell 2004, 116, 247–257. [Google Scholar] [CrossRef]
- Fukami, T.; Yokoi, T. The Emerging Role of Human Esterases. Drug Metab. Pharmacokinet. 2012, 27, 466–477. [Google Scholar] [CrossRef]
- De Libero, G.; Collmann, A.; Mori, L. The cellular and biochemical rules of lipid antigen presentation. Eur. J. Immunol. 2009, 39, 2648–2656. [Google Scholar] [CrossRef]
- Brown, R.L.; Strassmaier, T.; Brady, J.D.; Karpen, J.W. The Pharmacology of Cyclic Nucleotide-Gated Channels: Emerging from the Darkness. Curr. Pharm. Des. 2006, 12, 3597–3613. [Google Scholar] [CrossRef] [PubMed]
- Vazhacharickal, P.J. Bioactive Compounds from honey bee Products: An Overview of Therapeutic Properties. Int. J. Curr. Res. Aca. Rev. 2021, 9, 32–59. [Google Scholar]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honey bee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Jing, X.; Behmer, S.T. EN65CH13_Jing ARjats.cls Annual Review of Entomology Insect Sterol Nutrition: Physiological Mechanisms, Ecology, and Applications EN65CH13_Jing ARjats.cls. Annu. Rev. Entomol. 2020, 65, 251–271. [Google Scholar] [CrossRef]
- Zeng, B.-J.; Mortimer, B.-C.; Martins, I.J.; Seydel, U.; Redgrave, T.G. Chylomicron remnant uptake is regulated by the expression and function of heparan sulfate proteoglycan in hepatocytes Supplementary key words antisense antibody competitor inhib-itor receptor blocker metabolism lipoprotein glycosaminogly-can confocal microscope. J. Lipid Res. 1998, 39, 845–860. [Google Scholar]
- Li, Y.; Song, H.; Xie, L.; Tang, X.; Jiang, Y.; Yao, Y.; Peng, X.; Cui, J.; Zhou, Z.; Xu, J. Surviving high temperatures: The crucial role of vesicular inhibitory amino acid transporter in Asian honey bee, Apis cerana. Int. J. Biol. Macromol. 2024, 279, 135276. [Google Scholar] [CrossRef] [PubMed]
- Schier, A.C.; Taatjes, D.J. Structure and mechanism of the RNA polymerase II transcription machinery. Genes Dev. 2020, 34, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Heuser, T.; Song, K.; Fu, X.; Nicastro, D. One of the Nine Doublet Microtubules of Eukaryotic Flagella Exhibits Unique and Partially Conserved Structures. PLoS ONE 2012, 7, e46494. [Google Scholar] [CrossRef]
- Song, Q. Bursicon, a Neuropeptide Hormone that Controls Cuticle Tanning and Wing Expansion. In Insect Endocrinology; Academic Press: New York, NY, USA, 2012; pp. 93–105. [Google Scholar] [CrossRef]
- Kim, L.; Brudzynski, K. Identification of menaquinones (vitamin K2 homologues) as novel constituents of honey. Food Chem. 2018, 249, 184–192. [Google Scholar] [CrossRef]
- Chen, H.; Senda, T.; Emura, S.; Kubo, K.-Y. Send Orders of Reprints at reprints@benthamscience.net. Open Anat. J. 2013, 5, 1–9. [Google Scholar] [CrossRef]
- Peissig, K.; Condie, B.G.; Manley, N.R. Embryology of the Parathyroid Glands. Endocrinol. Metab. Clin. N. Am. 2018, 47, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase; StatPearls Publishing: Tampa, FL, USA, 2025. [Google Scholar]
- Deng, Y.; Yang, S.; Zhao, H.; Luo, J.; Lu, Z.; Hou, C. Peptidoglycan recognition protein S2 is crucial for activation the Toll pathway against Israeli acute paralysis virus infection in honey bee Apis mellifera. bioRxiv 2022. [Google Scholar] [CrossRef]
- Copeland, D.C.; Ricigliano, V.A.; Mott, B.M.; Kortenkamp, O.L.; Erickson, R.J.; Gorrochategui-Ortega, J.; Anderson, K.E. A longitudinal study of queen health in honey bees reveals tissue specific response to seasonal changes and pathogen pressure. Sci. Rep. 2024, 14, 8963. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.W.T.; Foster, L.J. Changes in protein expression during honey bee larval development. Genome Biol. 2008, 9, R156. [Google Scholar] [CrossRef]
- Fang, Y.; Feng, M.; Han, B.; Lu, X.; Ramadan, H.; Li, J. In-depth proteomics characterization of embryogenesis of the honey bee worker (Apis mellifera ligustica). Mol. Cell. Proteom. 2014, 13, 2306–2320. [Google Scholar] [CrossRef]
- Gao, J.; Yang, Y.; Ma, S.; Liu, F.; Wang, Q.; Wang, X.; Wu, Y.; Zhang, L.; Liu, Y.; Diao, Q.; et al. Combined transcriptome and metabolite profiling analyses provide insights into the chronic toxicity of carbaryl and acetamiprid to Apis mellifera larvae. Sci. Rep. 2022, 12, 16898. [Google Scholar] [CrossRef]
- Guo, Z.; Qin, J.; Zhou, X.; Zhang, Y. Insect transcription factors: A landscape of their structures and biological functions in Drosophila and beyond. Int. J. Mol. Sci. 2018, 19, 3691. [Google Scholar] [CrossRef] [PubMed]
- Giurfa, M. The amazing mini-brain: Lessons from a honey bee. Bee World 2003, 84, 5–18. [Google Scholar] [CrossRef]
- Zafra, F.; Giménez, C. Glycine transporters and synaptic function. IUBMB Life 2008, 60, 810–817. [Google Scholar] [CrossRef]
- Altstein, M.; Nässel, D.R. Chapter 8 Neuropeptide Signaling in Insects; Springer: Boston, MA, USA, 2010. [Google Scholar]
- Gao, Z.; Batool, R.; Xie, W.; Huang, X.; Wang, Z. Transcriptome and Metabolome Analysis Reveals the Importance of Amino-Acid Metabolism in Spodoptera Frugiperda Exposed to SPI. Insects 2022, 13, 852. [Google Scholar] [CrossRef]
- Vázquez, D.E.; Latorre-Estivalis, J.M.; Ons, S.; Farina, W.M. Chronic exposure to glyphosate induces transcriptional changes in honey bee larva: A toxicogenomic study. Environ. Pollut. 2020, 261, 114148. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Song, Y.; Zeng, R. The Role of Cytochrome P450-Mediated Detoxification in Insect Adaptation to Xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.K.; Streit, T.M.; Herring, K.L.; Xie, S. Carboxylesterases: Dual roles in lipid and pesticide metabolism. J. Pestic. Sci. 2010, 35, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Serrão, J.E. Ultrastructural Aspects of Epithelial Renewal in the Midgut of Adult Worker Bees (Hymenoptera, Apidae). 1996. Available online: https://www.researchgate.net/publication/292732535 (accessed on 25 January 2025).




| Insecticide | Toxicity Regression Equation | LC20 (mg·L−1) | 95% Confidence Interval | LC50 (mg·L−1) | 95% Confidence Interval | R2 |
|---|---|---|---|---|---|---|
| SPI | y = 1.043 x + 0.391 | 0.066 | 0.044 ~ 0.092 | 0.421 | 0.290 ~ 0.701 | 0.966 |
| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Clean Ratio | Q20 | Q30 | GC | Mapped Reads | Map Rate | Uniq |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CK_1 | 49,289,180 | 7,393,377,000 | 49,279,482 | 7,339,884,868 | 99.98% | 99.60% | 98.40% | 39.37% | 48,264,825 | 97.94% | 47,429,902 |
| CK_2 | 54,338,544 | 8,150,781,600 | 54,324,974 | 8,055,938,328 | 99.98% | 99.63% | 98.54% | 40.48% | 53,104,962 | 97.75% | 52,246,861 |
| CK_3 | 51,610,170 | 7,741,525,500 | 51,598,260 | 7,708,607,852 | 99.98% | 99.41% | 97.72% | 39.41% | 50,432,130 | 97.74% | 49,526,790 |
| LC20_1 | 55,398,272 | 8,309,740,800 | 55,384,436 | 8,280,072,910 | 99.98% | 99.40% | 97.72% | 39.39% | 54,172,283 | 97.81% | 53,181,246 |
| LC20_2 | 57,170,342 | 8,575,551,300 | 57,158,026 | 8,517,249,552 | 99.98% | 99.63% | 98.52% | 38.91% | 55,886,945 | 97.78% | 54,938,014 |
| LC20_3 | 40,079,318 | 6,011,897,700 | 40,070,638 | 5,947,681,080 | 99.98% | 99.62% | 98.49% | 39.19% | 39,209,575 | 97.85% | 38,644,603 |
| LC50_1 | 61,038,878 | 9,155,831,700 | 61,026,728 | 9,092,782,540 | 99.98% | 99.60% | 98.40% | 39.27% | 59,609,971 | 97.68% | 58,498,542 |
| LC50_2 | 48,208,610 | 7,231,291,500 | 48,197,838 | 7,179,127,732 | 99.98% | 99.63% | 98.52% | 38.94% | 47,156,526 | 97.84% | 46,412,287 |
| LC50_3 | 41,712,970 | 6,256,945,500 | 41,703,366 | 6,235,278,786 | 99.98% | 99.45% | 97.86% | 39.27% | 40,661,149 | 97.50% | 39,975,945 |
| SampleID | Total | UP | DOWN |
|---|---|---|---|
| LC20_vs_CK | 138 | 76 | 62 |
| LC50_vs_CK | 159 | 89 | 70 |
| LC50_vs_LC20 | 53 | 29 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; He, T.; Ding, L.; Lan, X.; Sun, J.; Xu, X.; Wu, H.; Zhou, D.; Huang, Z.; Zhou, T.; et al. Effect of Spinetoram Stress on Midgut Detoxification Enzyme and Gene Expression of Apis cerana cerana Fabricius. Insects 2025, 16, 492. https://doi.org/10.3390/insects16050492
Chen L, He T, Ding L, Lan X, Sun J, Xu X, Wu H, Zhou D, Huang Z, Zhou T, et al. Effect of Spinetoram Stress on Midgut Detoxification Enzyme and Gene Expression of Apis cerana cerana Fabricius. Insects. 2025; 16(5):492. https://doi.org/10.3390/insects16050492
Chicago/Turabian StyleChen, Lin, Tianjun He, Linglong Ding, Xinyan Lan, Jiahao Sun, Xiaoheng Xu, Huafen Wu, Dayun Zhou, Zhichu Huang, Tianxing Zhou, and et al. 2025. "Effect of Spinetoram Stress on Midgut Detoxification Enzyme and Gene Expression of Apis cerana cerana Fabricius" Insects 16, no. 5: 492. https://doi.org/10.3390/insects16050492
APA StyleChen, L., He, T., Ding, L., Lan, X., Sun, J., Xu, X., Wu, H., Zhou, D., Huang, Z., Zhou, T., Su, X., & Chen, L. (2025). Effect of Spinetoram Stress on Midgut Detoxification Enzyme and Gene Expression of Apis cerana cerana Fabricius. Insects, 16(5), 492. https://doi.org/10.3390/insects16050492





