Potential Distribution of Anoplophora horsfieldii Hope in China Based on MaxEnt and Its Response to Climate Change
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Selection of A. horsfieldii Distribution Data
2.2. Acquisition and Processing of Environmental Variables
2.3. The Optimization and Construction of MaxEnt Model Parameters
2.4. Centroid Migration Analysis
3. Results
3.1. MaxEnt Model Accuracy Assessment and Identification of Key Environmental Variables
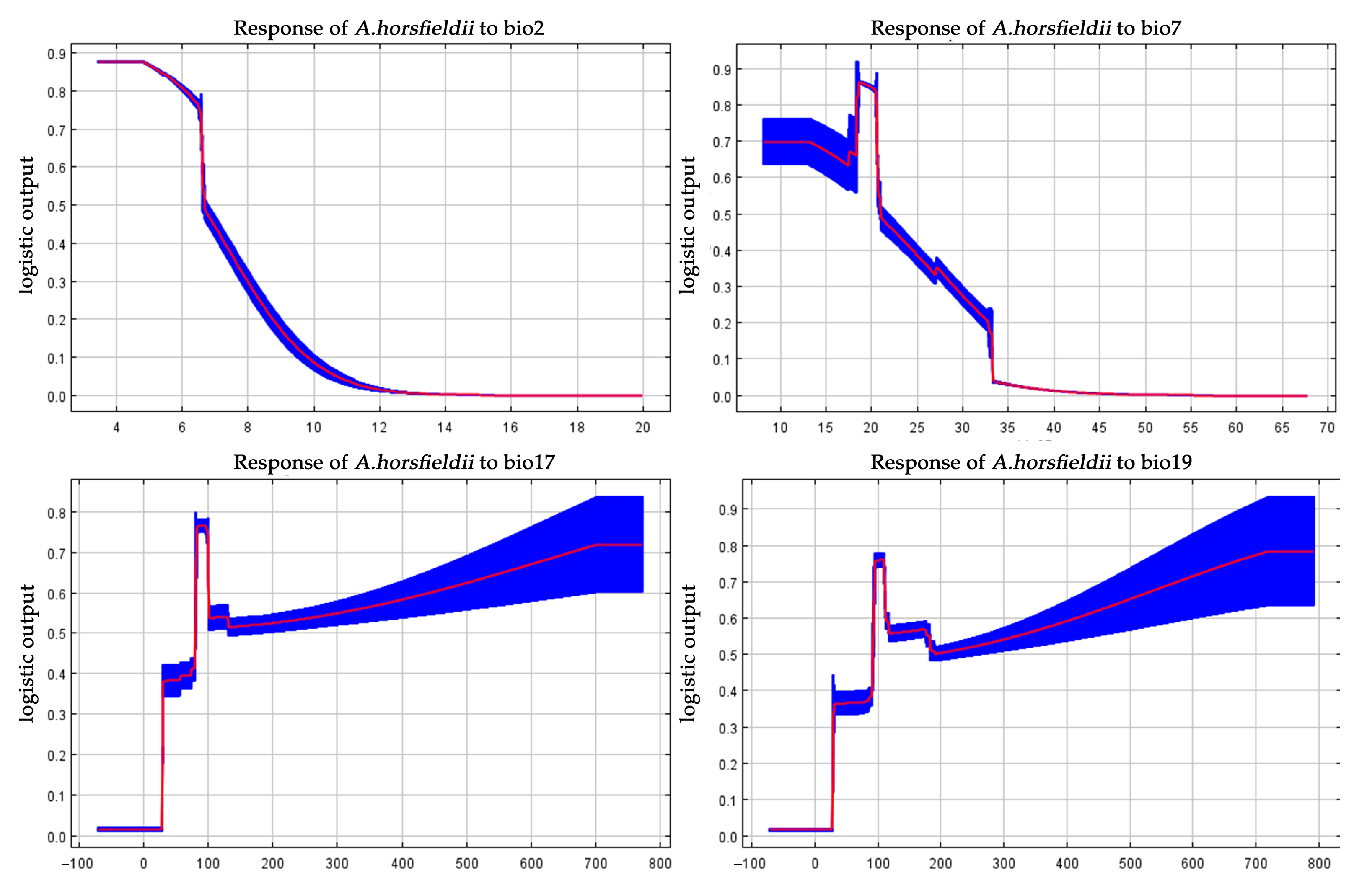
3.2. The Relationship Between Each Climate Variable and the Potential Distribution of A. horsfieldii
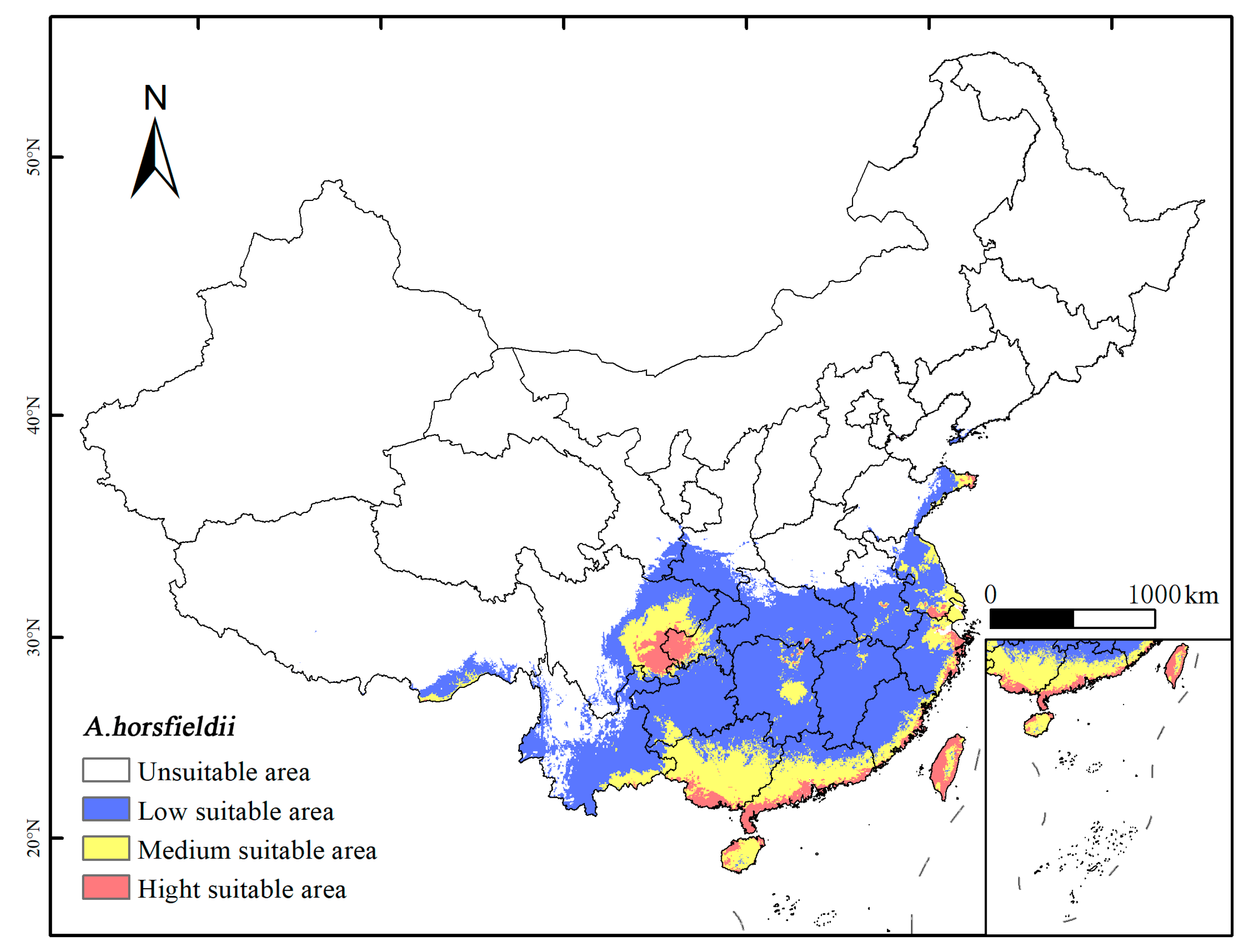
3.3. The Potential Distribution and Suitability Assessment of A. horsfieldii Under the Current Climate Scenario
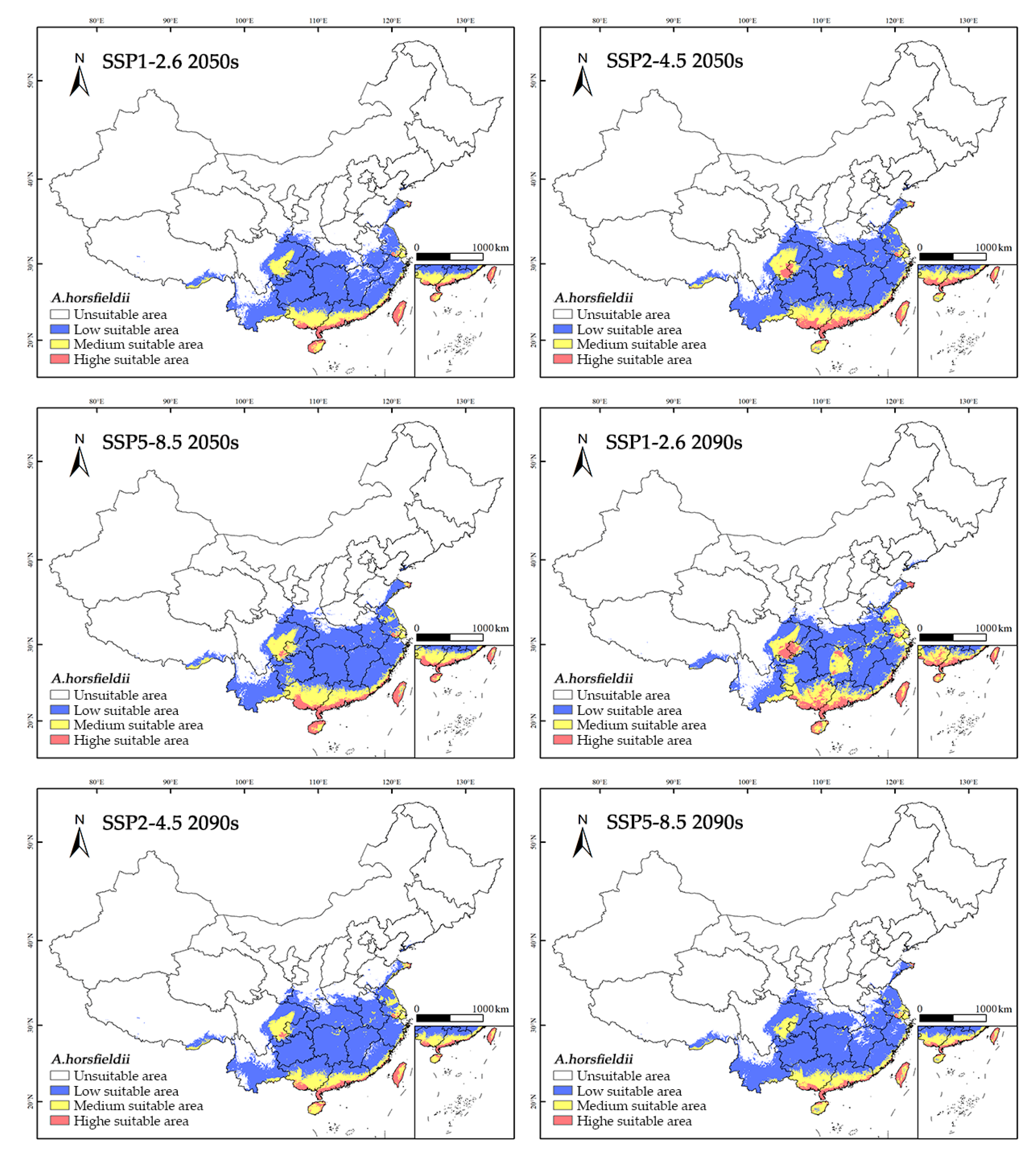
3.4. Prediction of the Suitable Habitat of A. horsfieldii Under Future Climate Scenarios
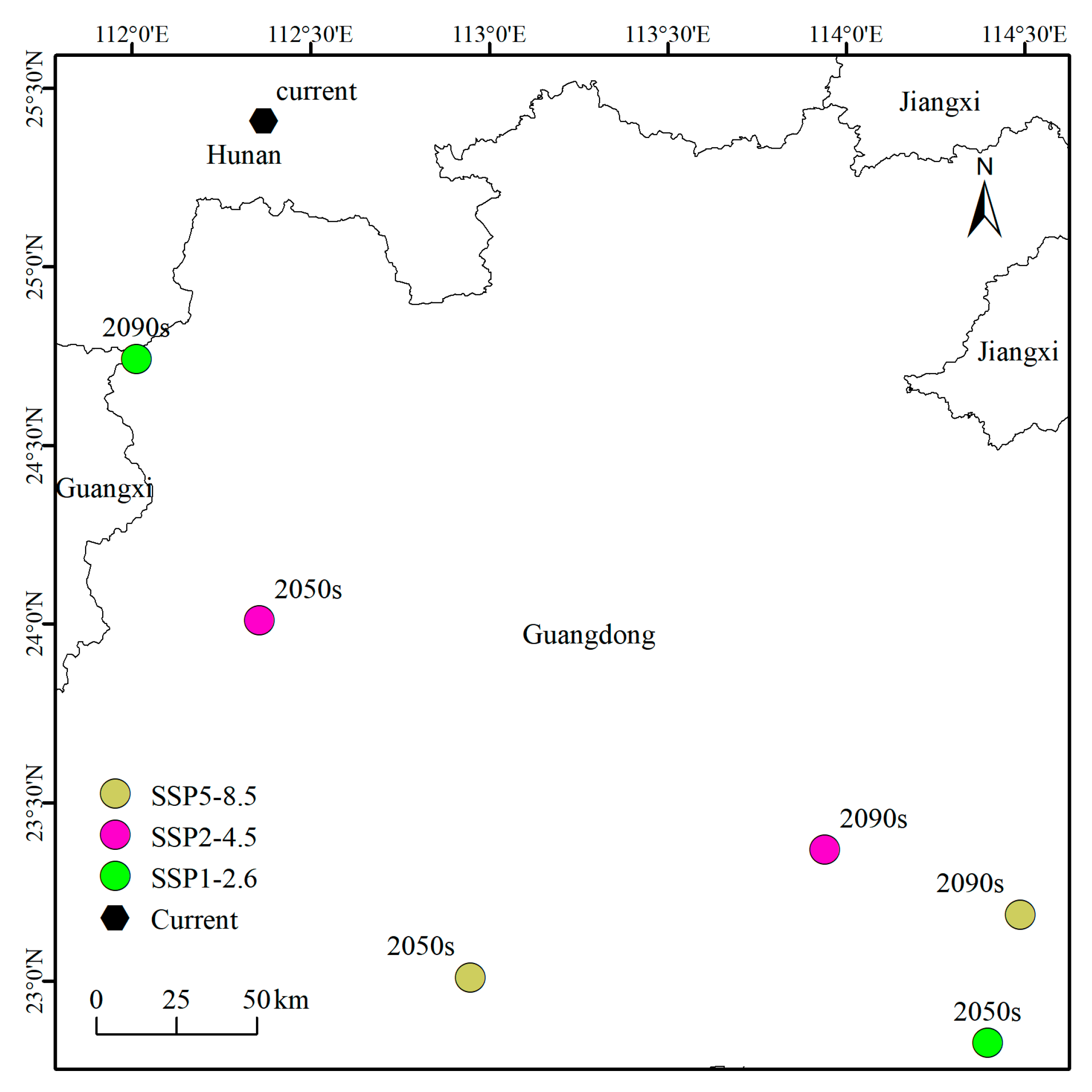
3.5. Analysis of Centroid Migration of A. horsfieldii’s Potential Suitable Habitat in China Under Climate Change Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.L.; Zhang, J.; Zhang, D.; Feng, Y.; Qiu, J.; Ye, X.J.; Wang, B.X. Complete mitochondrial genome of the longicorn Anoplophora horsfieldii Hope (Coleoptera: Cerambycidae). Mitochondrial DNA Part B 2023, 8, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Hoebeke, E. Revision of the Genus Anoplophora (Coleoptera: Cerambycidae); The Entomological Society of Washington: Washington, DC, USA, 2002. [Google Scholar]
- Lee, S.; Ko, G.; Lee, Y.; Lee, S.K.; Kim, M.; Lee, M.; Kang, K.; Ming, B.; Kim, D.; Lee, S. The third invasive Anoplophora: Citizen science facilitates uncovering massive abundance of non-native A. horsfieldii (Coleoptera: Cerambycidae) in South Korea. J. Asia-Pac. Entomol. 2024, 27, 102253. [Google Scholar] [CrossRef]
- Gressitt, J.L. Destructive Long-Horned Beetle Borers at Canton, China; Lingnan University: Guangzhou, China, 1942. [Google Scholar]
- Gressitt, J.L. Longicorn beetles of China. Longicornia 1951, 2, 22. [Google Scholar]
- Hua, L.; Nara, H.; Yu, C. Longicorn Beetles of Hainan & Guangdong; Muh-Sheng Museum of Entomology: Taiwan, China, 1993. [Google Scholar]
- Chou, W.I. Iconography of Longhorn Beetles in Taiwan; Owl Press: Taiwan, China, 2004. [Google Scholar]
- Tavakilian, G.L.; Chevillotte, H. Titan: Base de Données Internationales sur les Cerambycidae ou Longicornes; GBIF France: Paris, France, 2022. [Google Scholar]
- Lee, S.; Choi, J.; Jang, H.; Choi, W.; Kwon, W.; Kim, D.; Gim, J.; Park, J.; Park, S.; Kim, S.; et al. Establishment of non-native Anoplophora horsfieldii (Coleoptera: Cerambycidae) in South Korea. J. Integr. Pest. Manag. 2023, 14, 9. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.K.; Park, Y.; Kim, Y.; Lee, M.; Nam, Y. Climatic suitability and spread potential of Anoplophora horsfieldii (Coleoptera: Cerambycidae), a newly identified non-native insect on Jeju Island, Korea. Sci. Rep. 2025, 15, 10428. [Google Scholar] [CrossRef]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Renault, D.; Laparie, M.; McCauley, S.J.; Bonte, D. Environmental adaptations, ecological filtering, and dispersal central to insect invasions. Annu. Rev. Entomol. 2018, 63, 345–368. [Google Scholar] [CrossRef]
- Poland, T.M.; Patel-Weynand, T.; Finch, D.M.; Miniat, C.F.; Hayes, D.C.; Lopez, V.M. Invasive Species in Forests and Rangelands of the United States; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Cao, R.; Feng, J. Future Climate Change and Anthropogenic Disturbance Promote the Invasions of the World’s Worst Invasive Insect Pests. Insects 2024, 15, 280. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, M.; Li, M.; Jin, Z.; Yang, N.; Yu, H.; Liu, W. Climate Change Facilitates the Potentially Suitable Habitats of the Invasive Crop Insect Ectomyelois ceratoniae (Zeller). Atmosphere 2024, 15, 119. [Google Scholar] [CrossRef]
- Silva, D.P.; Andrade, A.F.A.; Oliveira, J.P.J.; Morais, D.M.; Vieira, J.E.A.; Engel, M.S. Current and future ranges of an elusive North American insect using species distribution models. J. Insect Conserv. 2019, 23, 175–186. [Google Scholar] [CrossRef]
- Wen, X.; Fang, G.; Chai, S.; He, C.; Sun, S.; Zhao, G.; Lin, X. Can ecological niche models be used to accurately predict the distribution of invasive insects? A case study of Hyphantria cunea in China. Ecol. Evol. 2024, 14, e11159. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, G.; Bu, W.; Gao, Y. The basic principles of niche models and their applications in biodiversity conservation biological diversity. Biodivers. Sci. 2013, 21, 90. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Alramadan, Y.; Mamay, M.; Farooq, S. Increased spread risk of citrus long-horned beetle [Anoplophora chinensis (Coleoptera: Cerambycidae)] under climate change in Türkiye: Implications for management. Crop Prot. 2025, 190, 107090. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Lei, Y. Predicting Distribution of the Asian Longhorned Beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae) and Its Natural Enemies in China. Insects 2022, 13, 687. [Google Scholar] [CrossRef]
- Xin, X.; Wu, T.; Zhang, J.; Zhang, F.; Li, W.; Zhang, Y.; Lu, Y.; Fang, Y.; Jie, W.; Zhang, L.; et al. Introduction to BCC Mode and CMIP6 Test Conducted. Adv. Clim. Change Res. 2019, 15, 533–539. [Google Scholar] [CrossRef]
- Kim, T.; Cho, Y. Predicting the potential distribution of an invasive species, Anoplophora horsfieldii (Coleoptera: Cerambycidae), under climate change using species distribution model. Proc. Korean Soc. Appl. Entomol. Conf. 2024, 180. [Google Scholar]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. Peerj 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, B.; Wan, F.; Xiao, Q.; Dai, L. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodivers. Sci. 2007, 15, 365. [Google Scholar]
- Qin, Z.; Zhang, J.E.; DiTommaso, A.; Wang, R.L.; Wu, R.S. Predicting invasions of Wedelia trilobata (L.) Hitchc. with Maxent and GARP models. J. Plant Res. 2015, 128, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Poutsma, J.; Loomans, A.J.M.; Aukema, B.; Heijerman, T. Predicting the potential geographical distribution of the harlequin ladybird, Harmonia axyridis, using the CLIMEX model. In From Biological Control to Invasion: The Ladybird Harmonia Axyridis as a Model Species; Springer: Berlin/Heidelberg, Germany, 2008; pp. 103–125. [Google Scholar]
- Wang, G.; Zhang, H.; Cao, X.; Zhang, X.; Wang, G.; He, Z.; Yu, C.; Zhao, T. Using GARP to predict the range of Aedes aegypti in China. Southeast Asian J. Trop. Med. 2014, 45, 290. [Google Scholar]
- Guisan, A.; Weiss, S.B.; Weiss, A.D. GLM versus CCA spatial modeling of plant species distribution. Plant Ecol. 1999, 143, 107–122. [Google Scholar] [CrossRef]
- Howse, M.W.; Haywood, J.; Lester, P.J. Bioclimatic modelling identifies suitable habitat for the establishment of the invasive European paper wasp (Hymenoptera: Vespidae) across the southern hemisphere. Insects 2020, 11, 784. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, F.; Lin, Y. Simulation analysis of ground temperature and precipitation changes in China in the 21st century under different greenhouse gas emission scenarios. Clim. Environ. Res. 2003, 8, 209–217. [Google Scholar] [CrossRef]
- Roth, T.; Plattner, M.; Amrhein, V. Plants, birds and butterflies: Short-term responses of species communities to climate warming vary by taxon and with altitude. PLoS ONE 2014, 9, e82490. [Google Scholar] [CrossRef]
- Ziter, C.; Robinson, E.A.; Newman, J.A. Climate change and voltinism in C alifornian insect pest species: Sensitivity to location, scenario and climate model choice. Global Change Biol. 2012, 18, 2771–2780. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Pajač Živković, I.; Lešić, V.; Lemić, D. Effect of climate change on introduced and native agricultural invasive insect pests in Europe. Insects 2021, 12, 985. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Arthur Woods, H.; Buckley, L.B.; Potter, K.A.; MacLean, H.J.; Higgins, J.K. Complex Life Cycles and the Responses of Insects to Climate Change; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Xing, K.; Hoffmann, A.A.; Ma, C. Does thermal variability experienced at the egg stage influence life history traits across life cycle stages in a small invertebrate? PLoS ONE 2014, 9, e99500. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef]
- Pareek, A.; Meena, B.M.; Sharma, S.; Tetarwal, M.L.; Kalyan, R.K.; Meena, B.L. Impact of climate change on insect pests and their management strategies. Clim. Chang. Sustain. Agric. 2017, 253–286. Available online: http://s.dic.cool/S/0vFiqKKM (accessed on 2 March 2025).
- Liu, Y.; Luo, J.; Zhang, D.; Wei, Y.; Zhou, Z. The effect of temperature and humidity on the developmental duration and hatching rate of Huangqi root nodule weevil eggs. Acta Phytophylacica Sin. 2019, 46, 1381–1382. [Google Scholar]
- Duffy, E.A.J. A monograph of the Immature Stages of Oriental Timber Beetles (Cerambycidae); Natural History Museum Publications: London, UK, 1968. [Google Scholar]
- Potter, K.A.; Arthur Woods, H.; Pincebourde, S. Microclimatic challenges in global change biology. Glob. Change Biol. 2013, 19, 2932–2939. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.M.; Khan, M.A.; Arshad, M.; Sagheer, M.; Sattar, Z.; Shafi, J.; Haq, E.U.; Ali, A.; Aslam, U.; Mushtaq, A.; et al. Impact of global warming on insects. Arch. Phytopathol. Plant Prot. 2015, 48, 84–94. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J. Assessing model performance of climate extremes in China: An intercomparison between CMIP5 and CMIP3. Clim. Change 2015, 129, 197–211. [Google Scholar] [CrossRef]
- Zhao, Z.; Luo, Y.; Jiang, Y.; Xu, Y. Detection and evaluation of global and Chinese precipitation, drought and flood changes. Sci. Technol. Rev. 2008, 6, 28–33. [Google Scholar]
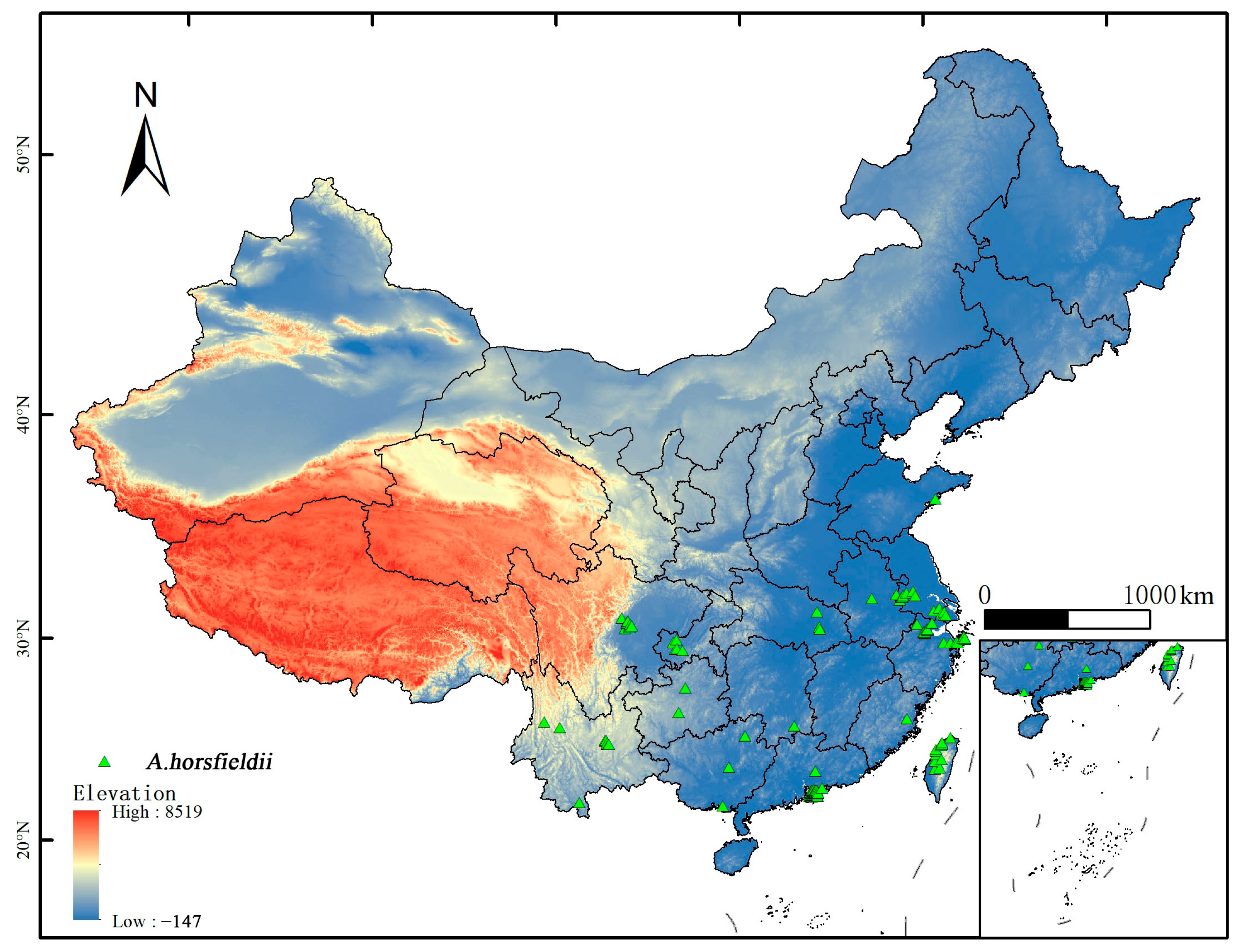
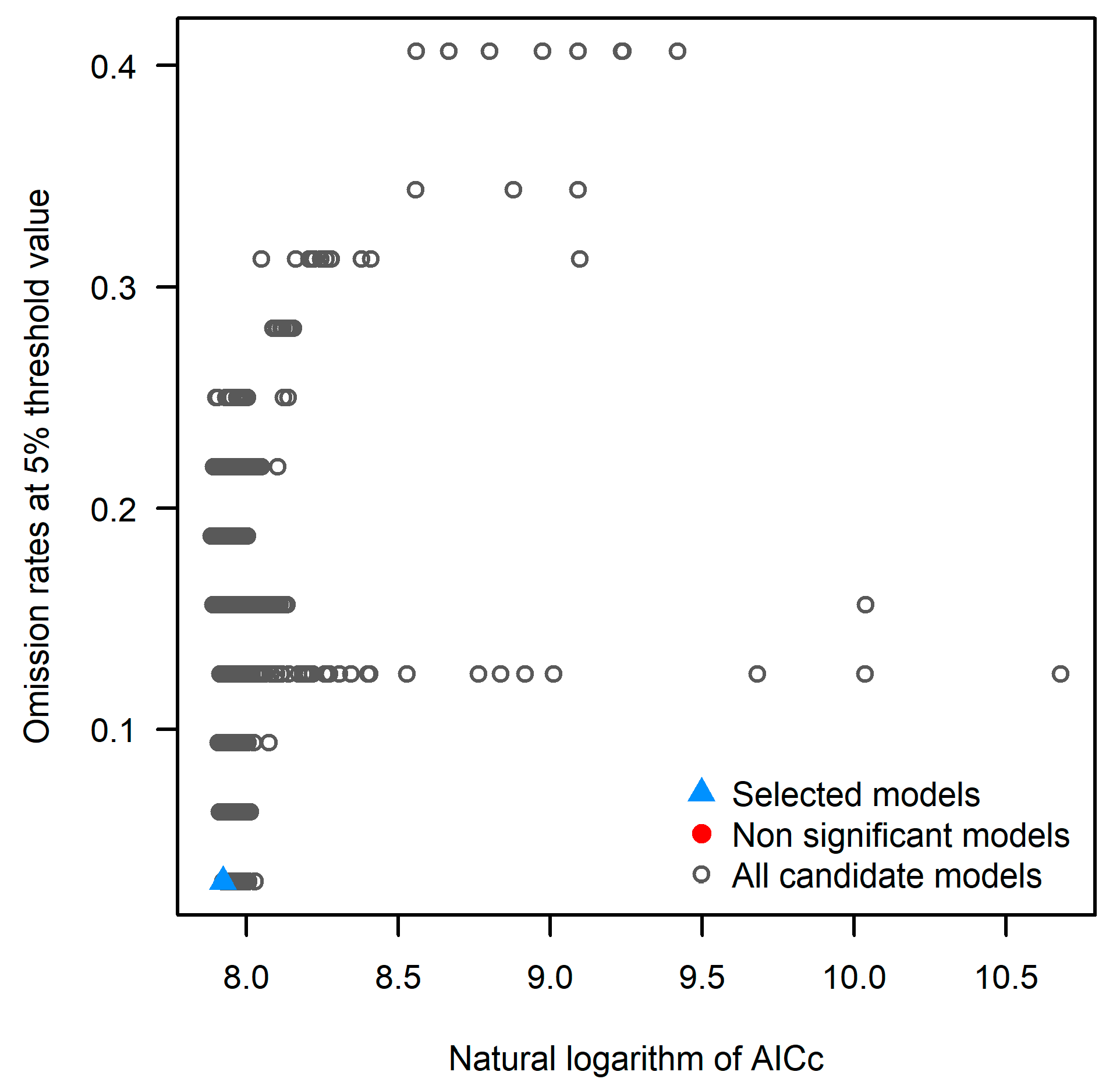
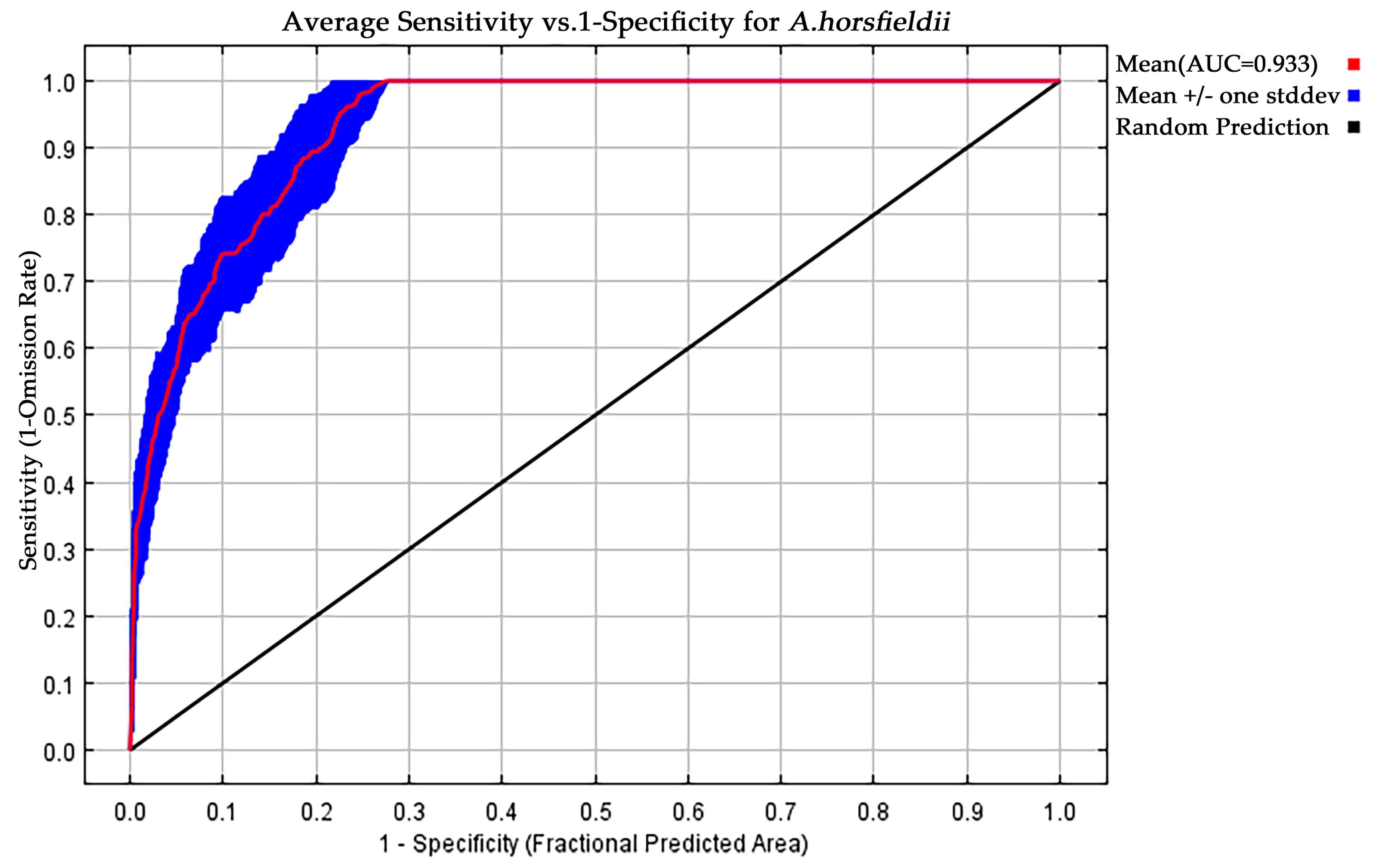
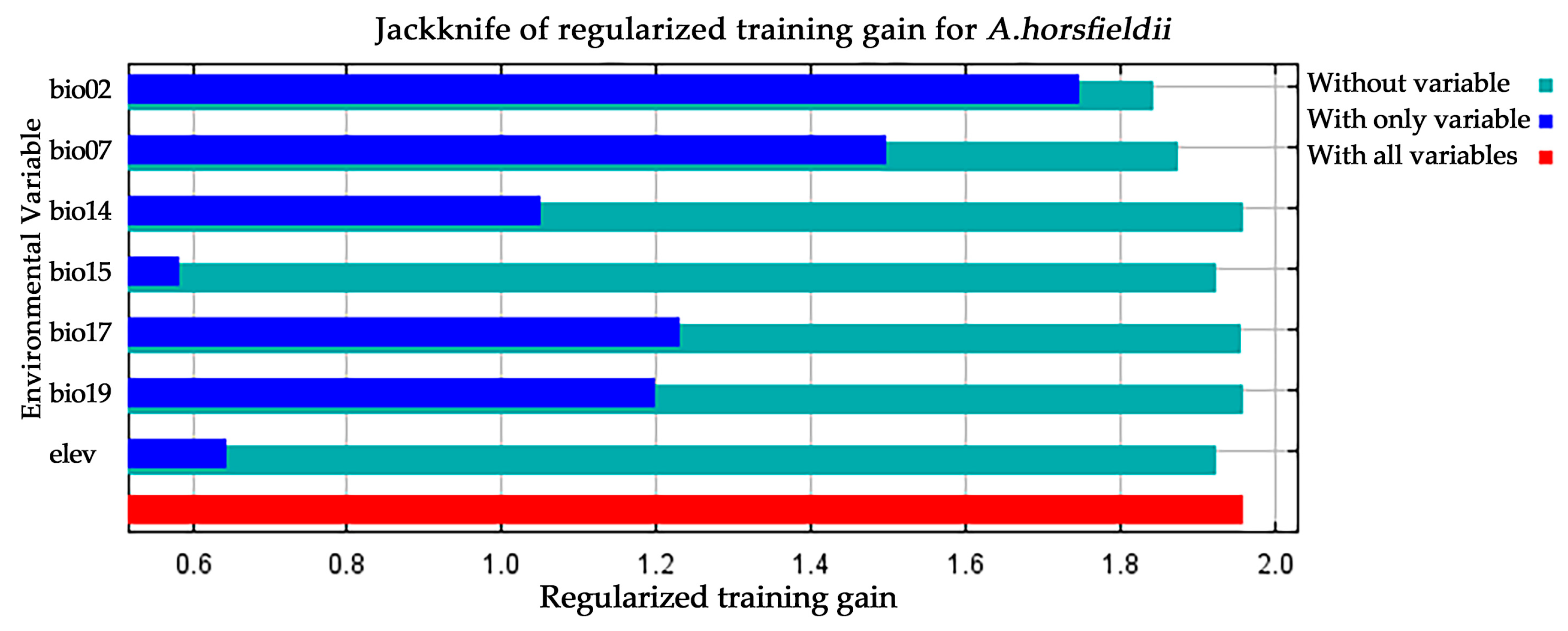
| Symbol | Environmental Variable | Unit |
|---|---|---|
| Mean diurnal range (mean of monthly) (max temp–min temp) | bio2 | °C |
| Temperature annual range (bio5–bio6) | bio7 | °C |
| Precipitation of driest quarter | bio17 | mm |
| Altitude | elev | m |
| Precipitation seasonality (coefficient of variation) | bio15 | % |
| Precipitation of coldest quarter | bio19 | mm |
| Precipitation of driest month | bio14 | mm |
| Variables | Percent Contribution/% | Permutation Importance/% |
|---|---|---|
| bio2 | 41.4 | 40.2 |
| bio07 | 28.2 | 41.9 |
| bio17 | 23.1 | 1.2 |
| elev | 5.3 | 10.1 |
| bio15 | 1.7 | 6.1 |
| bio19 | 0.1 | 0.3 |
| bio14 | 0.1 | 0.2 |
| Period | Current | 2050s | 2090s | ||||
|---|---|---|---|---|---|---|---|
| SSP1-2.6 | SSP2-4.5 | SSP5-8.5 | SSP1-2.6 | SSP2-4.5 | SSP5-8.5 | ||
| Low-suitable areas | 151.509 × 104 km2 | 149.347 × 104 km2 | 166.432 × 104 km2 | 158.859 × 104 km2 | 132.231 × 104 km2 | 167.411 × 104 km2 | 159.222 × 104 km2 |
| Moderately suitable areas | 44.970 × 104 km2 | 30.288 × 104 km2 | 40.175 × 104 km2 | 40.500 × 104 km2 | 52.161 × 104 km2 | 34.502 × 104 km2 | 31.266 × 104 km2 |
| High-suitable areas | 15.915 × 104 km2 | 10.929 × 104 km2 | 16.630 × 104 km2 | 14.705 × 104 km2 | 21.413 × 104 km2 | 9.903 × 104 km2 | 8.342 × 104 km2 |
| Total suitable areas | 212.394 × 104 km2 | 190.564 × 104 km2 | 223.238 × 104 km2 | 214.064 × 104 km2 | 205.806 × 104 km2 | 211.816 × 104 km2 | 198.830 × 104 km2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, D.; Xu, D.; Deng, X.; He, Z.; Zhuo, Z. Potential Distribution of Anoplophora horsfieldii Hope in China Based on MaxEnt and Its Response to Climate Change. Insects 2025, 16, 484. https://doi.org/10.3390/insects16050484
Yong D, Xu D, Deng X, He Z, Zhuo Z. Potential Distribution of Anoplophora horsfieldii Hope in China Based on MaxEnt and Its Response to Climate Change. Insects. 2025; 16(5):484. https://doi.org/10.3390/insects16050484
Chicago/Turabian StyleYong, Dan, Danping Xu, Xinqi Deng, Zhipeng He, and Zhihang Zhuo. 2025. "Potential Distribution of Anoplophora horsfieldii Hope in China Based on MaxEnt and Its Response to Climate Change" Insects 16, no. 5: 484. https://doi.org/10.3390/insects16050484
APA StyleYong, D., Xu, D., Deng, X., He, Z., & Zhuo, Z. (2025). Potential Distribution of Anoplophora horsfieldii Hope in China Based on MaxEnt and Its Response to Climate Change. Insects, 16(5), 484. https://doi.org/10.3390/insects16050484






