Estimating the Intra-Puparial Period of Chrysomya nigripes Aubertin Using Morphology and Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Study
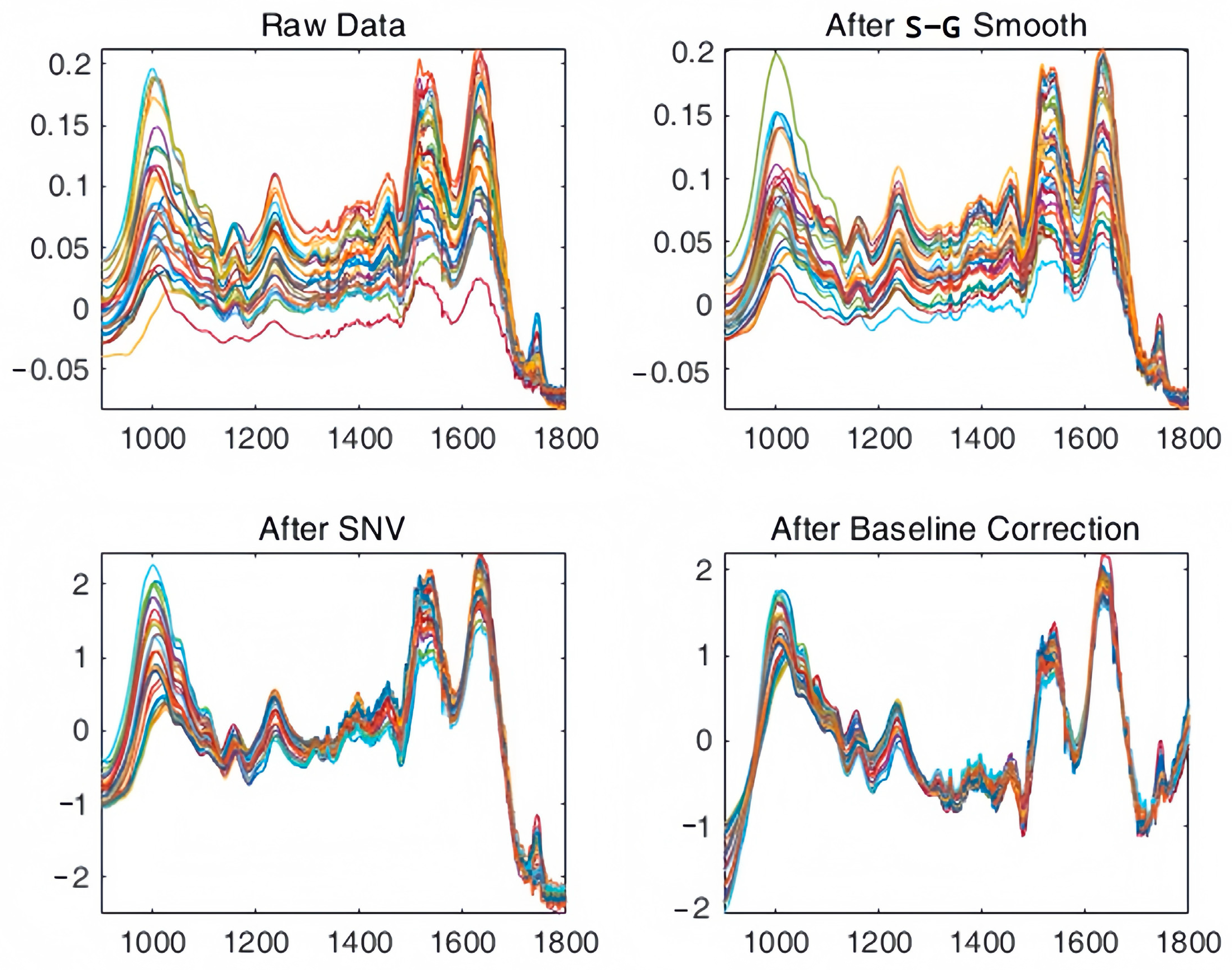
2.2. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR)
2.3. Analysis of Chemometric Methods
3. Results and Discussion
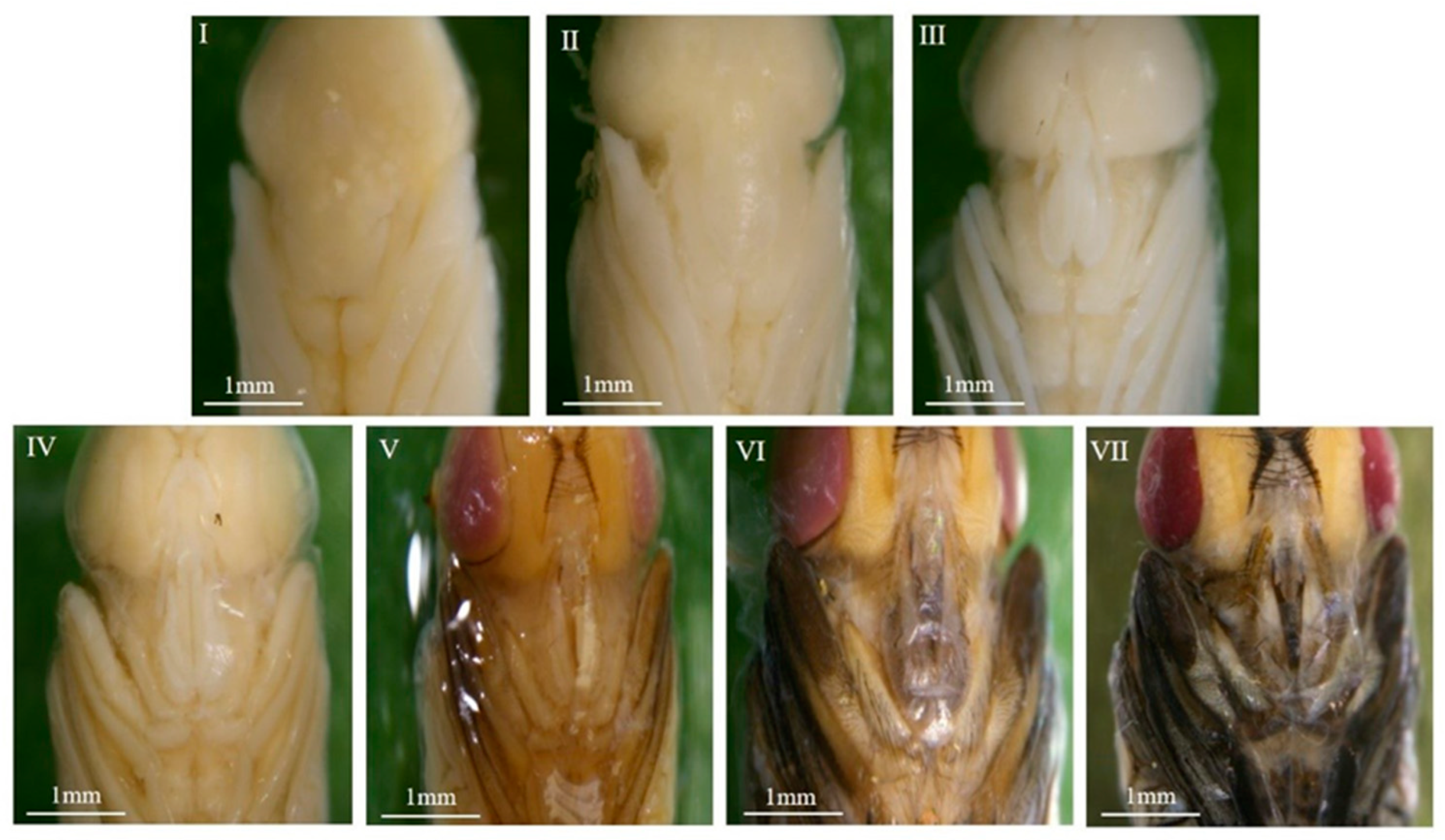
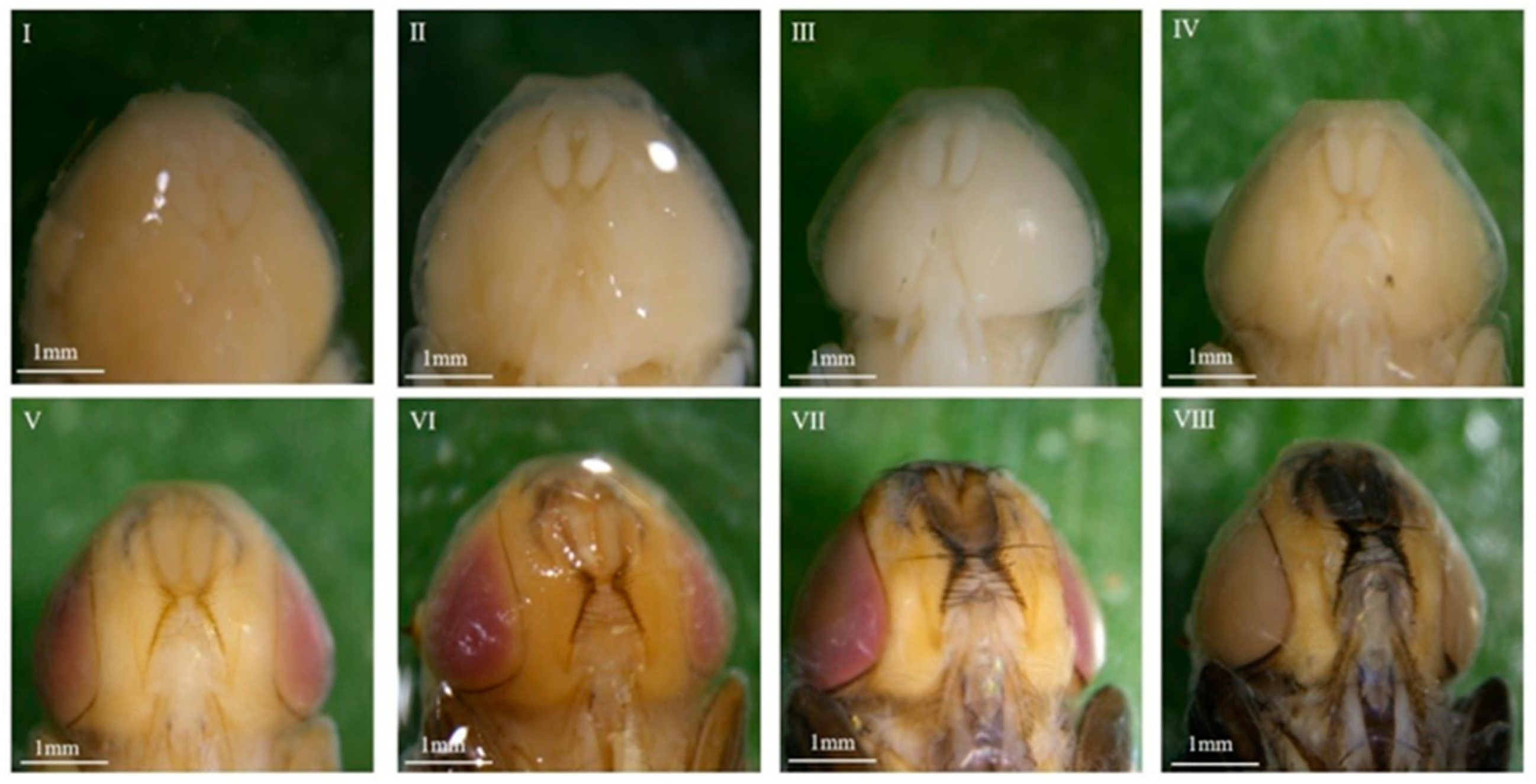
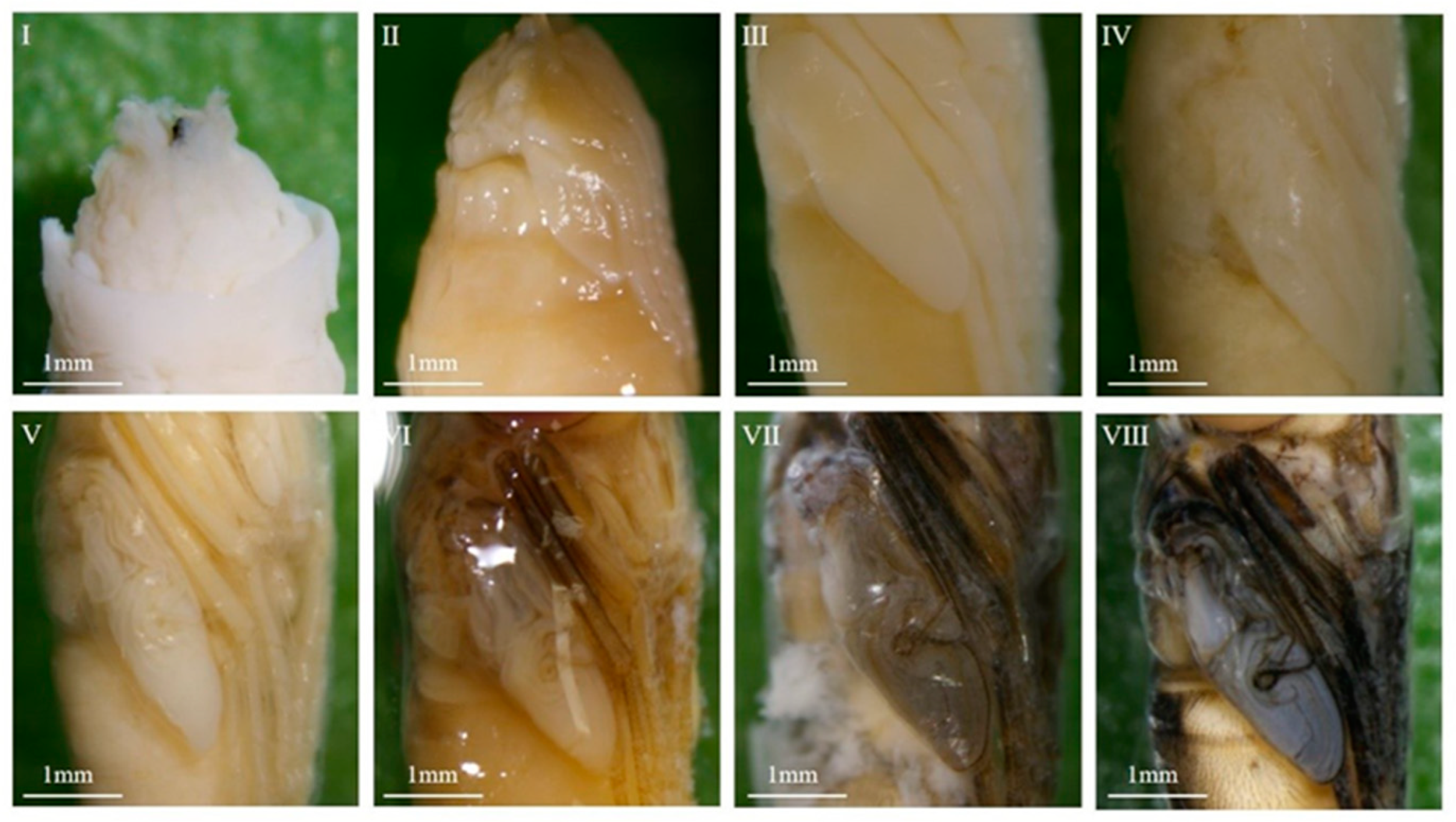
3.1. Morphological Observations
3.1.1. Overall Morphological Changes Within the Pupae
- A: Pre-pupal stage
- B: Early-cryptocephalic pupal stage
- C: Late-cryptocephalic pupal stage
- D: Phanerocephalic pupal stage
- E: Pharate adult stage I
- F: Pharate adult stage II
- G: Pharate adult stage III
- H: Pharate adult stage IV
- I: Pharate adult stage V
- J: Pharate adult stage VI
- K: Pharate adult stage VII
- L: Pharate adult stage VIII
3.1.2. Morphological Variations Within the Pupa: Compound Eyes, Thorax, and Abdomen
3.2. ATR-FTIR Variation over the Intra-Puparial Period
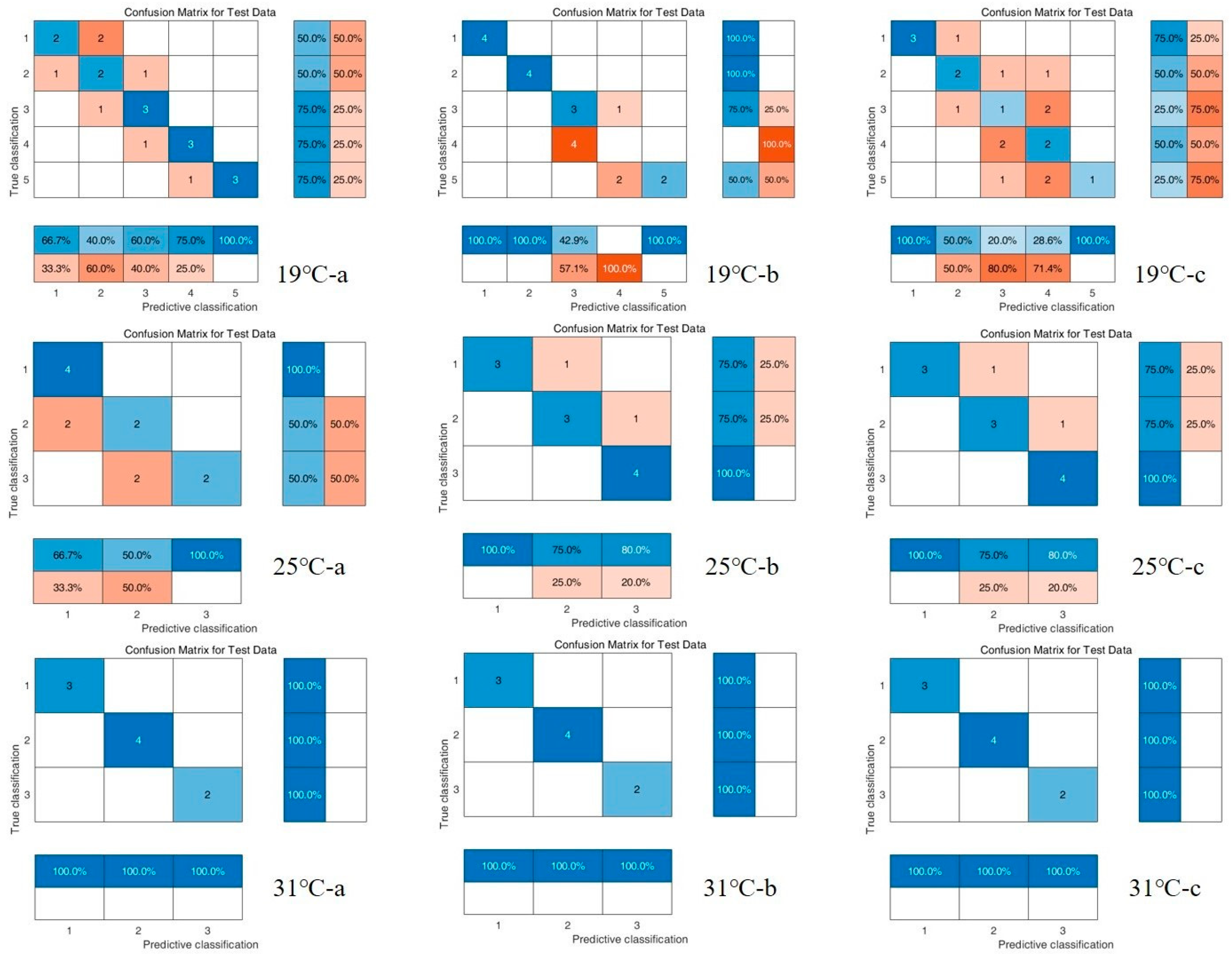
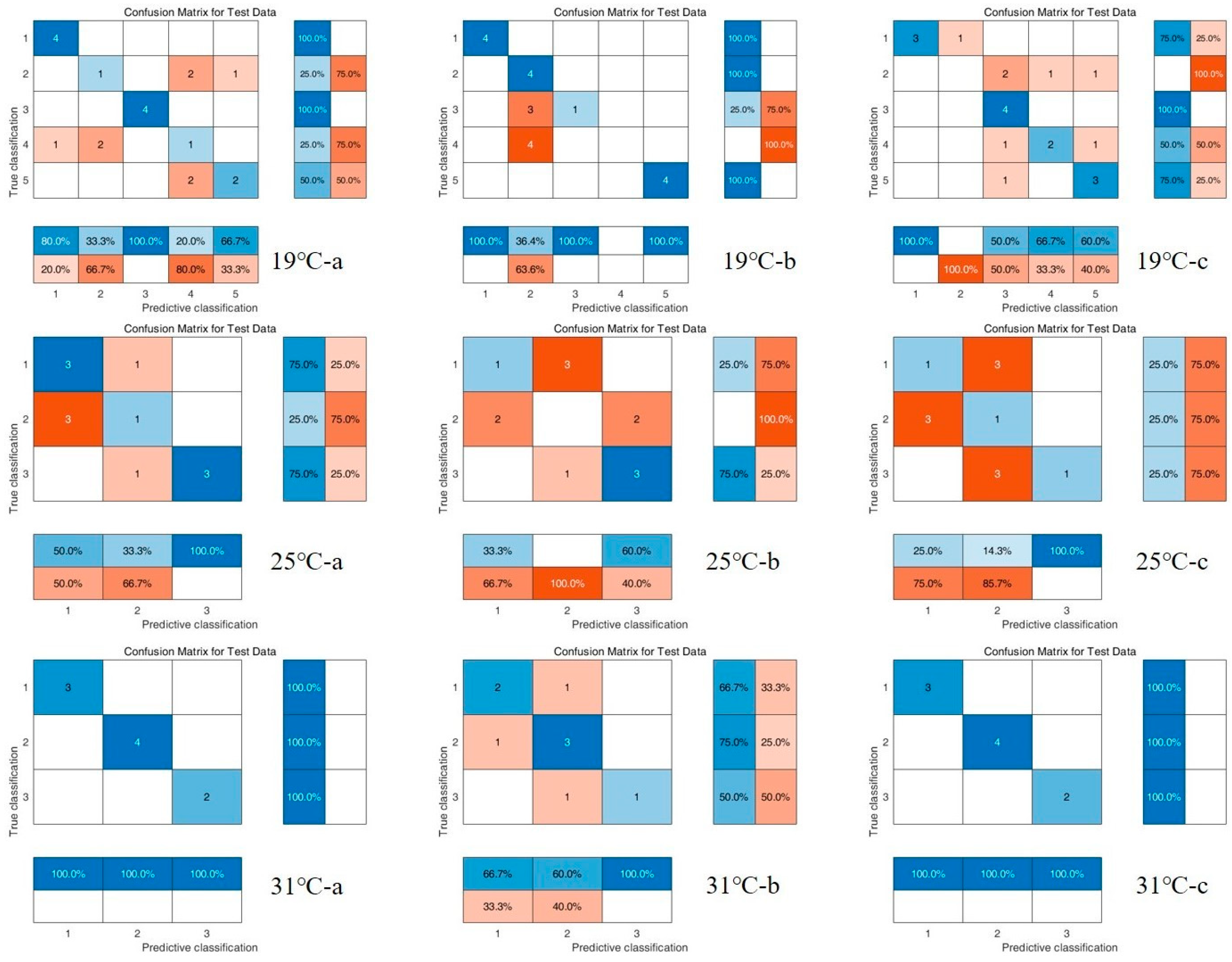
3.3. Chemometric Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR-FTIR | Attenuated Total Reflectance Fourier Transform Infrared |
| PMImin | minimum postmortem interval |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| RF | Random Forest |
| PMI | postmortem interval |
| PCA | principal component analysis |
References
- Tarone, A.M.; Sanford, M.R. Is PMI the hypothesis or the null hypothesis? J. Med. Entomol. 2017, 54, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S.; Byrd, J.; Castner, J. Insect Succession on Carrion and Its Relationship to Determining Time of Death; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Tomberlin, J.K.; Mohr, R.; Benbow, M.E.; Tarone, A.M.; Vanlaerhoven, S. A roadmap for bridging basic and applied research in forensic entomology. Annu. Rev. Entomol. 2011, 56, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Hu, C. Forensic Entomology; Chongqing Press: Chongqing, China, 2000; pp. 1–6. [Google Scholar]
- Smith, K.G.V. A manual of forensic entomology. Am. J. Archaeol. 1986, 92, 287. [Google Scholar]
- Sert, O.; Ergil, C. An examination of the intrapuparial development of Chrysomya albiceps (Wiedemann, 1819) (Calliphoridae: Diptera) at three different temperatures. Forensic Sci. Med. Pat. 2021, 17, 585–595. [Google Scholar] [CrossRef]
- Gruner, S.V.; Slone, D.H.; Capinera, J.L.; Turco, M.P. Development of the Oriental Latrine Fly, Chrysomya megacephala (Diptera: Calliphoridae), at five constant temperatures. J. Med. Entomol. 2017, 54, 290–298. [Google Scholar] [CrossRef]
- Yanmanee, S.; Husemann, M.; Benbow, M.E.; Suwannapong, G. Larval development rates of Chrysomya rufifacies Macquart, 1842 (Diptera: Calliphoridae) within its native range in South-East Asia. Forensic Sci. Int. 2016, 266, 63–67. [Google Scholar] [CrossRef]
- Arrighini, G.; Maestro, M.; Moccia, R. Magnetic properties of polyatomic molecules. I. Magnetic susceptibility of H2O, NH3, CH4, H2O2. J. Chem. Phys. 1968, 49, 882–889. [Google Scholar] [CrossRef]
- Greenberg, B.; Kunich, J.C. Entomology and the Law: Flies as Forensic Indicators; Cambridge University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Machado, D.S.S.; Osvaldo, M.M. Intrapuparial development of Hemilucilia semidiaphana (Diptera: Calliphoridae) and its use in forensic Entomology. J. Med. Entomol. 2019, 56, 1623–1635. [Google Scholar]
- Li, L.; Wang, Y.; Wang, J. Intra-puparial development and age estimation of forensically important Hermetia illucens (L.). J. Asia-Pac. Entomol. 2016, 19, 233–237. [Google Scholar] [CrossRef]
- Barros-Cordeiro, K.B.; Pujol-Luz, J.R.; Name, K.P.O.; Nair Báo, S. Intra-puparial development of the Cochliomyia macellaria and Lucilia cuprina (Diptera, Calliphoridae). Rev. Bras. Entomol. 2016, 60, 334–340. [Google Scholar] [CrossRef]
- Shao, S.; Hu, G.; Li, L.; Sheng, Y.; Wang, Y.; Zhang, Y.; Guo, Y.; Kang, C.; Xu, W.; Chen, J.; et al. Estimating the intra-puparial period of Hydrotaea spinigera (Stein, 1910) (Diptera: Muscidae) with morphological and gene expression changes. Acta Trop. 2023, 242, 106910. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Harvey, M. Optical coherence tomography: Age estimation of Calliphora vicina pupae in vivo? Forensic Sci. Int. 2014, 242, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vega, D.; Simonsen, T.J.; Wicklein, M.; Hall, M.J.R. Age estimation during the blow fly intra-puparial period: A qualitative and quantitative approach using micro-computed tomography. Int. J. Leg. Med. 2017, 131, 1429–1448. [Google Scholar] [CrossRef]
- Nur Aliah, N.; Heo, C.; Noor Shafini, M.; Mohd Hafizi, M. Age estimation of forensically important blowfly, Chrysomya megacephala (Diptera: Calliphoridae) pupae using micro-computed tomography imaging. Trop. Biomed. 2019, 36, 640–653. [Google Scholar]
- Voss, S.C.; Magni, P.; Dadour, I.; Nansen, C. Reflectance-based determination of age and species of blowfly puparia. Int. J. Leg. Med. 2017, 131, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Mahato, S. Intra-puparial development of flesh fly Sarcophaga dux (Thomson) (Diptera, Sarcophagidae). Curr. Sci. 2016, 6, 1063–1670. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Li, L.; Wang, J. Gene expression during the intra-puparial stage of Chrysomya megacephala: Implications for postmortem interval estimation. J. Asia-Pac. Entomol. 2019, 22, 841–846. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, Y.; Ren, L.; Qu, H.; Zhu, G.; Guo, Y. A study of cuticular hydrocarbons of all life stages in Sarcophaga peregrina (Diptera: Sarcophagidae). J. Med. Entomol. 2022, 59, 108–119. [Google Scholar] [CrossRef]
- Braga, M.V.; Pinto, Z.T.; Maria De Carvalho Queiroz, M.; Matsumoto, N.; Blomquist, G.J. Cuticular hydrocarbons as a tool for the identification of insect species: Puparial cases from Sarcophagidae. Acta Trop. 2013, 128, 479–485. [Google Scholar] [CrossRef]
- Barbosa, T.M.; de Lima, L.A.; Dos Santos, M.C.; Vasconcelos, S.D.; Gama, R.A.; Lima, K.M. A novel use of infra-red spectroscopy (NIRS and ATR-FTIR) coupled with variable selection algorithms for the identification of insect species (Diptera: Sarcophagidae) of medico-legal relevance. Acta Trop. 2018, 185, 1–12. [Google Scholar] [CrossRef]
- Levin, I.W.; Bhargava, R. Fourier transform infrared vibrational spectroscopic imaging: Integrating microscopy and molecular recognition. Annu. Rev. Phys. Chem. 2005, 56, 429–474. [Google Scholar] [CrossRef]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; Hall, M.J.R. Best practice in forensic entomology—Standards and guidelines. Int. J. Leg. Med. 2007, 121, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Alkhuder, K. Attenuated total reflection-fourier transform infrared spectroscopy: A universal analytical technique with promising applications in forensic analyses. Int. J. Leg. Med. 2022, 136, 1717–1736. [Google Scholar] [CrossRef]
- Manheim, J.; Doty, K.C.; McLaughlin, G.; Lednev, I.K. Forensic hair differentiation using attenuated total reflection fourier transform infrared (ATR FT-IR) spectroscopy. Appl. Spectrosc. 2016, 70, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Young, S.R. Testing the Use of Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy for Differentiating Forensic Soil Samples in Kansas. Ph.D. Thesis, Emporia State University, Emporia, KS, USA, 2020. [Google Scholar]
- Yang, X.; Wei, X.; Yu, K.; Wan, C.; Wan, Y.; Huang, S.; Sun, Q.; Huang, J. Identification of myocardial fibrosis by ATR-FTIR spectroscopy combined with chemometrics. Spectrochim. Acta A 2022, 264, 120–238. [Google Scholar] [CrossRef]
- Shang, Y.; Yang, F.; Ngando, F.J.; Zhang, X.; Feng, Y.; Ren, L.; Guo, Y. Development of forensically important Sarcophaga peregrina (Diptera: Sarcophagidae) and intra-puparial age estimation utilizing multiple methods at constant and fluctuating temperatures. Animals 2023, 13, 1607. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hu, G.; Li, L.; Liao, M.; Wang, J.; Wang, Y.; Tao, L. Developmental indicators of Chrysomya nigripes Aubertin under different constant temperature conditions and an application case for estimating the PMImin. Insects 2023, 14, 729. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Xia, S.; Wang, J.; Zhang, Y.; Tao, L. Estimating the age of Lucilia illustris during the intrapuparial period using two approaches: Morphological changes and differential gene expression. Forensic Sci. Int. 2018, 287, 1–11. [Google Scholar] [CrossRef]
- Bambaradeniya, T.B.; Magni, P.A.; Dadour, I.R. Morphological changes of larvae and pupae of Lucilia sericata (Diptera: Calliphoridae) reared at two temperatures and on three food types. J. Med. Entomol. 2024, 61, 521–529. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Baia, T.C.; Gama, R.A.; Lima, K.M. Development of a novel non-destructive method based on spectral fingerprint for determination of abused drug in insects: An alternative entomotoxicology approach. Microchem. J. 2014, 115, 39–46. [Google Scholar] [CrossRef]
- Morais, C.L.; Lima, K.M.; Singh, M.; Martin, F.L. Tutorial: Multivariate classification for vibrational spectroscopy in biological samples. Nat. Protoc. 2020, 15, 2143–2162. [Google Scholar] [CrossRef]
- Wang, G.; Cai, W.; Wu, H.; Yang, C.; Yu, K.; Liu, R.; Wei, X.; Lin, H.; Sun, Q.; Wang, Z. Identification of human and non-human bloodstains on rough carriers based on ATR-FTIR and chemometrics. Microchem. J. 2022, 180, 107620. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Wang, J.; Ma, M.; Lai, Y. Temperature-dependent development and the significance for estimating postmortem interval of Chrysomya nigripes Aubertin, a new forensically important species in China. Int. J. Leg. Med. 2016, 130, 1363–1370. [Google Scholar] [CrossRef]
- O’Flynn, M.A. The succession and rate of development of blowflies in carrion in southern Queensland and the application of these data to forensic entomology. Aust. J. Entomol. 1983, 22, 137–148. [Google Scholar] [CrossRef]
- Pujol-Luz, J.R.; Barros-Cordeiro, K.B. Intra-puparial development of the females of Chrysomya albiceps (Wiedemann) (Diptera, Calliphoridae). Rev. Bras. Entomol. 2012, 56, 269–272. [Google Scholar] [CrossRef]
- Brown, K.; Thorne, A.; Harvey, M. Calliphora vicina (Diptera: Calliphoridae) pupae: A timeline of external morphological development and a new age and PMI estimation tool. Int. J. Leg. Med. 2015, 129, 835–850. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Wang, M.; Wang, Y.; Wang, X.; Zhang, Y.; Wang, J. Intrapuparial development and age estimation of Calliphora grahami (Diptera: Calliphoridae) for postmortem interval estimation. J. Med. Entomol. 2022, 2, 2. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Chen, Y.; Guo, Y.; Wang, Y.; Hu, G.; Kang, C.; Wang, J.; Wang, Y. Intrapuparial development and age estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) for postmortem interval estimation. J. Asia-Pac. Entomol. 2023, 26, 102089. [Google Scholar] [CrossRef]
- Grabska, K.B.; Beć, J.; Huck, C.W. Biomolecular and bioanalytical applications of infrared spectroscopy—A review. Anal. Chim. Acta 2020, 1133, 150–177. [Google Scholar]
- Baker, M.J.; Hussain, S.R.; Lovergne, L.; Untereiner, V.; Hughes, C.; Lukaszewski, R.A.; Thiéfin, G.; Sockalingum, G.D. Developing and understanding biofluid vibrational spectroscopy: A critical review. Chem. Soc. Rev. 2016, 45, 1803–1818. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, X.; Huang, J.; Lin, H.; Deng, K.; Li, Z.; Shao, Y.; Zou, D.; Chen, Y.; Huang, P. Attenuated total reflectance fourier transform infrared (ATR-FTIR) spectral prediction of postmortem interval from vitreous humor samples. Anal. Bioanal. Chem. 2018, 410, 7611–7620. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.L.; Hands, J.R.; Fullwood, L.M.; Smith, J.A.; Baker, M.J. Rapid discrimination of maggots utilising ATR-FTIR spectroscopy. Forensic Sci. Int. 2015, 249, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Giffen, J.E.; Rosati, J.Y.; Longo, C.M.; Musah, R.A. Species identification of necrophagous insect eggs based on amino acid profile differences revealed by direct analysis in real time-high resolution mass spectrometry. Anal. Chem. 2017, 89, 7719–7726. [Google Scholar] [CrossRef] [PubMed]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Li, L.; Wu, H.; Xu, W.; Wang, Y.; Wang, J.; Wang, Y. New application of ATR-FTIR spectroscopy for postmortem interval estimation based on puparia of the sarcosaprophagous fly Chrysomya megacephala (Diptera: Calliphoridae). Forensic Chem. 2023, 33, 100484. [Google Scholar] [CrossRef]
- de Paula, M.C.; Michelutti, K.B.; Eulalio, A.D.; Mendonça, A.; Cardoso, C.A.; Andrade, L.H.; Lima, S.M.; Antonialli-Junior, W.F. New approach to application of mid-infrared photoacoustic spectroscopy in forensic analysis: Study with the necrophagous blow fly Chrysomya megacephala (Diptera: Calliphoridae). J. Photochem. Photobiol. B Biol. 2020, 209, 111934. [Google Scholar] [CrossRef]
- Tofolo, V.C.; Giannotti, E.; Neves, E.F.; Andrade, L.H.; MLima, S.; Súarez, Y.R.; Antonialli-Junior, W.F. Polydomy in the ant ectatomma opaciventre. J. Insect. Sci. 2014, 14, 21. [Google Scholar] [CrossRef]
- Bernardi, R.C.; Firmino, E.L.B.; Mendonça, A.; Sguarizi-Antonio, D.; Pereira, M.C.; da Cunha Andrade, L.H.; Antonialli-Junior, W.F.; Lima, S.M. Intraspecific variation and influence of diet on the venom chemical profile of the Ectatomma brunneum Smith (Formicidae) ant evaluated by photoacoustic spectroscopy. J. Photochem. Photobiol. B Biol. 2017, 175, 200–206. [Google Scholar] [CrossRef]
- Shang, Y.; Feng, Y.; Ren, L.; Zhang, X.; Yang, F.; Zhang, C.; Guo, Y. Pupal age estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) at different constant temperatures utilizing ATR-FTIR spectroscopy and cuticular hydrocarbons. Insects 2023, 14, 143. [Google Scholar] [CrossRef]
- Wang, H.; Ding, X.; He, X.; Guo, G.; Yang, J.; Zhang, Y.; Jia, Z.; Zhang, J.; Li, J.; Wang, Q. Application of ATR-FTIR spectroscopy and chemometrics for the forensic discrimination of aged peripheral and menstrual bloodstains. Microchem. J. 2024, 197, 109933. [Google Scholar] [CrossRef]
- Wu, H.; Liu, R.; Wang, G.; Shen, C.; Liang, X.; Chen, R.; Deng, M.; Wu, S.; Zhang, K.; Wang, Z. Accurate forensic identification of asphyxial deaths: Differentiating strangulation and drowning using ATR-FTIR spectroscopy. Sci. Justice 2025, 65, 101257. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, R.; Kumar, R.; Chauhan, R.; Sharma, V. Chemometric analysis of ATR-FTIR spectra of fingernail clippings for classification and prediction of sex in forensic context. Microchem. J. 2020, 159, 105504. [Google Scholar] [CrossRef]
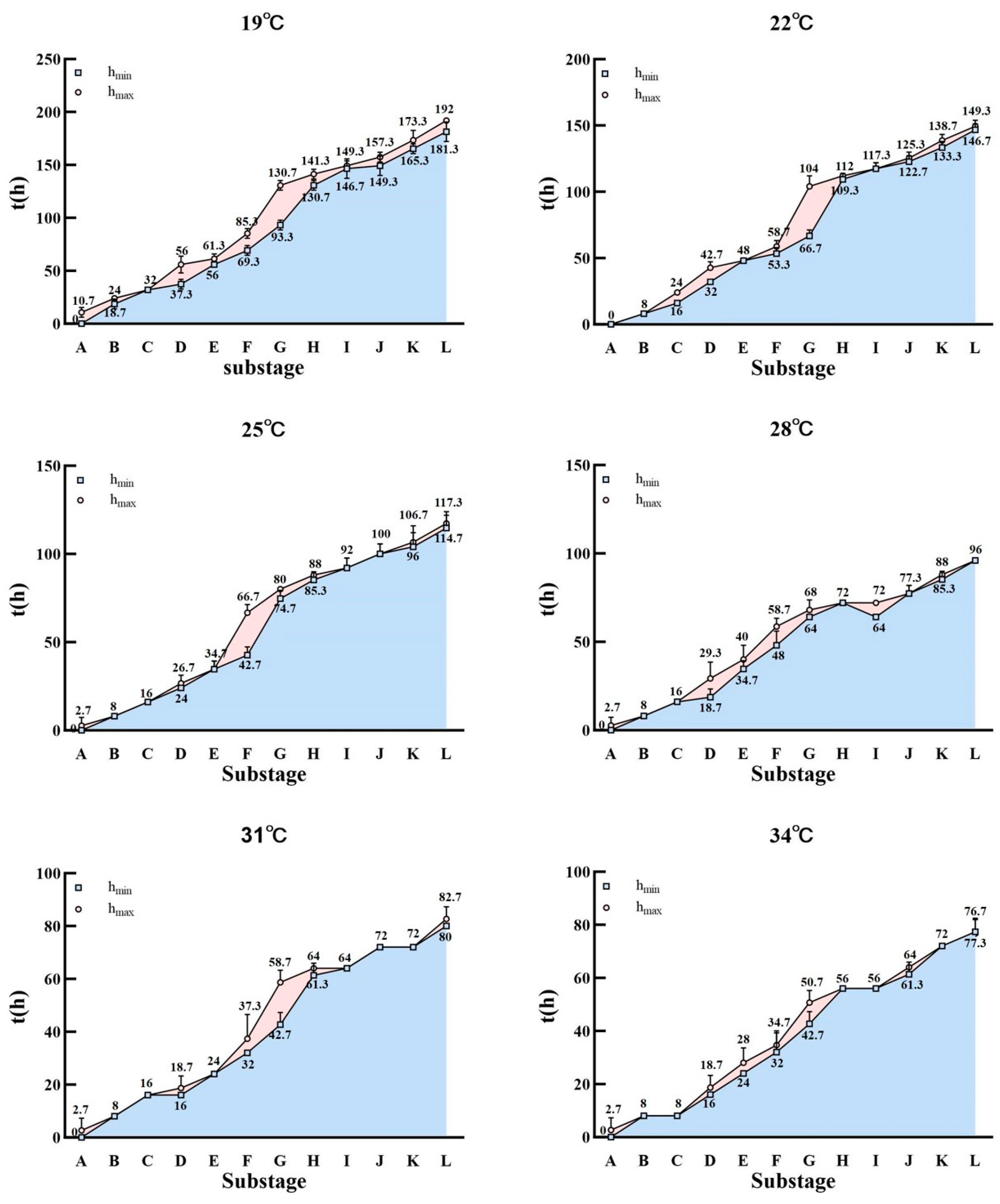







| Temperature | Compound Eyes (h) | Abdomen (h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | I | II | III | IV | V | VI | ||
| 19 °C | hmin | 0 | 37.3 | 56 | 130.7 | 144 | 176 | 0 | 56 | 93.3 | 122.7 | 144 | 176 |
| SD | 0 | 4.6 | 0 | 4.6 | 8 | 0 | 0 | 0 | 4.6 | 9.2 | 0 | 0 | |
| hmax | 29.3 | 56 | 130.7 | 141.3 | 170.7 | 192 | 56 | 85.3 | 120 | 144 | 168 | 192 | |
| SD | 4.6 | 8 | 4.6 | 4.6 | 4.6 | 0 | 8 | 4.6 | 13.9 | 0 | 0 | 0 | |
| 22 °C | hmin | 0 | 32 | 45.3 | 109.3 | 117.3 | 138.7 | 0 | 48 | 66.7 | 93.3 | 120 | 136 |
| SD | 0 | 0 | 4.6 | 4.6 | 4.6 | 4.6 | 0 | 0 | 4.6 | 16.7 | 0 | 8 | |
| hmax | 24 | 37.3 | 104 | 112 | 130.7 | 149.3 | 42.7 | 58.7 | 90.7 | 114.7 | 128 | 149.3 | |
| SD | 0 | 4.6 | 8 | 0 | 4.6 | 4.6 | 4.6 | 4.6 | 12.2 | 4.6 | 8 | 4.6 | |
| 25 °C | hmin | 0 | 24 | 32 | 85.3 | 96 | 109.3 | 0 | 34.7 | 74.7 | 88 | 96 | 109.3 |
| SD | 0 | 0 | 0 | 4.6 | 8 | 4.6 | 0 | 4.6 | 4.6 | 8 | 0 | 12.2 | |
| hmax | 16 | 24 | 80 | 93.3 | 104 | 117.3 | 26.7 | 66.7 | 85.3 | 90.7 | 108 | 117.3 | |
| SD | 0 | 0 | 0 | 9.2 | 8 | 4.6 | 4.6 | 4.6 | 4.6 | 4.6 | 5.7 | 4.6 | |
| 28 °C | hmin | 0 | 18.7 | 29.3 | 69.3 | 77.3 | 88 | 0 | 34.7 | 58.7 | 64 | 77.3 | 88 |
| SD | 0 | 4.6 | 4.6 | 4.6 | 4.6 | 0 | 0 | 4.6 | 9.2 | 8 | 4.6 | 0 | |
| hmax | 10.7 | 21.3 | 66.7 | 72 | 85.3 | 96 | 29.3 | 50.7 | 61.3 | 72 | 80 | 96 | |
| SD | 4.6 | 4.6 | 4.6 | 8 | 4.6 | 0 | 9.2 | 9.2 | 12.2 | 0 | 0 | 0 | |
| 31 °C | hmin | 0 | 16 | 24 | 61.3 | 72 | 80 | 0 | 24 | 42.7 | 58.7 | 72 | 80 |
| SD | 0 | 0 | 0 | 4.6 | 0 | 0 | 0 | 0 | 4.6 | 4.6 | 0 | 0 | |
| hmax | 16 | 18.7 | 58.7 | 64 | 72 | 85.3 | 18.7 | 37.3 | 53.3 | 64 | 72 | 85.3 | |
| SD | 0 | 4.6 | 4.6 | 0 | 0 | 4.6 | 4.6 | 9.2 | 9.2 | 0 | 0 | 4.6 | |
| 34 °C | hmin | 0 | 16 | 24 | 56 | 64 | 74.7 | 0 | 24 | 42.7 | 52 | 61.3 | 72 |
| SD | 0 | 0 | 0 | 0 | 0 | 4.6 | 0 | 0 | 4.6 | 5.7 | 4.6 | 0 | |
| hmax | 8 | 18.7 | 50.7 | 56 | 66.7 | 77.3 | 18.7 | 34.7 | 48 | 56 | 64 | 77.3 | |
| SD | 0 | 4.6 | 4.6 | 0 | 4.6 | 4.6 | 4.6 | 4.6 | 8 | 0 | 0 | 4.6 | |
| Temperature | Thorax (h) | Mouthparts (h) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | - | I | II | III | IV | V | VI | VII | ||
| 19 °C | hmin | 0 | 56 | 93.3 | 125.3 | 141.3 | 144 | 176 | 0 | 37.3 | 53.3 | 64 | 85.3 | 133.3 | 162.7 | 181.3 |
| SD | 0 | 0 | 4.6 | 4.6 | 4.6 | 0 | 8 | 0 | 4.6 | 4.6 | 8 | 12.2 | 9.2 | 4.6 | 4.6 | |
| hmax | 56 | 85.3 | 122.7 | 136 | 149.3 | 168 | 192 | 32 | 50.7 | 56 | 77.3 | 130.7 | 154.7 | 173.3 | 192 | |
| SD | 8 | 4.6 | 9.2 | 0 | 4.6 | 8 | 0 | 0 | 12.2 | 8 | 12.2 | 4.6 | 4.6 | 4.6 | 0 | |
| 22 °C | hmin | 0 | 34.7 | 66.7 | 98.7 | 120 | 122.7 | 136 | 0 | 32 | 44 | 50.7 | 72 | 114.7 | 122.7 | 146.7 |
| SD | 0 | 23.1 | 4.6 | 12.2 | 0 | 4.6 | 0 | 0 | 0 | 5.7 | 4.6 | 0 | 4.6 | 4.6 | 4.6 | |
| hmax | 42.7 | 58.7 | 93.3 | 112 | 120 | 125.3 | 149.3 | 24 | 40 | 44 | 64 | 106.7 | 117.3 | 138.7 | 149.3 | |
| SD | 4.6 | 4.6 | 9.2 | 0 | 0 | 4.6 | 4.6 | 0 | 8 | 5.7 | 0 | 4.6 | 4.6 | 4.6 | 4.6 | |
| 25 °C | hmin | 0 | 34.7 | 74.7 | 85.3 | 88 | 93.3 | 109.3 | 0 | 24 | 32 | 36 | 56 | 80 | 93.3 | 106.7 |
| SD | 0 | 4.6 | 4.6 | 4.6 | 0 | 4.6 | 12.2 | 0 | 0 | 0 | 5.7 | 21.2 | 13.9 | 4.6 | 12.2 | |
| hmax | 26.7 | 66.7 | 80 | 90.7 | 88 | 101.3 | 117.3 | 18.7 | 26.7 | 32 | 56 | 74.7 | 85.3 | 101.3 | 117.3 | |
| SD | 4.6 | 4.6 | 0 | 4.6 | 0 | 12.2 | 4.6 | 4.6 | 4.6 | 0 | 22.6 | 16.7 | 4.6 | 9.2 | 4.6 | |
| 28 °C | hmin | 0 | 34.7 | 58.7 | 64 | 72 | 77.3 | 85.3 | 0 | 18.7 | 26.7 | 37.3 | 48 | 69.3 | 77.3 | 93.3 |
| SD | 0 | 4.6 | 9.2 | 8 | 0 | 4.6 | 4.6 | 0 | 4.6 | 4.6 | 4.6 | 8 | 4.6 | 4.6 | 4.6 | |
| hmax | 29.3 | 50.7 | 58.7 | 72 | 72 | 80 | 96 | 10.7 | 18.7 | 32 | 40 | 69.3 | 72 | 85.3 | 96 | |
| SD | 9.2 | 9.2 | 9.2 | 0 | 0 | 0 | 0 | 4.6 | 4.6 | 8 | 8 | 4.6 | 0 | 4.6 | 0 | |
| 31 °C | hmin | 0 | 24 | 42.7 | 58.7 | 68 | 74.7 | 82.7 | 0 | 16 | 24 | 26.7 | 45.3 | 68 | 74.7 | 85.3 |
| SD | 0 | 0 | 4.6 | 4.6 | 5.7 | 4.6 | 4.6 | 0 | 0 | 0 | 4.6 | 12.2 | 5.7 | 4.6 | 4.6 | |
| hmax | 18.7 | 37.3 | 53.3 | 64 | 68 | 74.7 | 85.3 | 16 | 16 | 24 | 37.3 | 64 | 68 | 77.3 | 85.3 | |
| SD | 4.6 | 9.2 | 9.2 | 0 | 5.7 | 4.6 | 4.6 | 0 | 0 | 0 | 12.2 | 0 | 5.7 | 4.6 | 4.6 | |
| 34 °C | hmin | 0 | 24 | 42.7 | 48 | 56 | 64 | 69.3 | 0 | 16 | 24 | 29.3 | 36 | 58.7 | 69.3 | 77.3 |
| SD | 0 | 0 | 4.6 | 0 | 0 | 0 | 4.6 | 0 | 0 | 0 | 9.2 | 5.7 | 4.6 | 4.6 | 4.6 | |
| hmax | 18.7 | 34.7 | 45.3 | 52 | 56 | 64 | 77.3 | 8 | 18.7 | 32 | 37.3 | 52 | 61.3 | 69.3 | 77.3 | |
| SD | 4.6 | 4.6 | 9.2 | 5.7 | 0 | 0 | 4.6 | 0 | 4.6 | 0 | 16.7 | 5.7 | 4.6 | 4.6 | 4.6 | |
| Temperature | Antennae (h) | Wings (h) | Legs (h) | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | I | II | III | IV | V | VI | VII | VIII | - | I | II | III | IV | V | VI | VII | VIII | - | I | II | III | IV | V | VI | VII | VIII | ||
| 19 °C | hmin | 0 | 53.3 | 64 | 77.3 | 117.3 | 133.3 | 146.7 | 160 | 176 | 0 | 18.7 | 32 | 37.3 | 56 | 72 | 130.7 | 146.7 | 189.3 | 0 | 18.7 | 32 | 37.3 | 58.7 | 117.3 | 133.3 | 154.7 | 173.3 |
| SD | 0 | 4.6 | 8 | 4.6 | 4.6 | 4.6 | 4.6 | 8 | 0 | 0 | 4.6 | 0 | 4.6 | 0 | 8 | 4.6 | 4.6 | 4.6 | 0 | 4.6 | 0 | 4.6 | 4.6 | 12.2 | 4.6 | 9.2 | 4.6 | |
| hmax | 50.7 | 56 | 72 | 114.7 | 130.7 | 146.7 | 154.7 | 168 | 192 | 10.7 | 24 | 34.7 | 56 | 69.3 | 130.7 | 149.3 | 181.3 | 192 | 10.7 | 24 | 32 | 56 | 117.3 | 128 | 152 | 165.3 | 192 | |
| SD | 12.2 | 8 | 8 | 12.2 | 4.6 | 4.6 | 4.6 | 0 | 0 | 4.6 | 0 | 4.6 | 8 | 9.2 | 4.6 | 4.6 | 4.6 | 0 | 4.6 | 0 | 0 | 8 | 12.2 | 0 | 0 | 4.6 | 0 | |
| 22 °C | hmin | 0 | 48 | 53.3 | 69.3 | 96 | 114.7 | 120 | 128 | 138.7 | 0 | 8 | 16 | 37.3 | 48 | 69.3 | 114.7 | 125.3 | 149.3 | 0 | 8 | 16 | 32 | 48 | 88 | 109.3 | 125.3 | 136 |
| SD | 0 | 0 | 4.6 | 4.6 | 8 | 4.6 | 0 | 8 | 9.2 | 0 | 0 | 0 | 4.6 | 0 | 16.7 | 4.6 | 9.2 | 4.6 | 0 | 0 | 0 | 0 | 0 | 21.2 | 12.2 | 4.6 | 0 | |
| hmax | 42.7 | 48 | 61.3 | 93.3 | 112 | 114.7 | 124 | 130.7 | 149.3 | 0 | 8 | 29.3 | 45.3 | 61.3 | 109.3 | 120 | 141.3 | 149.3 | 0 | 8 | 24 | 42.7 | 82.7 | 101.3 | 117.3 | 128 | 149.3 | |
| SD | 4.6 | 0 | 4.6 | 12.2 | 0 | 4.6 | 5.7 | 9.2 | 4.6 | 0 | 0 | 4.6 | 4.6 | 16.7 | 4.6 | 8 | 4.6 | 4.6 | 0 | 0 | 0 | 4.6 | 16.7 | 12.2 | 4.6 | 0 | 4.6 | |
| 25 °C | hmin | 0 | 32 | 40 | 50.7 | 85.3 | 88 | 96 | 98.7 | 112 | 0 | 8 | 16 | 26.7 | 34.7 | 61.3 | 88 | 93.3 | 112 | 0 | 8 | 16 | 26.7 | 34.7 | 82.7 | 88 | 96 | 104 |
| SD | 0 | 0 | 0 | 4.6 | 4.6 | 0 | 0 | 4.6 | 8 | 0 | 0 | 0 | 4.6 | 4.6 | 16.7 | 0 | 4.6 | 8 | 0 | 0 | 0 | 4.6 | 4.6 | 4.6 | 0 | 0 | 0 | |
| hmax | 26.7 | 32 | 45.3 | 77.3 | 85.3 | 88 | 96 | 104 | 117.3 | 0 | 8 | 18.7 | 26.7 | 53.3 | 85.3 | 88 | 104 | 117.3 | 0 | 8 | 18.7 | 26.7 | 77.3 | 82.7 | 90.7 | 96 | 114.7 | |
| SD | 4.6 | 0 | 4.6 | 4.6 | 4.6 | 0 | 0 | 8 | 4.6 | 0 | 0 | 4.6 | 4.6 | 16.7 | 4.6 | 0 | 8 | 4.6 | 0 | 0 | 4.6 | 4.6 | 4.6 | 4.6 | 4.6 | 0 | 4.6 | |
| 28 °C | hmin | 0 | 34.7 | 48 | 44 | 61.3 | 66.7 | 72 | 77.3 | 90.7 | 0 | 8 | 16 | 18.7 | 34.7 | 50.7 | 68 | 77.3 | 96 | 0 | 8 | 16 | 18.7 | 34.7 | 64 | 72 | 77.3 | 90.7 |
| SD | 0 | 4.6 | 11.3 | 5.7 | 4.6 | 4.6 | 0 | 4.6 | 4.6 | 0 | 0 | 0 | 4.6 | 4.6 | 9.2 | 5.7 | 4.6 | 9 | 0 | 0 | 0 | 4.6 | 4.6 | 0 | 0 | 4.6 | 4.6 | |
| hmax | 29.3 | 37.3 | 48 | 52 | 64 | 72 | 72 | 82.7 | 96 | 2.7 | 8 | 16 | 29.3 | 50.7 | 69.3 | 72 | 88 | 96 | 0 | 8 | 16 | 29.3 | 58.7 | 66.7 | 72 | 82.7 | 96 | |
| SD | 9.2 | 9.2 | 11.3 | 5.7 | 0 | 0 | 0 | 4.6 | 0 | 4.6 | 0 | 0 | 9.2 | 9.2 | 4.6 | 0 | 0 | 0 | 0 | 0 | 0 | 9.2 | 4.6 | 4.6 | 0 | 4.6 | 0 | |
| 31 °C | hmin | 0 | 24 | 32 | 40 | 56 | 64 | 72 | 72 | 82.7 | 0 | 8 | 16 | 16 | 24 | 42.7 | 72 | 72 | 85.3 | 0 | 8 | 16 | 16 | 24 | 50.7 | 64 | 72 | 74.7 |
| SD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.6 | 0 | 0 | 0 | 0 | 0 | 4.6 | 0 | 0 | 4.6 | 0 | 0 | 0 | 0 | 0 | 9.2 | 11.3 | 0 | 4.6 | |
| hmax | 18.7 | 24 | 37.3 | 48 | 64 | 64 | 76 | 72 | 85.3 | 5.3 | 8 | 16 | 18.7 | 37.3 | 64 | 72 | 77.3 | 85.3 | 2.7 | 8 | 16 | 18.7 | 42.7 | 58.7 | 68 | 72 | 85.3 | |
| SD | 4.6 | 0 | 9.2 | 0 | 0 | 0 | 5.7 | 0 | 4.6 | 4.6 | 0 | 0 | 4.6 | 9.2 | 0 | 0 | 4.6 | 4.6 | 4.6 | 0 | 0 | 4.6 | 9.2 | 9.2 | 5.7 | 0 | 4.6 | |
| 34 °C | hmin | 0 | 24 | 29.3 | 37.3 | 48 | 56 | 64 | 68 | 74.7 | 0 | 8 | 8 | 16 | 24 | 40 | 58.7 | 61.3 | 77.3 | 0 | 8 | - | 16 | 24 | 48 | 56 | 61.3 | 72 |
| SD | 0 | 0 | 9.2 | 9.2 | 0 | 0 | 0 | 5.7 | 4.6 | 0 | 0 | 0 | 0 | 0 | 0 | 4.6 | 4.6 | 4.6 | 0 | 0 | - | 0 | 0 | 0 | 0 | 4.6 | 0 | |
| hmax | 18.7 | 32 | 29.3 | 48 | 56 | 56 | 64 | 68 | 77.3 | 0 | 8 | 8 | 18.7 | 32 | 53.3 | 58.7 | 69.3 | 77.3 | 0 | 8 | - | 18.7 | 42.7 | 53.3 | 56 | 64 | 77.3 | |
| SD | 4.6 | 0 | 9.2 | 8 | 0 | 0 | 0 | 5.7 | 4.6 | 0 | 0 | 0 | 4.6 | 0 | 4.6 | 4.6 | 4.6 | 4.6 | 0 | 0 | - | 4.6 | 4.6 | 4.6 | 0 | 0 | 4.6 | |
| Wave Number (cm−1) | Infrared Band |
|---|---|
| 1100~1000 | C-O(H) or C-C vibrational and PO2 symmetric stretching |
| 1180~1100 | C-O(H) stretching vibration |
| 1270~1230 | Two C-O telescopic vibration absorption |
| 1330–1277 | Amide III |
| 1420~1350 | C=O vibration of COO- of free fatty acids, free amino acids and peptides |
| 1580~1510 | Amide II (N-H bending coupled to C-N stretching) |
| 1680~1610 | Amide I (C=O stretching) |
| 1760~1730 | Lipids (C=O stretching vibration) |
| Accuracy (%) | 19 °C | 25 °C | 31 °C | ||||
|---|---|---|---|---|---|---|---|
| Group | PLS-DA | RF | PLS-DA | RF | PLS-DA | RF | |
| A | 58.3% | 63.3% | 77.8% | 41.7% | 100% | 89% | |
| P | 0.67 | 0.61 | 0.81 | 0.46 | 1 | 0.92 | |
| R | 0.58 | 0.60 | 0.78 | 0.39 | 1 | 0.88 | |
| F1-Score | 0.62 | 0.60 | 0.79 | 0.42 | 1 | 0.90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Gao, Y.; Chen, N.; Tang, X.; Li, L.; Hu, G.; Wang, J.; Wang, Y. Estimating the Intra-Puparial Period of Chrysomya nigripes Aubertin Using Morphology and Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy. Insects 2025, 16, 480. https://doi.org/10.3390/insects16050480
Guo Y, Gao Y, Chen N, Tang X, Li L, Hu G, Wang J, Wang Y. Estimating the Intra-Puparial Period of Chrysomya nigripes Aubertin Using Morphology and Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy. Insects. 2025; 16(5):480. https://doi.org/10.3390/insects16050480
Chicago/Turabian StyleGuo, Yi, Yundi Gao, Na Chen, Xin Tang, Liangliang Li, Gengwang Hu, Jiangfeng Wang, and Yu Wang. 2025. "Estimating the Intra-Puparial Period of Chrysomya nigripes Aubertin Using Morphology and Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy" Insects 16, no. 5: 480. https://doi.org/10.3390/insects16050480
APA StyleGuo, Y., Gao, Y., Chen, N., Tang, X., Li, L., Hu, G., Wang, J., & Wang, Y. (2025). Estimating the Intra-Puparial Period of Chrysomya nigripes Aubertin Using Morphology and Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy. Insects, 16(5), 480. https://doi.org/10.3390/insects16050480






