Inhibitory Effect of Fluralaner on GABA Receptor Subunit RDL of Bactrocera dorsalis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Chemicals
2.2. Bioassay
2.3. Cloning and Sequencing Analysis of BdRdl
2.4. Relative Expression Pattern of BdRdl
2.5. Characterization of BdRDL in Xenopus oocytes
2.6. Homology Modeling and Molecular Docking
3. Results
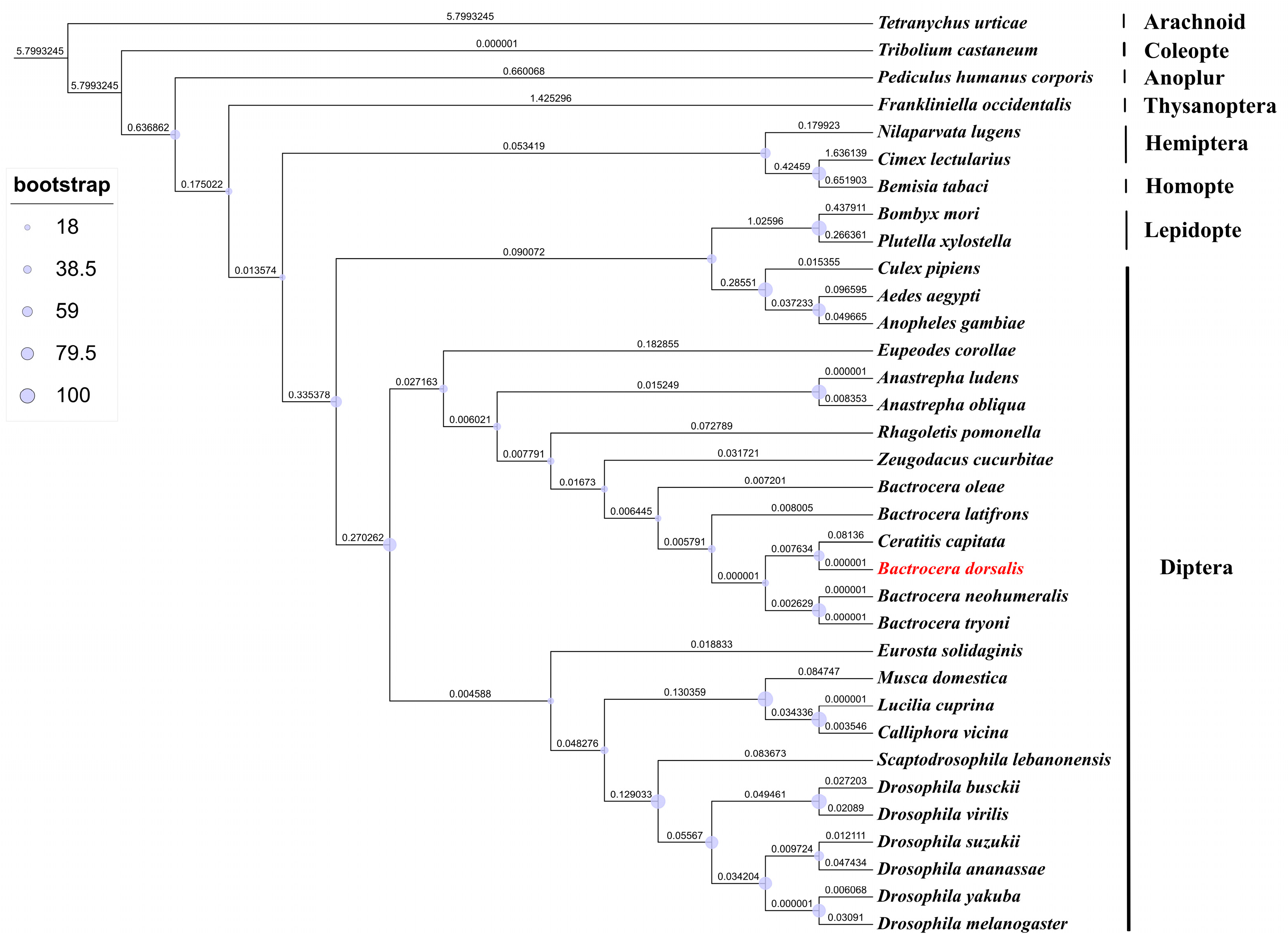
3.1. BdRdl Sequence Analysis
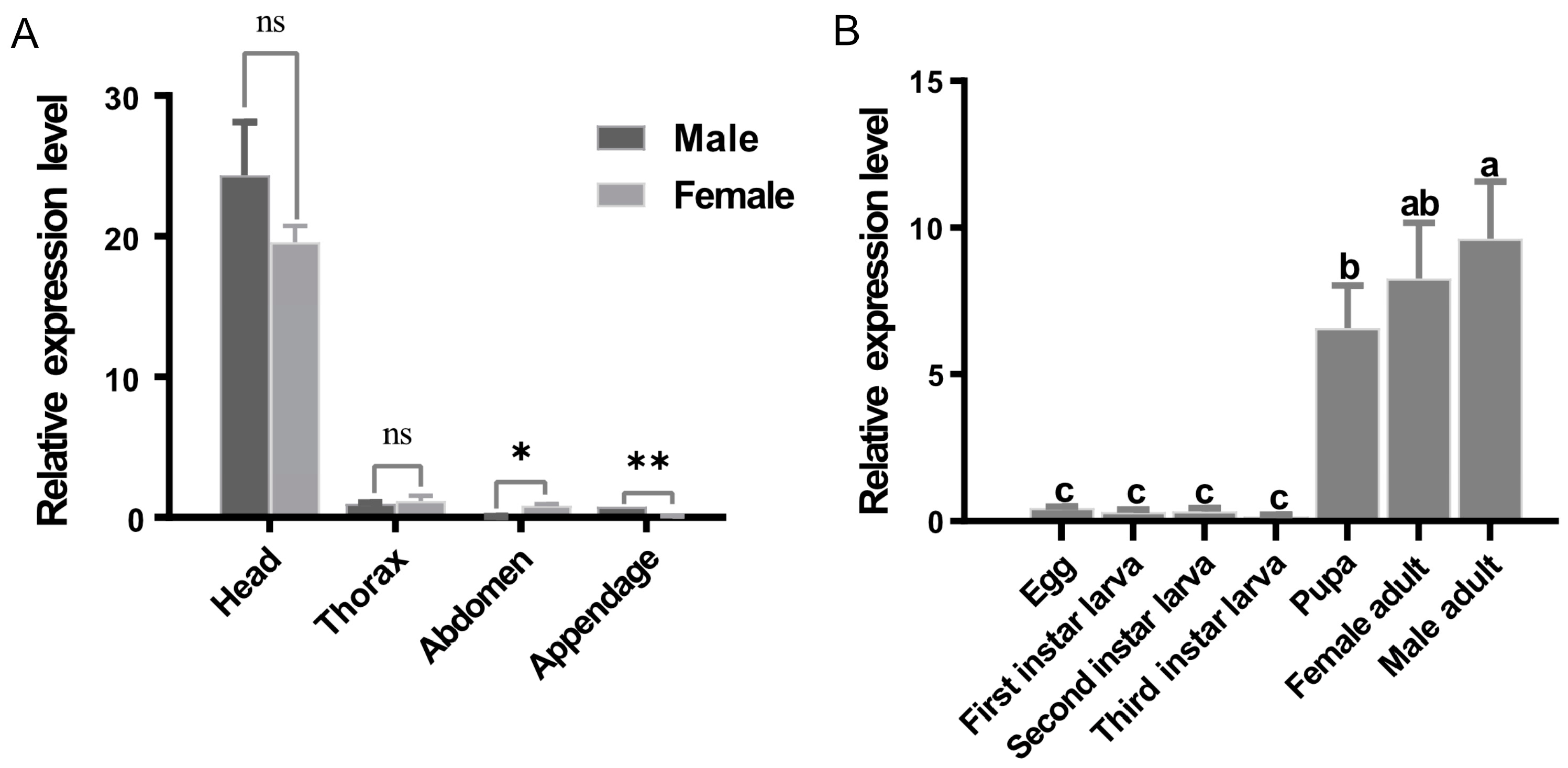
3.2. Expression Profiles of BdRdl
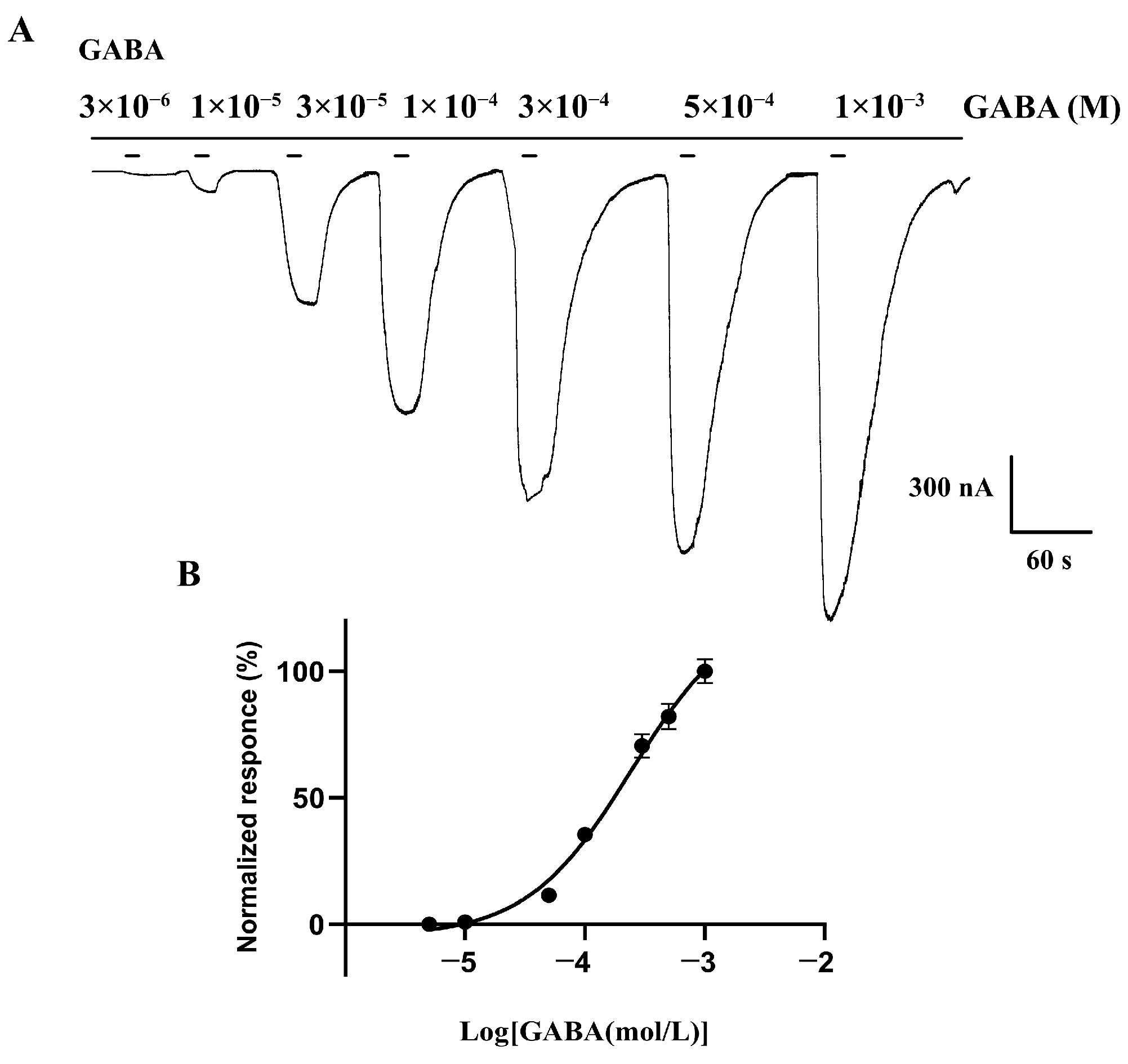
3.3. Functional Characterization of BdRDL in Xenopus oocytes
3.4. Inhibition Effects of Fluralaner BdRDL in Xenopus oocytes
3.5. Sensitivity of B. dorsalis Adults to Fluralaner
3.6. Modeling of Ligand Binding to BdRDL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sim, S.B.; Curbelo, K.M.; Manoukis, N.C.; Cha, D.H. Evaluating Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) response to methyl eugenol: Comparison of three common bioassay methods. J. Econ. Entomol. 2022, 115, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, Y.; Wang, D.; Xie, X.; Li, G.; Zheng, C.; Wen, J.; Su, H.; Liu, X.; Zeng, L.; et al. The effects of nine compounds on aldehyde-oxidase-related genes in Bactrocera dorsalis (Hendel). Genes 2023, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; He, Y.; Ren, Y.; Wang, G.; Chu, D. Seasonal and year-round distributions of Bactrocera dorsalis (Hendel) and its risk to temperate fruits under climate change. Insects 2022, 13, 550. [Google Scholar] [CrossRef]
- Fu, Q.; Zeng, T.; Xu, Y. Molecular cloning and expression profiling of CncC in Bactrocera dorsalis Hendel. Insects 2022, 13, 785. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Zhang, Y.; Chen, J.; Upadhyay, A.; Bhowmick, B.; Hang, J.; Wu, S.; Liao, C.; Han, Q. Functional analysis reveals ionotropic GABA receptor subunit RDL is a target site of ivermectin and fluralaner in the yellow fever mosquito, Aedes aegypti. Pest Manag. Sci. 2022, 78, 4173–4182. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Novel GABA receptor pesticide targets. Pestic. Biochem. Physiol. 2015, 121, 22–30. [Google Scholar] [CrossRef]
- McGonigle, I.; Lummis, S.C. RDL receptors. Biochem. Soc. Trans. 2009, 37, 1404–1406. [Google Scholar] [CrossRef]
- Meng, X.; Yang, X.; Zhang, N.; Jiang, H.; Ge, H.; Chen, M.; Qian, K.; Wang, J. Knockdown of the GABA receptor RDL genes decreases abamectin susceptibility in the rice stem borer, Chilo suppressalis. Pestic. Biochem. Physiol. 2019, 153, 171–175. [Google Scholar] [CrossRef]
- Nakao, T. Mechanisms of resistance to insecticides targeting RDL GABA receptors in planthoppers. Neurotoxicology 2017, 60, 293–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Q.; Sheng, C.; Liu, G.; Zhang, K.; Jia, Z.; Tang, T.; Mao, X.; Jones, A.K.; Han, Z.; et al. G3’MTMD3 in the insect GABA receptor subunit, RDL, confers resistance to broflanilide and fluralaner. PLoS Genet. 2023, 19, e1010814. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hohman, A.E.; Hsu, W.H. Current review of isoxazoline ectoparasiticides used in veterinary medicine. J. Vet. Pharmacol. Ther. 2021, 45, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tsikolia, M.; Bernier, U.R.; Bloomquist, J.R. Mosquitocidal activity and mode of action of the isoxazoline fluralaner. Int. J. Environ. Res. Public Health 2017, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Khan, M.M.; Fajar, A.; Chen, S.; Guo, M.; Chen, Y.; Yang, C.; Wu, J.; Qiu, B.; Zhou, X.; et al. Toxicity of fluralaner against vegetable pests and its sublethal impact on a biocontrol predatory ladybeetle. Ecotoxicol. Environ. Saf. 2021, 225, 112743. [Google Scholar] [CrossRef]
- Scott, J.G.; Baker, O.S.; Dressel, A.E.; Norris, R.H.; Burgess, E.R. Isolation and characterization of the decreased cuticular penetration mechanism of fluralaner resistance in the house fly, Musca domestica. Pestic. Biochem. Physiol. 2024, 205, 106154. [Google Scholar] [CrossRef]
- Shao, L.; Wang, W.; Gong, X.; Yu, Y.; Xue, J.; Zeng, X.; Liu, J. The Toxicity differences of fluralaner against the red imported fire ant (Solenopsis invicta) at different developmental stages. Int. J. Mol. Sci. 2023, 24, 15627. [Google Scholar] [CrossRef]
- Sheng, C.W.; Jia, Z.Q.; Liu, D.; Wu, H.-Z.; Luo, X.-M.; Song, P.P.; Xu, L.; Peng, Y.C.; Han, Z.J.; Zhao, C.Q. Insecticidal spectrum of fluralaner to agricultural and sanitary pests. J. Asia-Pac. Entomol. 2017, 20, 1213–1218. [Google Scholar] [CrossRef]
- Gassel, M.; Wolf, C.; Noack, S.; Williams, H.; Ilg, T. The novel isoxazoline ectoparasiticide fluralaner: Selective inhibition of arthropod γ-aminobutyric acid- and L-glutamate-gated chloride channels and insecticidal/acaricidal activity. Insect Biochem. Mol. Biol. 2014, 45, 111–124. [Google Scholar] [CrossRef]
- Kono, M.; Ozoe, F.; Asahi, M.; Ozoe, Y. State-dependent inhibition of GABA receptor channels by the ectoparasiticide fluralaner. Pestic. Biochem. Physiol. 2022, 181, 105008. [Google Scholar] [CrossRef]
- Thomaz, D.V.; Rodrigues, E.S.B.; Macedo, I.Y.L.D. Chemoinformatic approaches in the study of fluralaner and afoxolaner-mediated inhibition of l-glutamate-gated chloride channels. Path Sci. 2019, 5, 4001–4007. [Google Scholar] [CrossRef]
- Li, B.J.; Wang, K.K.; Chen, D.P.; Yan, Y.; Cai, X.L.; Chen, H.M.; Dong, K.; Lin, F.; Xu, H.H. Distinct roles of two RDL GABA receptors in fipronil action in the diamondback moth (Plutella xylostella). Insect Sci. 2021, 28, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Price, K.L.; Lummis, S.C.R. Characterisation of thymol effects on RDL receptors from the bee parasite Varroa destructor. Pestic. Biochem. Physiol. 2022, 183, 105064. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.W.; Huang, Q.T.; Liu, G.Y.; Ren, X.X.; Jiang, J.; Jia, Z.Q.; Han, Z.J.; Zhao, C.Q. Neurotoxicity and mode of action of fluralaner on honeybee Apis mellifera L. Pest Manag. Sci. 2019, 75, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, P.; Yan, R.; Chen, G.; Qian, J.; Zhu, G.; Chen, M.; Guo, Y. Antenna-biased odorant receptor PstrOR17 mediates attraction of Phyllotreta striolata to (S)-cis-verbenol and (-)-verbenone. Int. J. Mol. Sci. 2024, 25, 4362. [Google Scholar] [CrossRef]
- Alcântara, J.A.; de Araújo, F.S.A.; da Costa Paz, A.; Alencar, R.M.; de Albuquerque Caldas, B.Y.; Godoy, R.S.M.; Lacerda, M.V.G.; de Melo, G.C.; Monteiro, W.M.; de Souza Sampaio, V.; et al. Effect of fluralaner on the biology, survival, and reproductive fitness of the neotropical malaria vector Anopheles aquasalis. Malar. J. 2023, 22, 337. [Google Scholar] [CrossRef]
- Shah, H.K.; Srinivasan, V.; Venkatesan, S.; Balakrishnan, V.; Candasamy, S.; Mathew, N.; Kumar, A.; Kuttiatt, V.S. Evaluation of the mosquitocidal efficacy of fluralaner, a potential candidate for drug based vector control. Sci Rep. 2024, 14, 5628. [Google Scholar] [CrossRef]
- Liu, D.; Jia, Z.Q.; Peng, Y.C.; Sheng, C.W.; Tang, T.; Xu, L.; Han, Z.J.; Zhao, C.Q. Toxicity and sublethal effects of fluralaner on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2018, 152, 8–16. [Google Scholar] [CrossRef]
- Li, D.; Cai, X.; Qi, Y.; Lu, Y.; Li, X. Lethal, sublethal, and offspring effects of fluralaner and dinotefuran on three species of Bactrocera fruit flies. Insects 2024, 15, 440. [Google Scholar] [CrossRef]
- Eguchi, Y.; Ihara, M.; Ochi, E.; Shibata, Y.; Matsuda, K.; Fushiki, S.; Sugama, H.; Hamasaki, Y.; Niwa, H.; Wada, M.; et al. Functional characterization of Musca glutamate- and GABA-gated chloride channels expressed independently and coexpressed in Xenopus oocytes. Insect Mol. Biol. 2006, 15, 773–783. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Rocheleau, T.A. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature 1993, 363, 449–451. [Google Scholar] [CrossRef]
- Jones, A.K.; Sattelle, D.B. The cys-loop ligand-gated ion channel superfamily of the honeybee, Apis mellifera. Invertebr. Neurosci. 2006, 6, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Wells, J.; Senan, A.; Bermudez, I.; Jones, A.K. Species specific RNA A-to-I editing of mosquito RDL modulates GABA potency and influences agonistic, potentiating and antagonistic actions of ivermectin. Insect Biochem. Mol. Biol. 2018, 93, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, Y.; Hu, J.; Qi, C.; Zhang, M.; Xu, Q.; He, L. The interaction between abamectin and RDL in the carmine spider mite: A target site and resistant mechanism study. Pestic. Biochem. Physiol. 2020, 164, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Abbas, R.Z.; Mehmood, K.; Hussain, R.; Shah, S.; Faheem, M.; Zaheer, T.; Abbas, A.; Morales, B.; Aneva, I.; et al. Assessment of avermectins-induced toxicity in animals. Pharmaceuticals 2022, 15, 332. [Google Scholar] [CrossRef]
- Shoop, W.L.; Mrozik, H.; Fisher, M.H. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995, 59, 139–156. [Google Scholar] [CrossRef]
- Ozoe, Y. Chapter Four. γ-Aminobutyrate- and Glutamate-gated chloride channels as targets of insecticides. Adv. Insect Physiol. 2013, 44, 211–286. [Google Scholar] [CrossRef]
- Sun, X.; Hua, W.; Wang, K.; Song, J.; Zhu, B.; Gao, X.; Liang, P. A novel V263I mutation in the glutamate-gated chloride channel of Plutella xylostella (L.) confers a high level of resistance to abamectin. Int. J. Biol. Macromol. 2023, 230, 123389. [Google Scholar] [CrossRef]
- Nakatani, Y.; Furutani, S.; Ihara, M.; Matsuda, K. Ivermectin modulation of pH-sensitive chloride channels in the silkworm larvae of Bombyx mori. Pestic. Biochem. Physiol. 2016, 126, 1–5. [Google Scholar] [CrossRef]
- Yin, X.; Yang, G.F.; Niu, D.B.; Chen, J.; Liao, M.; Cao, H.Q.; Sheng, C.W. Identification and pharmacological characterization of histamine-gated chloride channels in the fall armyworm, Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2022, 140, 103698. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.-S.; He, P.-Y.; Zhang, M.-H.; Cao, H.-Q.; Palli, S.R.; Sheng, C.-W. Identification and pharmacological characterization of pH-sensitive chloride channels in the fall armyworm, Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2025, 177, 104243. [Google Scholar] [CrossRef]
- Bertaud, A.; Cens, T.; Mary, R.; Rousset, M.; Arel, E.; Thibaud, J.B.; Vignes, M.; Ménard, C.; Dutertre, S.; Collet, C.; et al. Xenopus Oocytes: A Tool to Decipher Molecular Specificity of insecticides towards mammalian and insect GABA-A receptors. Membranes 2022, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Asahi, M.; Kobayashi, M.; Matsui, H.; Nakahira, K. Differential mechanisms of action of the novel γ-aminobutyric acid receptor antagonist ectoparasiticides fluralaner (A1443) and fipronil. Pest Manag. Sci. 2015, 71, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, J.; Wang, Y.; Amponsah, P.; Tang, T.; Jones, A.K.; Zhao, C. N318L blocks the interaction of fluralaner but not broflanilide or fipronil with the insect GABA receptor in vivo. J. Agric. Food Chem. 2024, 72, 22554–22561. [Google Scholar] [CrossRef] [PubMed]
- Asahi, M.; Yamato, K.; Ozoe, F.; Ozoe, Y. External amino acid residues of insect GABA receptor channels dictate the action of the isoxazoline ectoparasiticide fluralaner. Pest Manag. Sci. 2023, 79, 4078–4082. [Google Scholar] [CrossRef]
- Norris, R.H.; Baker, O.S.; Burgess, E.R., IV; Tarone, A.; Gerry, A.; Fryxell, R.T.T.; Hinkle, N.C.; Olds, C.; Boxler, D.; Wise, K.; et al. Selection for, and characterization of, fluralaner resistance in the house fly, Musca domestica. Pestic. Biochem. Physiol. 2023, 191, 105355. [Google Scholar] [CrossRef]



| Primer Name | Primer Sequences (5′–3′) |
|---|---|
| For RT-qPCR | |
| BdorEF1a-F | CGTTGGTGTCAACAAGATGG |
| BdorEF1a-R | TGCCTTCAGCATTACCTTCC |
| BdorTublin-F | CGCATTCATGGTTGATAACG |
| BdorTublin-R | GGGCACCAAGTTAGTCTGGA |
| BdorRdlqPCR-F | GTGTTCATCATTCTGGAT |
| BdorRdlqPCR-R | ATGTCACGCACTTGTATAG |
| For amplification | |
| BdorRdl-F | GCCCCTCGTTATTGGTTATAT |
| BdorRdl-R | TAAGCTTGTATGCCAACTGTT |
| PGH19-F | AATTCTCTAGAGCAAGCTTGATC |
| PGH19-R | CGGATCCCCGGGGAATTGATCTG |
| BdorRdlPGH19-F | ATCAATTCCCCGGGGATCCGGCCACCATGGCCTTTAGCAGCAGCAG |
| BdorRdlPGH19-R | CAAGCTTGCTCTAGAGAATTTTACTTCTCCTCGCCGAGCAA |
| Compounds | Regression Equation | R2 | LC50 (95%CI) (mg/L) a | p-Value b | χ2 (df) |
|---|---|---|---|---|---|
| Fluralaner | y = 4.8267x − 4.8267 | 0.9989 | 8.281 (6.341–10.775) | 0.562 | 2.975 (4) |
| Avermectin | y = 3.2056x − 2.0328 | 0.9869 | 4.307 (3.042–6.760) | 0.464 | 2.563 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Chen, G.; Chen, K.; Xu, Z.; Qian, J.; Yan, R.; Chen, B.; Wu, H.; Chen, M. Inhibitory Effect of Fluralaner on GABA Receptor Subunit RDL of Bactrocera dorsalis. Insects 2025, 16, 479. https://doi.org/10.3390/insects16050479
Zhou X, Chen G, Chen K, Xu Z, Qian J, Yan R, Chen B, Wu H, Chen M. Inhibitory Effect of Fluralaner on GABA Receptor Subunit RDL of Bactrocera dorsalis. Insects. 2025; 16(5):479. https://doi.org/10.3390/insects16050479
Chicago/Turabian StyleZhou, Xiangyi, Guoxing Chen, Keying Chen, Zhanyi Xu, Jiali Qian, Ru Yan, Bosheng Chen, Huiming Wu, and Mengli Chen. 2025. "Inhibitory Effect of Fluralaner on GABA Receptor Subunit RDL of Bactrocera dorsalis" Insects 16, no. 5: 479. https://doi.org/10.3390/insects16050479
APA StyleZhou, X., Chen, G., Chen, K., Xu, Z., Qian, J., Yan, R., Chen, B., Wu, H., & Chen, M. (2025). Inhibitory Effect of Fluralaner on GABA Receptor Subunit RDL of Bactrocera dorsalis. Insects, 16(5), 479. https://doi.org/10.3390/insects16050479





