Morphology of External Genitalia in the Genus Acanthoponera Mayr, with Redescription of A. mucronata (Roger) Male (Hymenoptera: Formicidae: Ectatomminae)
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Morphological Analysis
2.2. Measurements
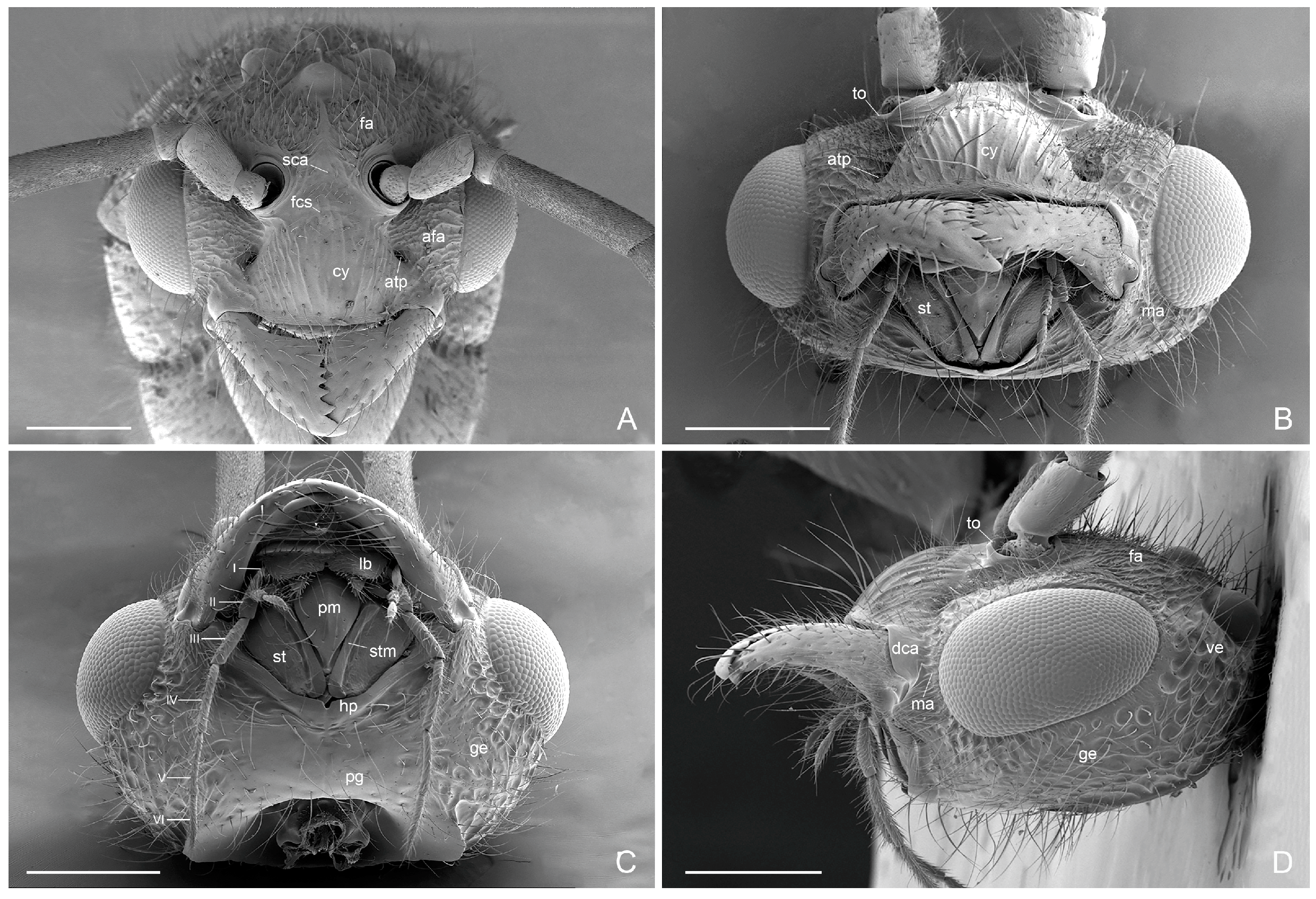
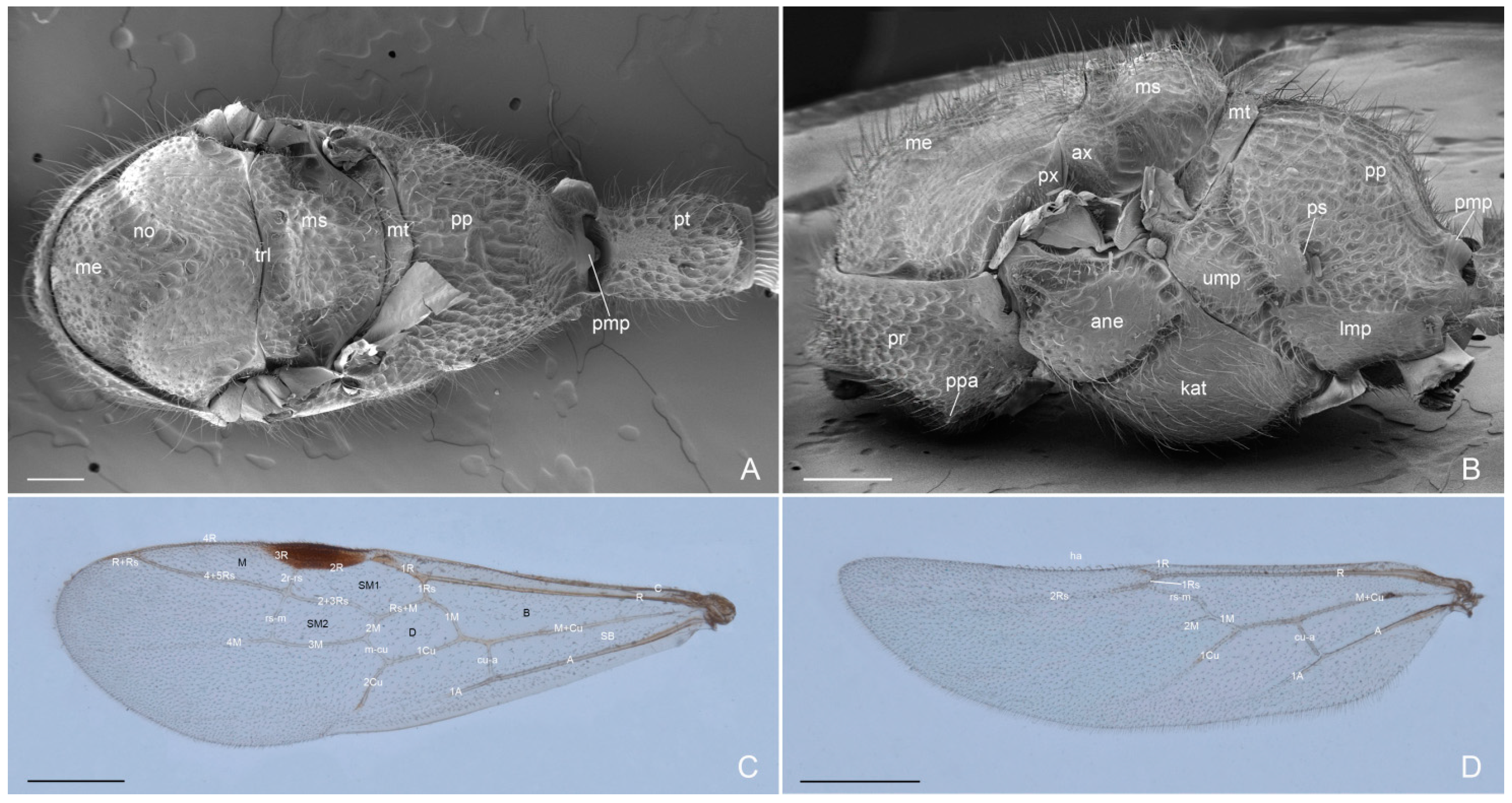
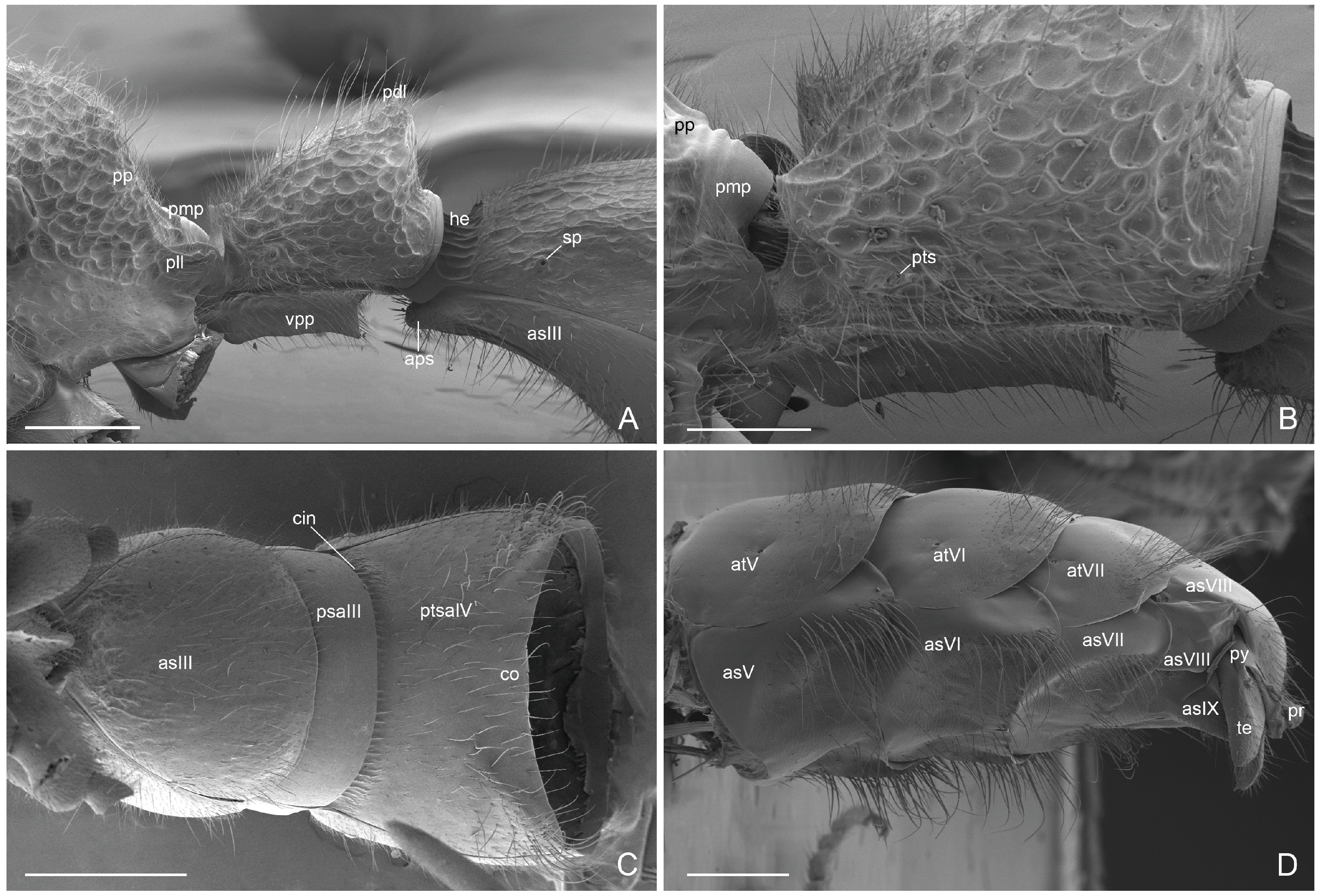
3. Results
3.1. Male-Based Morphological Diagnosis of the Genus Acanthoponera
3.2. Acanthoponera mucronata ♂ (Roger, 1860)
4. Discussion
- (i)
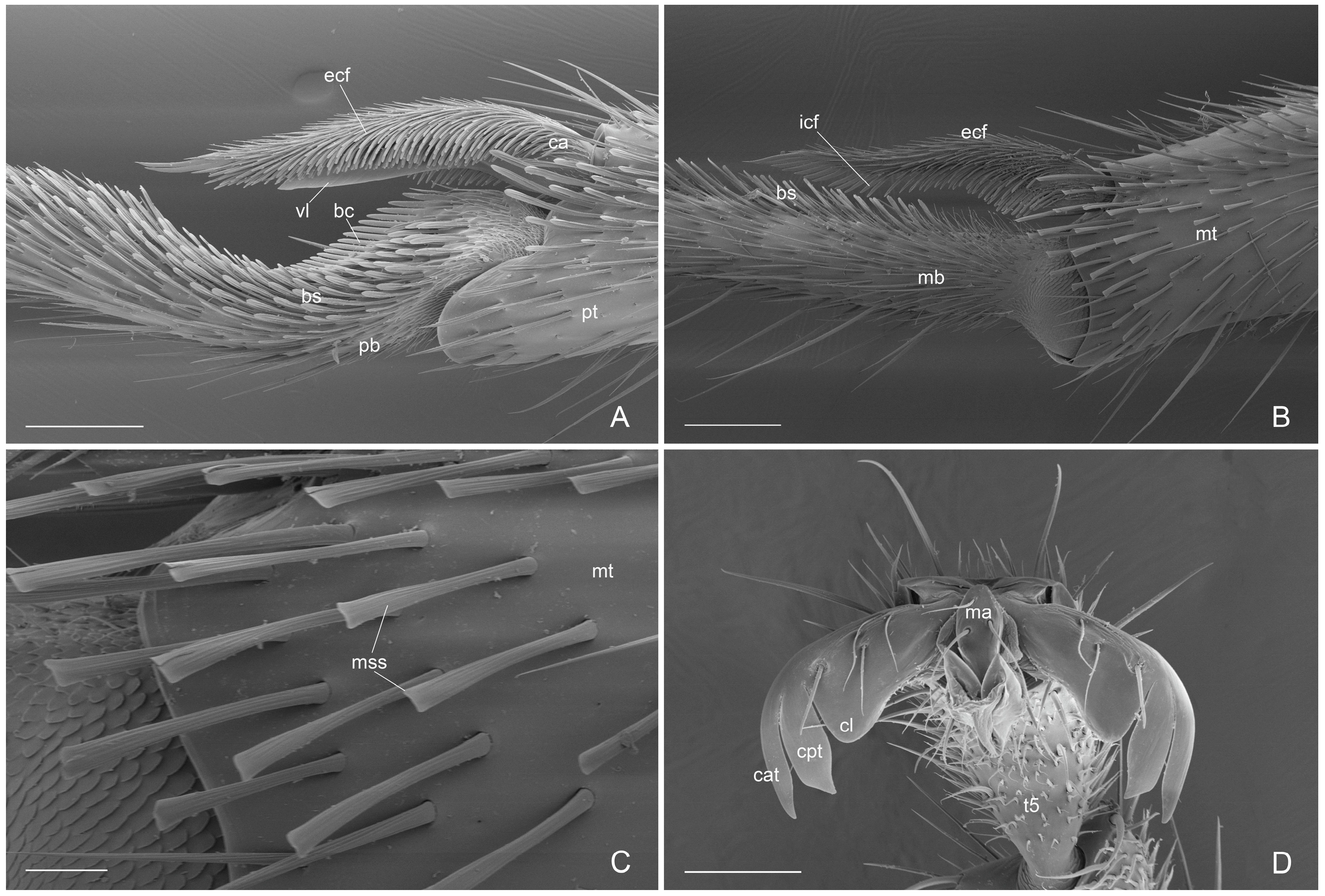
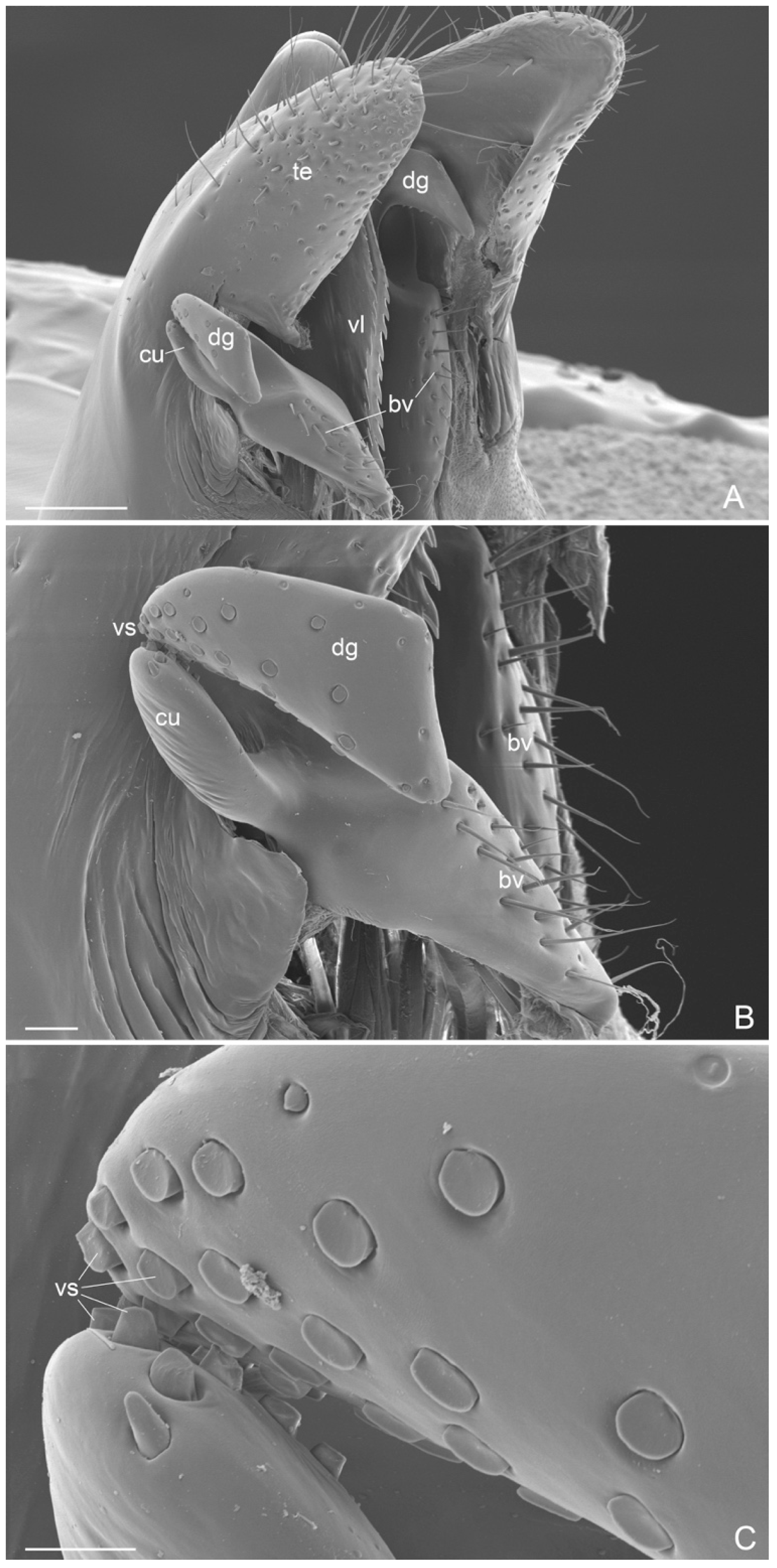
- antennal cleaning with slender and long velum (lamella) in the calcar, without the calcar comb (Figure 4A). Similar velum morphology is described in some genera of the subfamily Amblyoponinae (Amblyopone, Myopopone, Stigmatomma); velum of different morphology, characterized by a basal notch, is present in some genera of the subfamilies Ectatomminae (Rhytidoponera, Ectatomma), Leptanillinae (Leptanilla), Ponerinae (Anochetus, Dinoponera, Megaponera, Odontomachus, Pachycondyla, Palthothyreus, Platythyrea, Ponera, Pseudoponera), Paraponerinae (Paraponera) and, Pseudomyrmecinae (Pseudomyrmex, Tetraponera), [16,18,19].
- (ii)
- (iii)
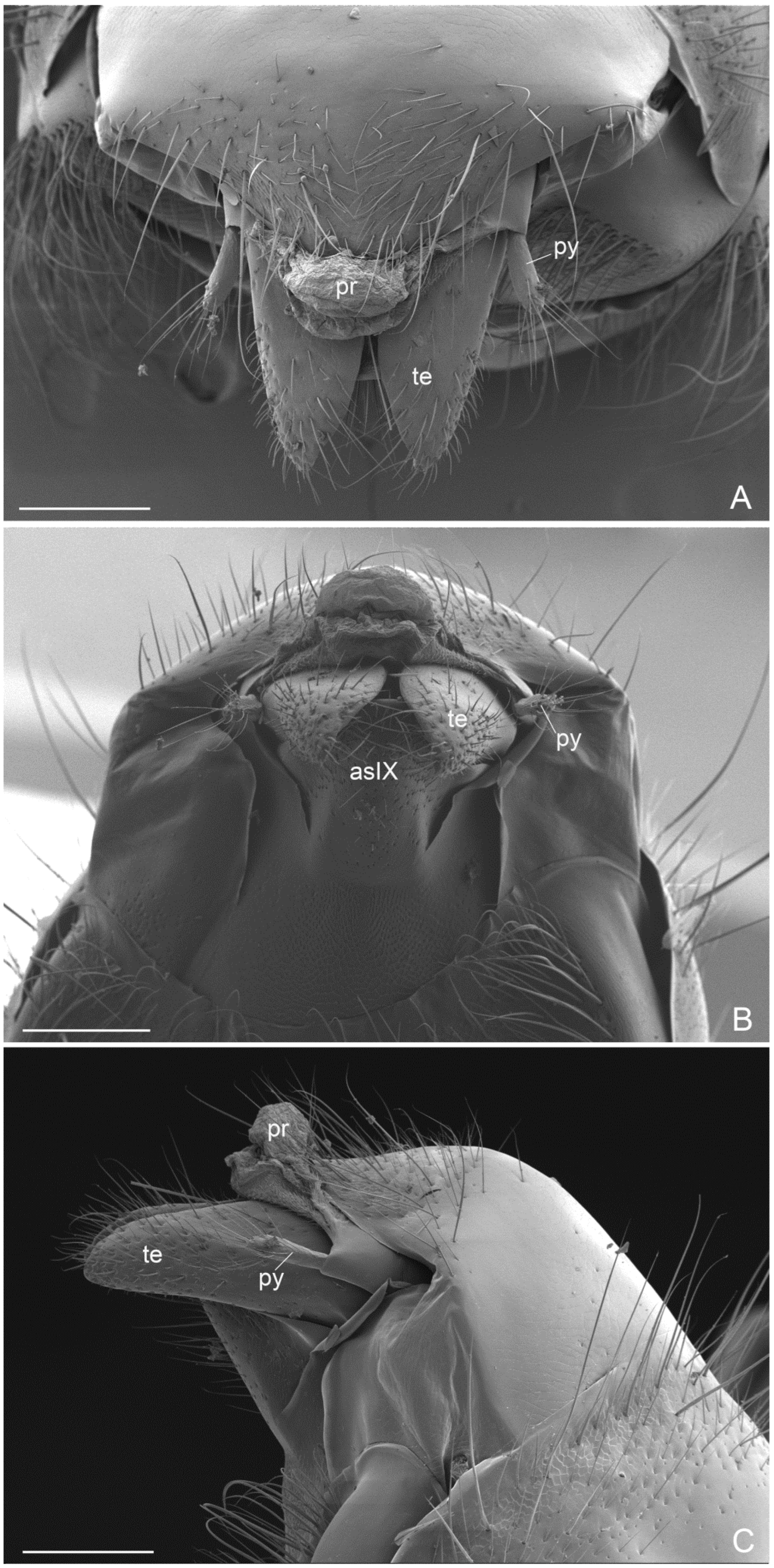
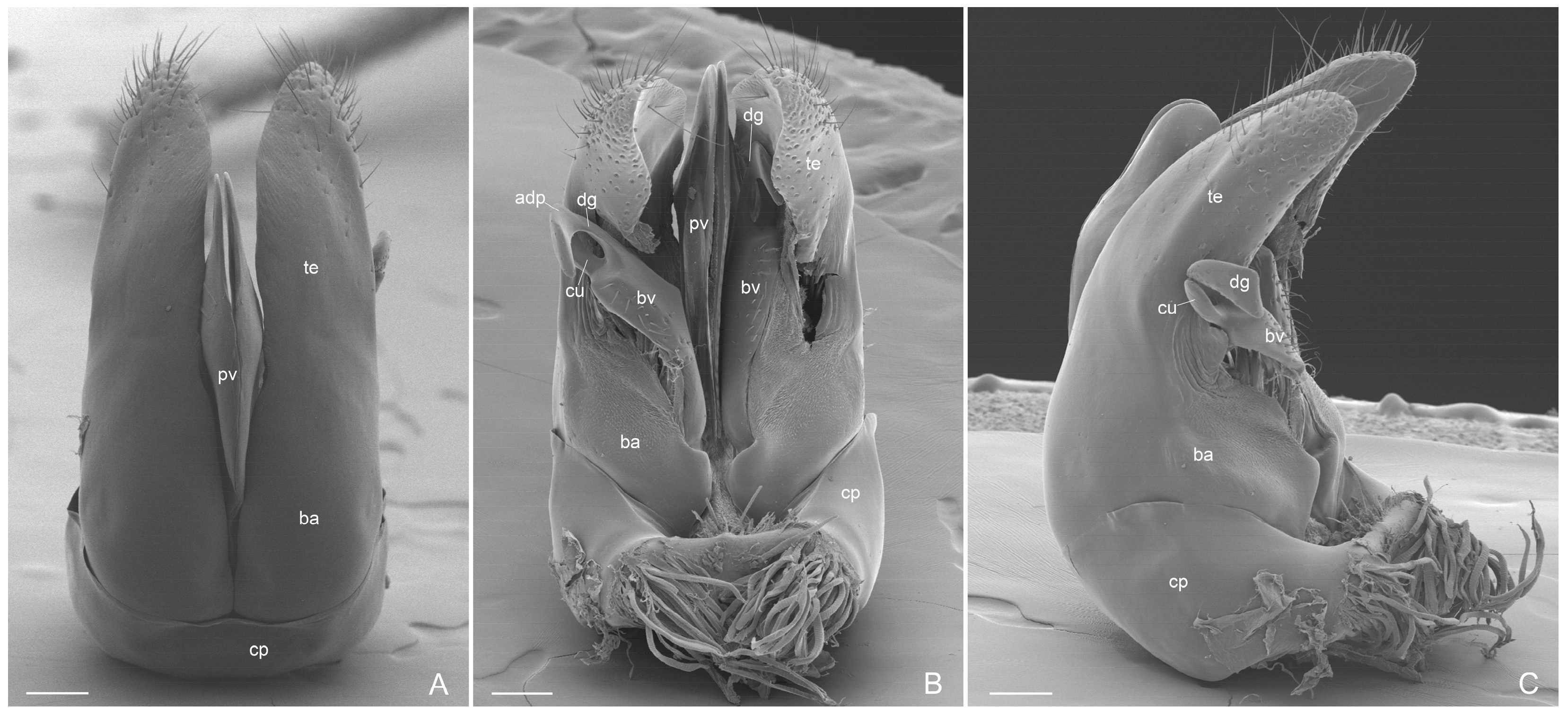
- External genitalia. They show features that can be used for the diagnosis of genus, like: telomere lobiform, presence of a cuspis developed and lobiform and digitus falciform with a apicodorsal process (Figure 6C and Figure 7A). In A. mucronata the lateral digitus and the median apical cuspis present the button-like sensilla, which constitute the “volsella sensorium” [26] (Figure 8B,C). The button-like sensilla of the cuspis are in correspondence with some button-like sensilla of the digitus apicodorsal process, showing that they have a mechanosensorial function during mating; in fact, the introduction of the penisvalve is favored by the volsella (cuspis and digitus), which has the function of keeping the last abdominal segments of the female [21,22,24]. In the subfamily Ectatomminae the external genitalia have been described in: Rhytidioponera metallica [23], showing similarity of parassiculus structures with A. mucronata; Typhlomyrmex meire [35]; and in four species of the genus Ectatomma [36].
- (iv)
- Apex of metatibiae and proximal part of metabasitarsus with spatulate setae, truncate at apex.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Camacho, G.P.; Franco, W.; Branstetter, M.G.; Pie, M.R.; Longino, J.T.; Schultz, T.R.; Feitosa, R.M. UCE Phylogenomics Resolves Major Relationships Among Ectaheteromorph Ants (Hymenoptera: Formicidae: Ectatomminae, Heteroponerinae): A New Classification For the Subfamilies and Description of a New Genus. Insect Syst. Divers. 2022, 6, 5. [Google Scholar] [CrossRef]
- Feitosa, R.M.; Prada-Achiardi, F.C. Subfamilia Ectatomminae. In Hormigas de Colombia; Fernandez, F., Guerrero, R.J., Delsine, T., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2019; Capitulo 22; pp. 659–679. [Google Scholar]
- Fernandez, F. Hormigas de Colombia III: Los generous Acanthoponera Mayr, Heteroponera Mayr y Paraponera Fr. Smith (Formicidae: Ponerinae: Ectatommini). Caldasia 1993, 17, 249–258. [Google Scholar]
- Feitosa, R.M. Revisão taxonômica e análise filogenética de Heteroponerinae (Hymenoptera, Formicidae) 2011, XIII+297p. Tese de Doutorado—Faculdade de Filosofia Ciências e Letras de Ribeirão Preto da USP. Departamento de Biologia. Programa de Pós-Graduação em Entomologia. Available online: https://www.teses.usp.br/teses/disponiveis/59/59131/tde-17072012-114907/pt-br.php (accessed on 6 April 2025).
- Bolton, B. An Online Catalog of the Ants of the World. 2024. Available online: http://antcat.org (accessed on 1 March 2025).
- Emery, C. Hymenoptera, Fam. Formicidae, Subfam. Ponerinae. Genera Insectorum 1911, 118, 1–125. [Google Scholar]
- Brown, W.L. Contribution toward a reclassification of the Formicidae. II. Tribe Ectatommini (Hymenoptera). Bull. Mus. Comp. Zool. Harv. Coll. 1958, 118, 5. [Google Scholar]
- Kusnezov, N. El ala posterior de las hormigas. Acta Zool. Lilloana 1962, 18, 367–378. [Google Scholar]
- Ketterl, J.; Verhaagh, M. Acanthoponera mucronata (Roger, 1860) (Hymenoptera: Formicidae), first record in Peru and Rio Grande do Sul, Brazil, with description of its male. Rev. Peru. Entomol. 2004, 44, 65–68. [Google Scholar]
- Boudinot, B.E. Contribution to the knowledge of Formicidae (Hymenoptera, Aculeata): A new diagnosis of the family, the first global male-based key to subfamilies, and a treatment of early branching lineages. Eur. J. Taxon. 2015, 120, 1–62. [Google Scholar] [CrossRef]
- Cantone, S. Winged Ants, The Male: Dichotomous Key to Genera of Winged ♂♂ Ants in the World; Behavioral Ecology of Mating Flight; Stefano Cantone Editor: Catania, Italy, 2017; ISBN 9791220023948. Available online: https://antcat.org/references/143115 (accessed on 6 April 2025).
- Cantone, S. Wings of the Ants (Hymenoptera, Formicidae): Comparative Study and Identification Keys for Winged Castes. Universidade Estadual Paulista (UNESP), São Paulo. 2019. Available online: https://repositorio.unesp.br/items/d488bfc2-e4bd-4d5e-b35cf2901f3286 (accessed on 6 April 2025).
- Cantone, S.; Von Zuben, J.C. The Hindwings of Ants: A Phylogenetic analysis. Psyche 2019, 2019, 7929717. [Google Scholar] [CrossRef]
- Yoshimura, M.; Fisher, B.L. A revision of male ants of the Malagasy region (Hymenoptera: Formicidae): Key to subfamilies and treatment of the genera of Ponerinae. Zootaxa 2007, 1654, 21–40. [Google Scholar] [CrossRef]
- Yoshimura, M.; Fisher, B.L. A revision of male ants of the Malagasy region (Hymenoptera: Formicidae): Key to genera of the subfamily Dolichoderinae. Zootaxa 2011, 2794, 1–34. [Google Scholar]
- Keller, R.A. A Phylogenetic Analysis of Ant Morphology (Hymenoptera: Formicidae) with Special Reference to the Poneromorph Subfamilies. Bull. Am. Mus. Nat. Hist. 2011, 2011, 1–90. [Google Scholar] [CrossRef]
- Delsine, T.; Serna, F.J.; Leponce, M.; Boudinot, B.E. Glossario de morfologia. In Hormigas de Colombia; Fernandez, F., Guerrero, R.J., Delsine, T., Eds.; Universidad Nacional de Colombia: Bogotà, Colombia, 2019; Capitulo 13; pp. 387–457. ISBN 978-958-783-765-0. [Google Scholar]
- Schönitzer, K.; Lawitzky, G. A phylogenetic study of the antenna cleaner in Formicidae, Mutillidae, and Tiphiidae (Insecta, Hymenoptera). Zoomorphology 1987, 107, 272–285. [Google Scholar] [CrossRef]
- Francoeur, A.; Loiselle, R. Evolution du stringile chez les formicides (Hymenopteres). Le Nat. Can. 1988, 115, 333–353. [Google Scholar]
- Beutel, R.G.; Richter, A.; Keller, R.A.; Hita Garcia, F.; Matsumura, Y.; Economo, E.P.; Gorb, S.N. Distal leg structures of the Aculeata (Hymenoptera): A comparative evolutionary study of Sceliphron (Phecidae) and Formica (Formicidae). J. Morfol. 2020, 281, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Snograss, R.E. The male genitalia of Hymenoptera. Smithson. Misc. Collect. 1941, 99, 14. [Google Scholar]
- Snograss, R.E. A revised interpretation of the external reproductive organs of male insects. Smithson. Misc. Collect. 1957, 135, 6. [Google Scholar]
- Forbes, J.; Hagopian, M. The Male Genitalia and Terminal Segment of the Ponerine Ant Rhytidoponera metallica F. Smith (Hymenoptera: Formicidae). J. N. Y. Entomol. Soc. 1965, 73, 190–194. [Google Scholar]
- Boudinot, B.E. The male genitalia of ants: Musculature, homology, and functional morphology (Hymenoptera, Aculeata, Formicidae). J. Hymenopt. Res. JHR 2013, 30, 29–49. [Google Scholar] [CrossRef]
- Cantone, S.; Di Giulio, A. A new Neotropical ant species of genus Linepithema Mayr (Hymenoptera, Formicidae, Dolichoderinae) with partial revision of the L. fuscum group based on males. Zookeys 2023, 1160, 125–144. [Google Scholar] [CrossRef]
- Cantone, S.; Di Giulio, A. A Fine Morphological Study of the Rare Anillidris bruchi Santschi (Hymenoptera: Formicidae: Dolichoderinae) Male and Queen. Insects 2023, 14, 723. [Google Scholar] [CrossRef]
- Dlussky, G.M.; Brothers, D.J.; Rasnitsyn, A.P. The first Late Cretaceous ants (Hymenoptera: Formicidae) from souther Africa, with comment on the origin of the Myrmicinae. Insect Syst. Evol. 2004, 35, 1–13. [Google Scholar]
- Perfilieva, K.S. Trend in Evolution of Ant Wing Venation (Hymenoptera, Formicidae). Entomol. Rev. 2010, 90, 857–870. [Google Scholar] [CrossRef]
- Richter AKeller, R.A.; Rosumek, F.B.; Economo, E.P.; Hita Garcia, F.; Beutel, R.G. The cephalic anatomy of workers of the ant species Wasmannia affinis (Formicidae, Hymenoptera, Insecta) and its evolutionary implications. Arthropod Struct. Dev. 2019, 49, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Hita Garcia, F.; Keller, R.A.; Billen, J.; Economo, E.P.; Beutel, R.G. Comparative analysis of worker head anatomy of Formica and Brachyponera (Hymenoptera: Formicidae). Arthropod Struct. Phylogeny 2020, 78, 133–170. [Google Scholar] [CrossRef]
- Hellenbrand, J.P.; Penick, C.A. Ant cuticle microsculpturing: Diversity, classification, and evolution. Myrmecol. News 2023, 33, 123–138. [Google Scholar] [CrossRef]
- Lieberman, Z.E.; Billen, J.; van de Kamp, T.; Boudinot, B.E. The ant abdomen: The skeletomuscolar and soft tissue anatomy of Amblyopone australis worker (Hymenoptera: Formicidae). J. Morphol. 2022, 283, 693–770. [Google Scholar] [CrossRef]
- Hölldobler, B.; Engel-Siegel, H. On the metapleural gland of ants. Psyche 1984, 91, 3–4. [Google Scholar] [CrossRef]
- Yek, S.H.; Mueller, U.G. The metapleural gland of ants. Biol. Rev. 2011, 86, 774–791. [Google Scholar] [CrossRef]
- Lacau, S.; Villemant, C.; Delabie, J.H.C. Typhlomyrmex meire, a remarkable new species endemic to Southern Bahia, Brazil (Formicidae: Ectatomminae). Zootaxa 2004, 678, 1–23. [Google Scholar] [CrossRef]
- Almeida, A.J. Descrição de quarto machos do genero Ectatomma Smith, 1858 (Hymenoptera, Formicidae, Ponerinae). Quid Teresina 1986, 6, 24–38. [Google Scholar]
- Silva, N.S.; Maciel, E.A.; Prado, L.P.; Silva, O.G.M.; Barbosa, D.A.; Andrade-Silva, J.; Souza-Campana, D.R.; Silva, R.R.; Brandão, C.R.F.; Delabie, J.H.C.; et al. Ant rarity and vulnerability in Brazilian Atlantic Forest fragments. Biol. Conserv. 2024, 296, 110640. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantone, S.; Di Giulio, A. Morphology of External Genitalia in the Genus Acanthoponera Mayr, with Redescription of A. mucronata (Roger) Male (Hymenoptera: Formicidae: Ectatomminae). Insects 2025, 16, 436. https://doi.org/10.3390/insects16040436
Cantone S, Di Giulio A. Morphology of External Genitalia in the Genus Acanthoponera Mayr, with Redescription of A. mucronata (Roger) Male (Hymenoptera: Formicidae: Ectatomminae). Insects. 2025; 16(4):436. https://doi.org/10.3390/insects16040436
Chicago/Turabian StyleCantone, Stefano, and Andrea Di Giulio. 2025. "Morphology of External Genitalia in the Genus Acanthoponera Mayr, with Redescription of A. mucronata (Roger) Male (Hymenoptera: Formicidae: Ectatomminae)" Insects 16, no. 4: 436. https://doi.org/10.3390/insects16040436
APA StyleCantone, S., & Di Giulio, A. (2025). Morphology of External Genitalia in the Genus Acanthoponera Mayr, with Redescription of A. mucronata (Roger) Male (Hymenoptera: Formicidae: Ectatomminae). Insects, 16(4), 436. https://doi.org/10.3390/insects16040436






