Life Table Parameters and Digestive Enzyme Activity of Araecerus fasciculatus (Coleoptera: Anthribidae) Feeding on Different Stored Products
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Stored Products
2.3. Development and Survival of Araecerus fasciculatus
2.4. Fecundity
2.5. Life Table Parameters
2.6. Activity Assays of Digestive Enzymes
2.6.1. Enzyme Extract Preparation
2.6.2. Digestive Enzyme Activity Assay
3. Statistical Analyses
4. Results
4.1. Development
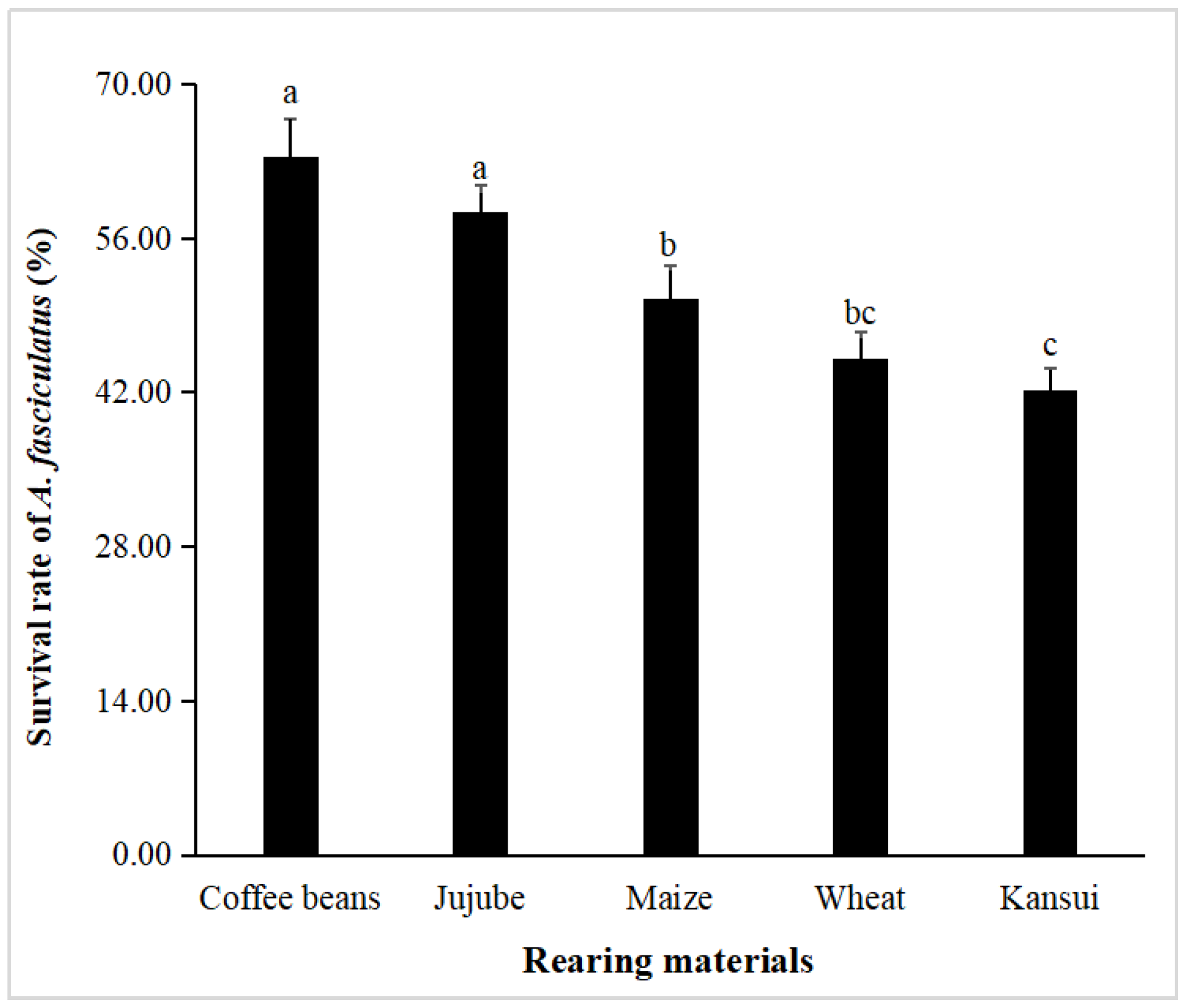
4.2. Survival
4.3. Oviposition and Adult Longevity
4.4. Life Table Parameters
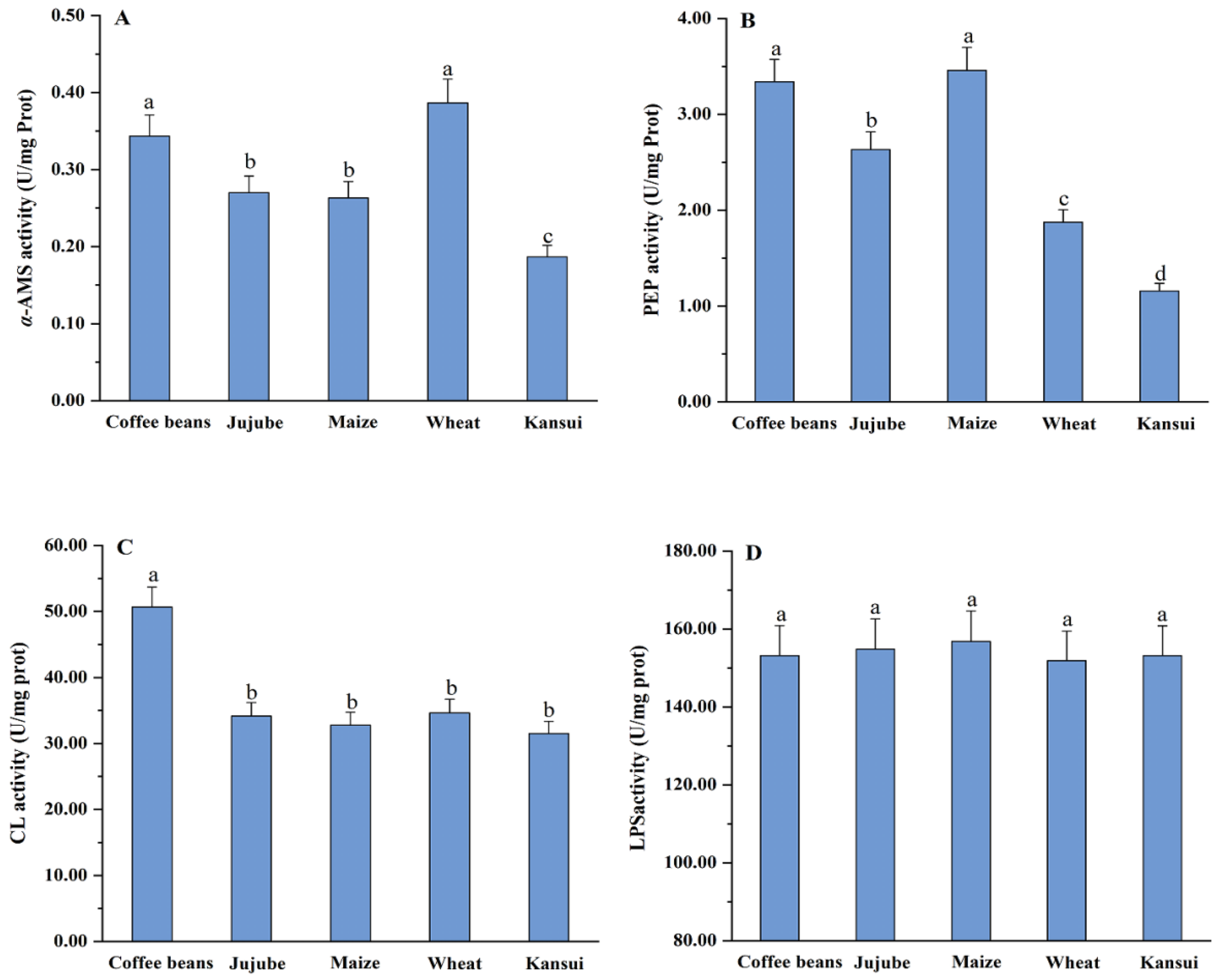
4.5. Digestive Enzyme Activity Bioassays
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salbiah; Hidayat, H.; Sudarjat. Sulfuryl fluoride fumigation to eliminate all life stages of Araecerus fasciculatus (Coleoptera: Anthribidae). J. Stored Prod. Res. 2023, 103, 102152. [Google Scholar] [CrossRef]
- Alba-Alejandre, I.; Alba-Tercedor, J.; Vega, E.F. Micro-CT to Document the Coffee Bean Weevil, Araecerus fasciculatus (Coleoptera: Anthribidae), Inside Field-Collected Coffee Berries (Coffea canephora). Insects 2018, 9, 100. [Google Scholar] [CrossRef]
- Caasi-Lit, T.M.; Lit, L.I., Jr. First Report of the Coffee Bean Weevil Araecerus fasciculatus (De Geer) (Coleoptera: Anthribidae) as Pest of Papaya in the Philippines. Philipp. Agric. Sci. 2011, 94, 415–420. [Google Scholar]
- Chijindu, E.N.; Boateng, B.A. Preference of and damage to processed cassava chips by Araecerus fasciculatus (Degeer). J. Appl. Sci. 2008, 4, 939–944. [Google Scholar]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef]
- Jovanović, Z.; Kostić, M.; Popović, Z. Grain protective properties of herbal extracts against the bean weevil Acanthoscelides obtectus say. Ind. Crops Prod. 2007, 26, 100–104. [Google Scholar] [CrossRef]
- Nayak, M.K.; Holloway, J.C.; Emery, R.N.; Pavic, H.; Bartlet, J.; Collins, P.J. Strong resistance to phosphine in the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae): Its characterisation, a rapid assay for diagnosis and its distribution in Australia. Pest Manag. Sci. 2013, 69, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Agrafioti, P.; Athanassiou, C.G.; Subramanyam, B. Efficacy of heat treatment on phosphine resistant and susceptible populations of stored product insects. J. Stored Prod. Res. 2019, 81, 100–106. [Google Scholar] [CrossRef]
- Jagadeesan, R.; Singarayan, V.T.; Nayak, M.K. A Co-fumigation strategy utilizing reduced rates of phosphine (PH3) and sulfuryl fluoride (SF) to control strongly resistant rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae). Pest Manag. Sci. 2021, 77, 4009–4015. [Google Scholar] [CrossRef] [PubMed]
- Chougourou, D.C.; Togola, A.; Nwilene, F.E.; Adeliossi, J.; Bachabi, F.; Oyetunji, O.E. Susceptibility of some rice varieties to the lesser grain borer, Rhyzopertha dominica Fab. (Coleoptera: Bostrichidae) in Benin. J. Appl. Sci. 2013, 13, 173–177. [Google Scholar] [CrossRef]
- Majd-Marani, S.; Naseri, B.; Nouri-Ganbalani, G.; Borzoui, E. The effect of maize hybrid on biology and life table parameters of the Khapra beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae). J. Econ. Entomol. 2017, 110, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Naseri, B.; Majd-Marani, S. Assessment of eight rice cultivars flour for feeding resistance to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2020, 88, 101650. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, T.; Wang, C.; D’Isita, I.; Hu, Q.; Germinara, G.S.; Cao, Y. Population development of Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) on different stored products. Entomol. Res. 2023, 53, 359366. [Google Scholar] [CrossRef]
- Patankar, A.G.; Giri, A.P.; Harsulkar, A.M.; Sainani, M.N.; Deshpande, V.V.; Ranjekar, P.K.; Gupta, V.S. Complexity in specificities and expression of Helicoverpa armigera gut proteinases explains polyphagous nature of the insect pest. Insect Biochem. Mol. Biol. 2001, 31, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Borzoui, E.; Naseri, B.; Nouri-Ganbalani, G. Effects of food quality on biology and physiological traits of Sitotroga cerealella (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2017, 110, 266–273. [Google Scholar]
- Naseri, B.; Borzoui, E. Life cycle and digestive physiology of Trogoderma granarium (Coleoptera: Dermestidae) on various wheat cultivars. Ann. Entomol. Soc. Am. 2016, 109, 831–838. [Google Scholar] [CrossRef]
- Naseri, B.; Borzoui, E.; Majd, S.H.; Mozaffar Mansouri, S. Influence of different food commodities on life history, feeding efficiency, and digestive enzymatic activity of Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2017, 110, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Nemati-Kalkhoran, M.; Razmjou, J.; Borzoui, E.; Naseri, B. Comparison of life table parameters and digestive physiology of Rhyzopertha dominica (Coleoptera: Bostrichidae) fed on various barley cultivars. J. Insect Sci. 2018, 18, 31. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Borzoui, E. Growth performance and digestive enzymes activity of Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) feeding on six rice cultivars. J. Stored Prod. Res. 2019, 82, 48–53. [Google Scholar]
- Naseri, B.; Majd-Marani, S. Different cereal grains affect demographic traits and digestive enzyme activity of Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae). J. Stored Prod. Res. 2022, 95, 101898. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.Z. Effect of controlled atmosphere on the activity and kinetics of three detoxification enzymes in Araecerus fasciculatus (Coleoptera: Anthribidae). Acta Entomol. Sin. 2012, 55, 950–957. [Google Scholar]
- Zhang, T.; Hu, Q.; Wang, J.; Chen, L.; Zhang, Y.; Shen, M.; Rumbos, C.I.; Li, C.; Athanassiou, C.G.; Cao, Y. The rice cultivar affects the population growth and physiological enzyme activity of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) reared on rice grains. J. Stored Prod. Res. 2023, 104, 102163. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Borzoui, E.; Naseri, B. Wheat cultivars affecting life history and digestive amylolytic activity of Sitotroga cerealella Olivier (Lepidoptera: Gelechiidae). Bull. Entomol. Res. 2016, 106, 464–473. [Google Scholar] [CrossRef]
- Golizadeh, A.; Abedi, Z. Feeding performance and life table parameters of Khapra Beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae) on various barley cultivars. Bull. Entomol. Res. 2017, 107, 689–698. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Subramanyam, B. Stored-Product Insect Resource; AACC International: Nashville, TN, USA, 2009; 509p. [Google Scholar]
- Li, C.; Li, Z.Z. Influence of temperature on development and reproduction of experimental populations of Araecerus fasciculatus (Coleoptera: Anthribidae). Acta Entomol. Sin. 2009, 52, 1385–1389. [Google Scholar]
- Yang, S.; Zhang, T.; Gao, Y.L.; Mei, X.; Ning, J. Effects of relative humidity on the development, reproduction and population growth of Araecerus fascilatus (Coleoptera: Anthribidae). Chin. J. Appl. Entomol. 2016, 53, 121–127. [Google Scholar]
- Namin, F.R.; Nouri-Ganbalani, G.; Naseri, B.; Razmjou, J. Effect of different barley cultivars on nutritional physiology of Tribolium castaneum (Coleoptera: Tenebrionidae). J. Crop Prot. 2019, 8, 389–402. [Google Scholar]
- Gachoka, K.K.; Obeng-Ofori, D.; Danquah, E.Y. Host suitability of two Ghanaian biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on five common tropical weeds. Int. J. Trop. Insect Sci. 2005, 25, 236–244. [Google Scholar] [CrossRef]
- Terra, W.R. Evolution of digestive systems of insects. Annu. Rev. Entomol. 1990, 35, 181–200. [Google Scholar] [CrossRef]
- Gatehouse, A.M.; Norton, E.; Davison, G.M.; Babbé, S.M.; Newell, C.A.; Gatehouse, J.A. Digestive proteolytic activity in larvae of tomato moth, Lacanobia oleracea, effects of plant protease inhibitors in vitro and in vivo. J. Insect Physiol. 1999, 45, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Rekha, M.R.; Sasikiran, K.; Padmaja, G. Inhibitor potential of protease and α-amylase inhibitors of sweet potato and taro on the digestive enzymes of root crop storage pests. J. Stored Prod. Res. 2004, 40, 461–470. [Google Scholar] [CrossRef]
- Parde, V.D.; Sharma, H.C.; Kachole, M.S. In vivo inhibition of Helicoverpa armigera gut pro–proteinase activation by non–host plant protease inhibitors. J. Insect Physiol. 2010, 56, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef]
- AlJabr, A.M.; Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H. Toxicity of plant secondary metabolites modulating detoxification genes expression for natural red palm weevil pesticide development. Molecules 2017, 22, 169. [Google Scholar] [CrossRef]
| Diet | Egg Incubation | Larval Period | Pupal Period | Immature Period |
|---|---|---|---|---|
| Coffee beans | 6.12 ± 0.10 d | 39.24 ± 0.48 e | 6.77 ± 0.12 d | 51.41 ± 0.33 e |
| Jujube | 6.48 ± 0.07 c | 43.36 ± 0.36 d | 7.05 ± 0.04 cd | 56.16 ± 0.24 d |
| Maize | 6.82 ± 0.08 b | 45.93 ± 0.30 c | 7.26 ± 0.04 bc | 59.67 ± 0.30 c |
| Wheat | 7.13 ± 0.05 ab | 49.82 ± 0.38 b | 7.45 ± 0.03 b | 64.25 ± 0.17 b |
| Kansui | 7.23 ± 0.08 a | 55.12 ± 0.23 a | 7.76 ± 0.04 a | 69.65 ± 0.24 a |
| Diet | Fecundity (Eggs per Female) | Male Longevity (d) | Female Longevity (d) |
|---|---|---|---|
| Coffee beans | 80.78 ± 1.47 a | 36.56 ± 0.40 a | 37.49 ± 0.40 a |
| Jujube | 75.62 ± 0.70 b | 35.57 ± 0.52 a | 36.15 ± 0.35 ab |
| Maize | 68.04 ± 0.90 c | 35.17 ± 0.16 a | 35.74 ± 0.33 ab |
| Wheat | 60.46 ± 0.90 d | 34.50 ± 0.45 ab | 34.87 ± 0.43 b |
| Kansui | 50.43 ± 1.19 e | 32.80 ± 0.77 b | 32.20 ± 0.39 c |
| Diet | R0 | rm | λ | T |
|---|---|---|---|---|
| Coffee bean | 48.42 ± 0.48 a | 0.141 ± 0.001 a | 1.152 ± 0.001 a | 27.51 ± 0.32 c |
| Jujube | 42.53 ± 0.54 b | 0.129 ± 0.002 b | 1.137 ± 0.002 b | 29.12 ± 0.44 bc |
| Maize | 35.39 ± 0.52 c | 0.117 ± 0.002 c | 1.125 ± 0.002 c | 30.39 ± 0.37 ab |
| Wheat | 27.53 ± 0.32 d | 0.105 ± 0.001 d | 1.111 ± 0.001 cd | 31.48 ± 0.31 a |
| Kansui | 21.47 ± 0.56 e | 0.097 ± 0.001 e | 1.101 ± 0.001 d | 31.86 ± 0.31 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, L.; Yang, Y.; Xie, S.; Lou, Y.; Chen, L.; Dai, F.; Agrafioti, P.; Cao, Y.; Athanassiou, C.G.; Li, C. Life Table Parameters and Digestive Enzyme Activity of Araecerus fasciculatus (Coleoptera: Anthribidae) Feeding on Different Stored Products. Insects 2025, 16, 428. https://doi.org/10.3390/insects16040428
Jian L, Yang Y, Xie S, Lou Y, Chen L, Dai F, Agrafioti P, Cao Y, Athanassiou CG, Li C. Life Table Parameters and Digestive Enzyme Activity of Araecerus fasciculatus (Coleoptera: Anthribidae) Feeding on Different Stored Products. Insects. 2025; 16(4):428. https://doi.org/10.3390/insects16040428
Chicago/Turabian StyleJian, Lingyan, Yuping Yang, Songhai Xie, Yibin Lou, Ling Chen, Fanglian Dai, Paraskevi Agrafioti, Yu Cao, Christos G. Athanassiou, and Can Li. 2025. "Life Table Parameters and Digestive Enzyme Activity of Araecerus fasciculatus (Coleoptera: Anthribidae) Feeding on Different Stored Products" Insects 16, no. 4: 428. https://doi.org/10.3390/insects16040428
APA StyleJian, L., Yang, Y., Xie, S., Lou, Y., Chen, L., Dai, F., Agrafioti, P., Cao, Y., Athanassiou, C. G., & Li, C. (2025). Life Table Parameters and Digestive Enzyme Activity of Araecerus fasciculatus (Coleoptera: Anthribidae) Feeding on Different Stored Products. Insects, 16(4), 428. https://doi.org/10.3390/insects16040428









