Acute Toxicity of Dinotefuran to Picromerus lewisi Scott (Hemiptera: Pentatomidae) and Its Impact on Offspring Growth and Predation Ability in Integrated Pest Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insecticides
2.2. Test Insects
2.3. Bioassays
2.3.1. Concentration Mortality Response in 72 h Toxicity Contact Test
2.3.2. Half-Lethal Toxicity Bioassays
2.4. Functional Response of the P. lewisi F1 Generation to S. exigua and S. litura
2.5. Data Analysis
3. Results
3.1. Effects of 72 h Toxicity Contact Test
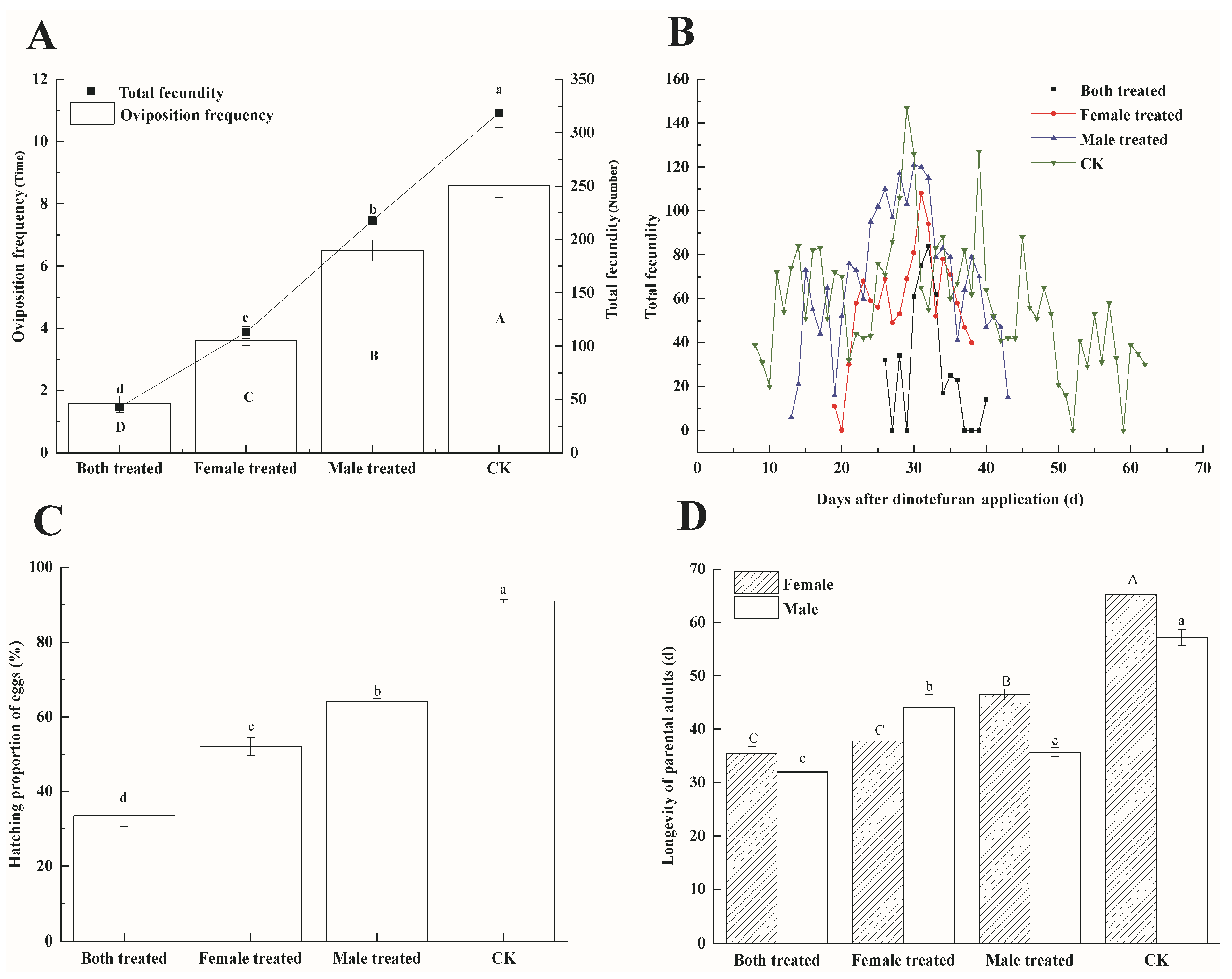
3.2. Effects of Dinotefuran on Parental (F0) Fecundity and Longevity of P. lewisi
3.3. Effects of Dinotefuran on Progeny (F1) Developmental Duration and Survival of P. lewisi
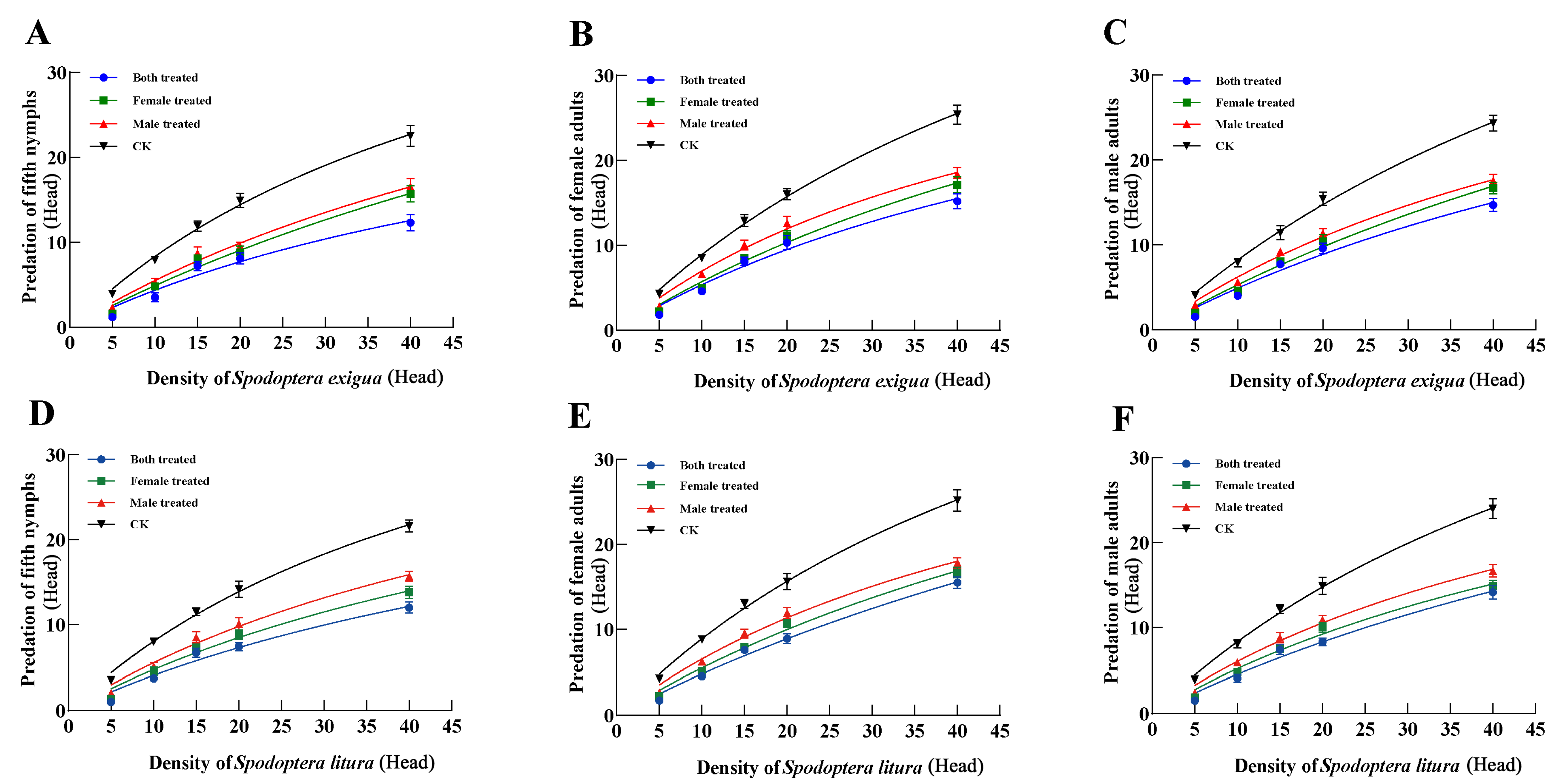
3.4. Effects of Dinotefuran on Progeny (F1) Predation Functional Response of P. lewisi
3.5. Effects of Dinotefuran on Progeny (F1) Searching Efficiency of P. lewisi
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Driesche, R.; Bellows, T.S. Pest Origins, Pesticides, and the History of Biological Control. In Biological Control; Springer: Berlin/Heidelberg, Germany, 1996; pp. 3–20. [Google Scholar]
- Hajek, A.E.; Eilenberg, J. Natural Enemies: An Introduction to Biological Control; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Overton, K.; Hoffmann, A.A.; Reynolds, O.L.; Umina, P. Toxicity of Insecticides and Miticides to Natural Enemies in Australian Grains: A Review. Insects 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Patel, L. Laboratory contact effect of some insecticides on predatory assassin bug, Rhynocoris marginatus Fabricius (Reduviidae: Hemiptera). Int. J. Chem. Stud. 2020, 8, 767–770. [Google Scholar] [CrossRef]
- Schmidt, R.; Beers, E.; Sater, C. Meta-analysis and review of pesticide non-target effects on phytoseiids, key biological control agents. Pest Manag. Sci. 2021, 77, 4848–4862. [Google Scholar] [CrossRef] [PubMed]
- Duso, C.; Van, L.T.; Pozzebon, A. Improving the compatibility of pesticides and predatory mites: Recent findings on physiological and ecological selectivity. Curr. Opin. Insect Sci. 2020, 39, 63–68. [Google Scholar] [CrossRef]
- Bredeson, M.M.; Reese, R.N.; Lundgren, J.G. The effects of insecticide dose and herbivore density on tri-trophic effects of thiamethoxam in a system involving wheat, aphids, and ladybeetles. Crop Prot. 2015, 69, 70–76. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, R.; Zhang, N.; Yuan, S.; Zhou, X.; Huang, J.; Ren, X.; Wang, S.; Jiang, H.; Yu, C. Toxicity of six insecticides to predatory mite Amblyseius cucumeris (Oudemans) (Acari: Phytoseiidae) in-and off-field. Ecotoxicol. Environ. Saf. 2018, 161, 715–720. [Google Scholar] [CrossRef]
- Silva, D.E.; do Nascimento, J.M.; da Silva, R.T.; Juchem, C.F.; Ruffatto, K.; da Silva, G.L.; Johann, L.; Corrêa, L.L.; Ferla, N.J. Impact of vineyard agrochemicals against Panonychus ulmi (Acari: Tetranychidae) and its natural enemy, Neoseiulus californicus (Acari: Phytoseiidae) in Brazil. Crop Prot. 2019, 123, 5–11. [Google Scholar] [CrossRef]
- Lima, A.P.; Santana, E.D.; Santos, A.C.; Silva, J.E.; Ribeiro, G.T.; Pinheiro, A.M.; Santos, Í.T.; Blank, A.F.; Araújo, A.P.; Bacci, L. Insecticide activity of botanical compounds against Spodoptera frugiperda and selectivity to the predatory bug Podisus nigrispinus. Crop Prot. 2020, 136, 105230. [Google Scholar] [CrossRef]
- Passos, L.; Soares, M.; Collares, L.; Malagoli, I.; Desneux, N.; Carvalho, G. Lethal, sublethal and transgenerational effects of insecticides on Macrolophus basicornis, predator of Tuta absoluta. Entomol. Gen. 2018, 38, 127–143. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Yu, X.; Yu, C.; Liu, F.; Mu, W. Sublethal and transgenerational effects of thiamethoxam on the demographic fitness and predation performance of the seven-spot ladybeetle Coccinella septempunctata L. (Coleoptera: Coccinellidae). Chemosphere 2019, 216, 168–178. [Google Scholar] [CrossRef]
- Mostafiz, M.; Hassan, E.; Shim, J.; Lee, K. Lethal and sublethal effects of methyl benzoate on the predatory bug Nesidiocoris tenuis. Insects 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Field, L.M. Neonicotinoids. Curr. Biol. 2018, 28, R772–R773. [Google Scholar] [CrossRef] [PubMed]
- Corbel, V.; Duchon, S.; Zaim, M.; Hougard, J. Dinotefuran: A potential neonicotinoid insecticide against resistant mosquitoes. J. Med. Entomol. 2004, 41, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dong, F.; Li, S.; Zheng, Z.; Xu, Y.; Xu, J.; Liu, X.; Zheng, Y. Response surface methodology for the enantioseparation of dinotefuran and its chiral metabolite in bee products and environmental samples by supercritical fluid chromatography/tandem mass spectrometry. J. Chromatogr. A 2015, 1410, 181–189. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, X.; Dong, F.; Duan, H.; Shao, X.; Chen, X.; Yang, T.; Wang, G.; Zheng, Y. Ecological toxicity reduction of dinotefuran to honeybee: New perspective from an enantiomeric level. Environ. Int. 2019, 130, 104854. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Juliano, S.A. Nonlinear curve fitting: Predation and functional response curves. In Design and Analysis of Ecological Experiments; Chapman and Hall/CRC: London, UK, 2020; pp. 159–182. [Google Scholar]
- Ding, Y.Q. Insect Mathematical Ecology; Science Press: Beijing, China, 1994; Volume 257–258, pp. 303–304. [Google Scholar]
- Zhu, W.; Fan, R.; Liu, M.; Wang, J.; Zhang, Y.; Ma, R. Toxic Effects of Five Insecticides on the Development and Enzymatic Activities of Trichogramma ostriniae. J. Appl. Entomol. 2025, 149, 56–64. [Google Scholar] [CrossRef]
- Rahaman, M.; Stout, M. Comparative efficacies of next-generation insecticides against yellow stem borer and their effects on natural enemies in rice ecosystem. Rice Sci. 2019, 26, 157–166. [Google Scholar] [CrossRef]
- Lin, R.; He, D.; Men, X.; Zheng, L.; Cheng, S.; Tao, L.; Yu, C. Sublethal and transgenerational effects of acetamiprid and imidacloprid on the predatory bug Orius sauteri (Poppius) (Hemiptera: Anthocoridae). Chemosphere 2020, 255, 126778. [Google Scholar] [CrossRef]
- Yu, C.; Lin, R.; Fu, M.; Zhou, Y.; Zong, F.; Jiang, H.; Lv, N.; Piao, X.; Zhang, J.; Liu, Y.; et al. Impact of imidacloprid on life-cycle development of Coccinella septempunctata in laboratory microcosms. Ecotoxicol. Environ. Saf. 2014, 110, 168–173. [Google Scholar] [CrossRef]
- Holz, C.; Streil, G.; Dettner, K.; Dütemeyer, J.; Boland, W. Intersexual transfer of a toxic terpenoid during copulation and its paternal allocation to developmental stages: Quantification of cantharidin in cantharidin-producing oedemerids (Coleoptera: Oedemeridae) and canthariphilous pyrochroids (Coleoptera: Pyrochroidae). Z. Für Naturforschung C 1994, 49, 856–864. [Google Scholar]
- Yu, Y.L.; Huang, L.J.; Wang, L.P.; Wu, J.C. Effects of Development and Fecundity of Nilaparvata lugens (stål) (Hemiptera: Delphacidae). Crop Prot. 2012, 34, 59–64. [Google Scholar] [CrossRef]
- Wang, L.P.; Shen, J.; Ge, L.Q.; Wu, J.C.; Yang, G.Q.; Jahn, G.C. Insecticide-induced increase in the protein content of male accessory glands and its effect on the fecundity of females in the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Crop Prot. 2010, 29, 1280–1285. [Google Scholar] [CrossRef]
- Cheng, X.; Li, F.; Chen, J.; Wang, H.; Mao, T.; Li, J.; Hu, J.; Li, B. The mechanism of trace acetamiprid-caused reproductive disorders in silkworm, Bombyx mori. Pest Manag. Sci. 2019, 75, 2672–2681. [Google Scholar] [CrossRef]
- Sun, R.R. Regulation Mechanism of Infertility Induced by Azadirachtin in Spodoptera litura (Fabricius). Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar]
- Li, B.X.; Wang, W.C.; Zhang, X.P.; Zhang, D.X.; Ren, Y.P.; Gao, Y.; Mu, W.; Liu, F. Using coordination assembly as the microencapsulation strategy to promote the efficacy and environmental safety of pyraclostrobin. Adv. Funct. Mater. 2017, 27, 1701841. [Google Scholar] [CrossRef]


| Treatment | Pre-Oviposition Duration (d) |
|---|---|
| Both treated | 30.40 ± 0.65 a |
| Female treated | 22.30 ± 0.47 b |
| Male treated | 16.00 ± 0.58 c |
| CK | 11.50 ± 0.65 d |
| Treatments | Emergence Proportion of Adults (%) | Female–Male Sex Ratio |
|---|---|---|
| Both treated | 0.50 ± 0.10 c | 1:2 |
| Female treated | 0.60 ± 0.17 bc | 1:1.57 |
| Male treated | 0.83 ± 0.06 ab | 1:1.78 |
| CK | 1.00 ± 0 a | 1:1.5 |
| Treatments | Instar | Types | Functional Response Equation | R2 | Instantaneous Attack Rate (a) | Handling Time (Th)/d | Daily Maximum Predition Number (1/Th)/Individual | Control Efficiency (a/Th) |
|---|---|---|---|---|---|---|---|---|
| Both treated | 5th instar nymph | Type II | Na = 0.499N/(1 + 0.014N) | 0.764 | 0.499 ± 0.016 b | 0.029 ± 0.004 a | 34.483 ± 4.864 a | 17.207 ± 1.863 b |
| Female adult | Type II | Na = 0.613N/(1 + 0.015N) | 0.838 | 0.613 ± 0.022 a | 0.024 ± 0.003 a | 41.667 ± 5.305 a | 25.542 ± 2.318 a | |
| Male adult | Type II | Na = 0.545N/(1 + 0.011N) | 0.868 | 0.545 ± 0.026 b | 0.021 ± 0.003 a | 47.619 ± 6.968 a | 25.952 ± 2.529 a | |
| Female treated | 5th instar nymph | Type II | Na = 0.560N/(1 + 0.015N) | 0.839 | 0.560 ± 0.035 b | 0.026 ± 0.005 a | 38.462 ± 7.728 a | 21.538 ± 2.921 b |
| Female adult | Type II | Na = 0.649N/(1 + 0.014N) | 0.911 | 0.649 ± 0.021 a | 0.021 ± 0.002 a | 47.619 ± 4.584 a | 30.901 ± 1.964 a | |
| Male adult | Type II | Na = 0.583N/(1 + 0.011N) | 0.900 | 0.583 ± 0.032 b | 0.019 ± 0.002 a | 52.632 ± 5.613 a | 30.684 ± 1.566 a | |
| Male treated | 5th instar nymph | Type II | Na = 0.616N/(1 + 0.012N) | 0.845 | 0.616 ± 0.051 b | 0.020 ± 0.003 a | 50.000 ± 7.701 a | 30.800 ± 2.126 a |
| Female adult | Type II | Na = 0.834N/(1 + 0.020N) | 0.874 | 0.834 ± 0.035 a | 0.024 ± 0.004 a | 41.667 ± 7.176 a | 34.750 ± 4.478 a | |
| Male adult | Type II | Na = 0.718N/(1 + 0.016N) | 0.901 | 0.718 ± 0.036 b | 0.022 ± 0.002 a | 45.454 ± 4.172 a | 32.636 ± 1.344 a | |
| CK | 5th instar nymph | Type II | Na = 0.985N/(1 + 0.019N) | 0.882 | 0.985 ± 0.064 a | 0.019 ± 0.004 a | 52.632 ± 11.680 a | 51.842 ± 7.954 b |
| Female adult | Type II | Na = 1.024N/(1 + 0.015N) | 0.918 | 1.024 ± 0.072 a | 0.015 ± 0.002 a | 66.667 ± 9.077 a | 68.267 ± 4.393 a | |
| Male adult | Type II | Na = 0.925N/(1 + 0.013N) | 0.911 | 0.925 ± 0.068 a | 0.014 ± 0.001 a | 71.429 ± 5.133 a | 66.071 ± 0.139 ab |
| Treatments | Instar | Types | Functional Response Equation | R2 | Instantaneous Attack Rate (a) | Handling Time (Th)/d | Daily Maximum Predition Number (1/Th)/individual | Control Efficiency (a/Th) |
|---|---|---|---|---|---|---|---|---|
| Both treated | 5th instar nymph | Type II | Na = 0.459N/(1 + 0.013N) | 0.834 | 0.459 ± 0.016 b | 0.028 ± 0.004 a | 35.714 ± 5.226 b | 16.393 ± 1.813 b |
| Female adult | Type II | Na = 0.519N/(1 + 0.008N) | 0.902 | 0.519 ± 0.022 a | 0.016 ± 0.003 b | 62.500 ± 12.217 a | 32.438 ± 4.907 a | |
| Male adult | Type II | Na = 0.498N/(1 + 0.010N) | 0.855 | 0.498 ± 0.026 ab | 0.020 ± 0.003 ab | 50.000 ± 7.701 ab | 24.900 ± 2.500 a | |
| Female treated | 5th instar nymph | Type II | Na = 0.539N/(1 + 0.013N) | 0.857 | 0.539 ± 0.035 a | 0.025 ± 0.005 a | 40.000 ± 8.389 a | 21.560 ± 3.053 b |
| Female adult | Type II | Na = 0.611N/(1 + 0.011N) | 0.922 | 0.611 ± 0.021 a | 0.018 ± 0.002 a | 55.556 ± 6.263 a | 33.944 ± 2.643 a | |
| Male adult | Type II | Na = 0.593N/(1 + 0.014N) | 0.884 | 0.593 ± 0.032 a | 0.024 ± 0.002 a | 41.667 ± 3.501 a | 24.708 ± 0.732 b | |
| Male treated | 5th instar nymph | Type II | Na = 0.638N/(1 + 0.015N) | 0.862 | 0.638 ± 0.051 b | 0.024 ± 0.003 a | 41.667 ± 5.305 a | 26.583 ± 1.220 b |
| Female adult | Type II | Na = 0.768N/(1 + 0.018N) | 0.902 | 0.768 ± 0.035 a | 0.023 ± 0.004 a | 43.478 ± 7.836 a | 33.391 ± 4.441 a | |
| Male adult | Type II | Na = 0.706N/(1 + 0.017N) | 0.887 | 0.706 ± 0.036 ab | 0.024 ± 0.002 a | 41.667 ± 3.501 a | 29.417 ± 0.959 ab | |
| CK | 5th instar nymph | Type II | Na = 0.958N/(1 + 0.019N) | 0.909 | 0.958 ± 0.064 a | 0.020 ± 0.004 a | 50.000 ± 10.486 a | 47.900 ± 6.690 b |
| Female adult | Type II | Na = 1.030N/(1 + 0.015N) | 0.901 | 1.030 ± 0.072 a | 0.015 ± 0.002 a | 66.667 ± 9.077 a | 68.667 ± 4.447 a | |
| Male adult | Type II | Na = 0.956N/(1 + 0.014N) | 0.895 | 0.956 ± 0.068 a | 0.015 ± 0.001 a | 66.667 ± 4.468 a | 63.733 ± 0.286 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Wang, M.; Xue, C.; Mao, J.; Li, Y.; Zhang, L. Acute Toxicity of Dinotefuran to Picromerus lewisi Scott (Hemiptera: Pentatomidae) and Its Impact on Offspring Growth and Predation Ability in Integrated Pest Management. Insects 2025, 16, 404. https://doi.org/10.3390/insects16040404
Ji Y, Wang M, Xue C, Mao J, Li Y, Zhang L. Acute Toxicity of Dinotefuran to Picromerus lewisi Scott (Hemiptera: Pentatomidae) and Its Impact on Offspring Growth and Predation Ability in Integrated Pest Management. Insects. 2025; 16(4):404. https://doi.org/10.3390/insects16040404
Chicago/Turabian StyleJi, Yutong, Mengqing Wang, Chuanzhen Xue, Jianjun Mao, Yuyan Li, and Lisheng Zhang. 2025. "Acute Toxicity of Dinotefuran to Picromerus lewisi Scott (Hemiptera: Pentatomidae) and Its Impact on Offspring Growth and Predation Ability in Integrated Pest Management" Insects 16, no. 4: 404. https://doi.org/10.3390/insects16040404
APA StyleJi, Y., Wang, M., Xue, C., Mao, J., Li, Y., & Zhang, L. (2025). Acute Toxicity of Dinotefuran to Picromerus lewisi Scott (Hemiptera: Pentatomidae) and Its Impact on Offspring Growth and Predation Ability in Integrated Pest Management. Insects, 16(4), 404. https://doi.org/10.3390/insects16040404







