Pupal Development and Adult Acclimation Temperatures Influence the Cold and Heat Tolerance in Tenebrio molitor (Coleoptera: Tenebrionidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Cold and Heat Tolerance
2.3. Chill-Coma Test
2.4. Heat-Tolerance Test
2.5. Statistical Analysis
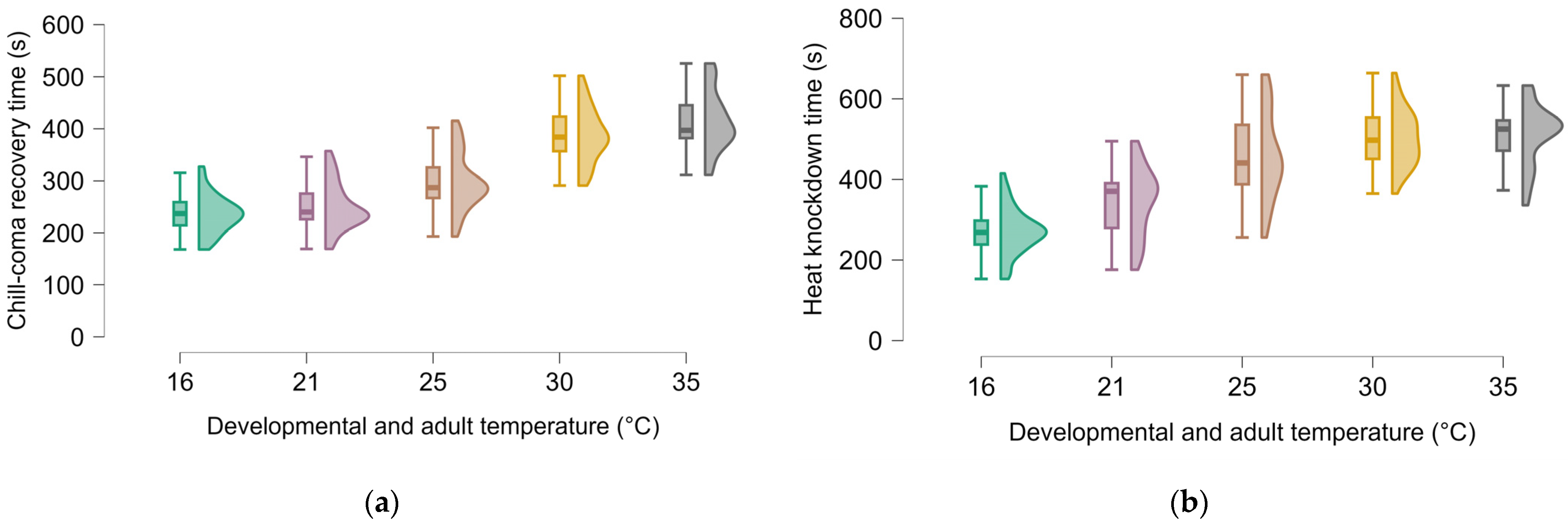
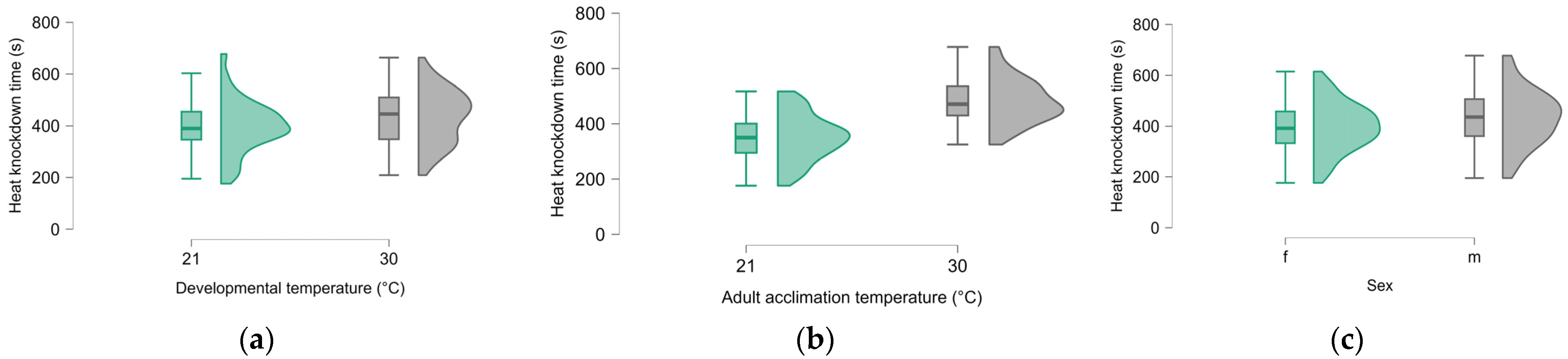
3. Results
4. Discussion
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| BAH | Beneficial acclimation hypothesis |
References
- Teets, N.M.; Denlinger, D.L. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 2013, 38, 105–116. [Google Scholar] [CrossRef]
- Bodlah, M.A.; Iqbal, J.; Ashiq, A.; Bodlah, I.; Jiang, S.; Mudassir, M.A.; Rasheed, M.T.; Fareen, A.G.E. Insect behavioral restraint and adaptation strategies under heat stress: An inclusive review. J. Saudi Soc. Agri. Sci. 2023, 22, 327–350. [Google Scholar] [CrossRef]
- Scheiner, S.M. The Theory of the Evolution of Phenotypic Plasticity. In The Theory of Evolution: Principles, Concepts, and Assumptions; Scheiner, S.M., Mindell, D.P., Eds.; Chicago Scholarship Online: Chicago, IL, USA, 2020; pp. 254–272. [Google Scholar] [CrossRef]
- Randall, D.; Burggren, W.; French, K. Eckert Animal Physiology: Mechanisms and Adaptations, 5th ed.; W.H. Freeman: New York, NY, USA, 2001; p. 752. [Google Scholar]
- Wilson, R.S.; Franklin, C.E. Testing the beneficial acclimation hypothesis. Trends Ecol. Evol. 2002, 17, 66–70. [Google Scholar] [CrossRef]
- Willmer, P.; Stone, G.; Johnston, I.A. Environmental Physiology of Animals, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2000; p. 784. [Google Scholar]
- Colinet, H.; Hoffmann, A.A. Comparing phenotypic effects and molecular correlates of developmental, gradual and rapid cold acclimation responses in Drosophila melanogaster. Funct. Ecol. 2012, 26, 84–93. [Google Scholar] [CrossRef]
- Scharf, I.; Galkin, N.; Halle, S. Disentangling the Consequences of Growth Temperature and Adult Acclimation Temperature on Starvation and Thermal Tolerance in the Red Flour Beetle. Evol. Biol. 2015, 42, 54–62. [Google Scholar] [CrossRef]
- Stazione, L.; Norry, F.M.; Sambucetti, P. Heat-hardening effects on mating success at high temperature in Drosophila melanogaster. J. Therm. Biol. 2019, 80, 172–177. [Google Scholar] [CrossRef]
- Bowler, K. Acclimation, heat shock and hardening. J. Therm. Biol. 2005, 30, 125–130. [Google Scholar] [CrossRef]
- Bubliy, O.A.; Kristensen, T.N.; Kellermann, V.; Loeschcke, V. Plastic responses to four environmental stresses and cross-resistance in a laboratory population of Drosophila melanogaster. Funct. Ecol. 2012, 26, 245–253. [Google Scholar] [CrossRef]
- Abdelghany, A.Y.; Suthisut, D.; Fields, P.G. The effect of diapause and cold acclimation on the cold-hardiness of the warehouse beetle, Trogoderma variabile (Coleoptera: Dermestidae). Can. Entomol. 2015, 147, 158–168. [Google Scholar] [CrossRef]
- Modlmeier, A.P.; Pamminger, T.; Foitzik, S.; Scharf, I. Cold resistance depends on acclimation and behavioral caste in a temperate ant. Naturwissenschaften 2012, 99, 811–819. [Google Scholar] [CrossRef]
- Chen, C.P.; Lee, R.E.; Denlinger, D.L. Cold shock and heat shock: A comparison of the protection generated by brief pretreatment at less severe temperatures. Physiol. Entomol. 1991, 16, 19–26. [Google Scholar] [CrossRef]
- Sejerkilde, M.; Sørensen, J.G.; Loeschcke, V. Effects of cold- and heat hardening on thermal resistance in Drosophila melanogaster. J. Insect Physiol. 2003, 49, 719–726. [Google Scholar] [CrossRef]
- Leroi, A.M.; Bennett, A.F.; Lenski, R.E. Temperature acclimation and competitive fitness: An experimental test of the beneficial acclimation assumption. Proc. Natl. Acad. Sci. USA 1994, 91, 1917–1921. [Google Scholar] [CrossRef]
- Huey, R.B.; Berrigan, D.A. Testing evolutionary hypotheses of acclimation. In Animals and Temperature: Phenotypic and Evolutionary Adaptation; Society for Experimental Biology Seminar Series; Johnston, I.A., Bennett, A.F., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 203–237. [Google Scholar] [CrossRef]
- Huey, R.B.; Berrigan, D.; Gilchrist, G.W.; Herron, J.C. Testing the adaptive significance of acclimation: A strong inference hypothesis. Am. Zool. 1999, 39, 323–336. [Google Scholar] [CrossRef]
- Nunney, L.; Cheung, W. The effect of temperature on body size and fecundity in female Drosophila melanogaster: Evidence for adaptive plasticity. Evolution 1997, 51, 1529–1535. [Google Scholar] [CrossRef]
- Woods, H.A.; Harrison, J.F. Interpreting rejections of the beneficial acclimation hypothesis: When is physiological plasticity adaptive? Evolution 2002, 56, 1863–1866. [Google Scholar] [CrossRef]
- Krebs, R.A.; Loeschcke, V. Costs and benefits of activation of the heat shock response in Drosophila melanogaster. Funct. Ecol. 1994, 8, 730–737. [Google Scholar] [CrossRef]
- Zamudio, K.R.; Huey, R.B.; Crill, W.D. Bigger isn’t always better: Body size, temperature and male territorial success in Drosophila melanogaster. Anim. Behav. 1995, 49, 671–677. [Google Scholar] [CrossRef]
- Bennett, A.F.; Lenski, R.E. Evolutionary adaptation to temperature. VI. Phenotypic acclimation and its evolution in Escherichia coli. Evolution 1997, 51, 36–44. [Google Scholar] [CrossRef]
- Gibbs, A.G.; Louie, A.K.; Ayala, J.A. Effects of temperature on cuticular lipids and water balance in a desert Drosophila: Is thermal acclimation beneficial? J. Exp. Biol. 1998, 201, 71–80. [Google Scholar] [CrossRef]
- Woods, H.A. Patterns and mechanisms of growth of fifth- instar Manduca sexta caterpillars following exposure to low- or high-protein food during early instars. Physiol. Biochem. Zool. 1999, 72, 445–454. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U. Temperature effects on egg size and their fitness consequences in the yellow dung fly Scathophaga stercoraria. Evol. Ecol. 2000, 14, 627–643. [Google Scholar] [CrossRef]
- Woods, H.A.; Harrison, J.F. The beneficial acclimation hypothesis versus acclimation of specific traits: Physiological change in water-stressed Manduca sexta caterpillars. Physiol. Biochem. Zool. 2001, 74, 32–44. [Google Scholar] [CrossRef]
- Gibert, P.; Huey, R.B.; Gilchrist, G.W. Locomotor performance of Drosophila melanogaster: Interactions among developmental and adult temperatures, age, and geography. Evolution 2001, 55, 205–209. [Google Scholar] [CrossRef]
- Gilchrist, G.W.; Huey, R.B. Parental and developmental temperature effects on the thermal dependence of fitness in Drosophila melanogaster. Evolution 2001, 55, 209–214. [Google Scholar] [CrossRef]
- Santos, M.A.; Carromeu-Santos, A.; Quina, A.S.; Santos, M.; Matos, M.; Simões, P. High developmental temperature leads to low reproduction despite adult temperature. J. Therm. Biol. 2021, 95, 102794. [Google Scholar] [CrossRef] [PubMed]
- Vainikka, A.; Seppäla, O.; Löytynoja, K.; Rantala, M.J. Fitness consequences of female preference for male pheromones in Tenebrio molitor. Evol. Ecol. Res. 2006, 8, 943–957. [Google Scholar]
- Makkar, P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Adamaki-Sotiraki, C.; Rumbos, C.I.; Athanassiou, C.G. From a stored-product pest to a promising protein source: A U-turn of human perspective for the yellow mealworm Tenebrio molitor. J. Pest Sci. 2024, 98, 113–129. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; Avila Gonzalez, E.; Rocha Hernandez, A.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef]
- Ribeiro, N.; Abelho, M.; Costa, R.A. Review of the Scientific Literature for Optimal Conditions for Mass Rearing Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2018, 53, 434–454. [Google Scholar] [CrossRef]
- Punzo, F.; Mutchmor, J.A. Effects of temperature, relative humidity and period of exposure on the survival capacity of Tenebrio molitor (Coleoptera: Tenebrionidae). J. Kans. Entomol. Soc. 1980, 53, 260–270. [Google Scholar]
- Damborsky, M.; Bar, P.S.T.; Oscherov, M.E. Ciclo de vida de Tenebrio molitor (Coleoptera, Tenebrionidae) en condiciones experimentales. Comun. Cient. y Tecnol. UNNE 2000, 6, 35–38. [Google Scholar]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef]
- Stevens, M.M.; Jackson, S.; Bester, S.A.; Terblanche, J.S.; Chown, S.L. Oxygen limitation and thermal tolerance in two terrestrial arthropod species. J. Exp. Biol. 2010, 213, 2209–2218. [Google Scholar] [CrossRef]
- Koo, H.; Kim, S.; Oh, H.; Kim, J.; Choi, D.; Kim, D.; Kim, I. Temperature-dependent development model of larvae of mealworm beetle, Tenebrio molitor L. (Coleoptera: Tenebrionidae). Korean J. Appl. Entomol. 2013, 52, 387–394. [Google Scholar] [CrossRef]
- Ludwig, D. Effects of temperature and parental age on the life cycle of the mealworm, Tenebrio molitor Linnaeus (Coleoptera, Tenebrionidae). Ann. Entomol. Soc. Am. 1956, 49, 12–15. [Google Scholar] [CrossRef]
- Mutchmor, J.A.; Richards, A.G. Low temperature tolerance of insects in relation to the influence of temperature on muscle apyrase activity. J. Insect Physiol. 1961, 7, 141–158. [Google Scholar] [CrossRef]
- Martin, R.D.; Rivers, J.P.W.; Cowgill, U.M. Culturing mealworms as food for animals in captivity. Int. Zoo Yearb. 1976, 16, 63–70. [Google Scholar] [CrossRef]
- Allen, J.L.; Clusella-Trullas, S.; Chown, S.L. The effects of acclimation and rates of temperature change on critical thermal limits in Tenebrio molitor (Tenebrionidae) and Cyrtobagous salviniae (Curculionidae). J. Insect Physiol. 2012, 58, 669–678. [Google Scholar] [CrossRef]
- David, R.J.; Gibert, P.; Pla, E.; Petavy, G.; Karan, D.; Moreteau, B. Cold stress tolerance in Drosophila: Analysis of chill coma recovery in D. melanogaster. J. Therm. Biol. 1998, 23, 291–299. [Google Scholar] [CrossRef]
- MacMillan, H.A.; Sinclair, B.J. Mechanisms underlying insect chill-coma. J. Insect Physiol. 2011, 57, 12–20. [Google Scholar] [CrossRef]
- Findsen, A.; Pedersen, T.H.; Petersen, A.G.; Nielsen, O.B.; Overgaard, J. Why do insects enter and recover from chill coma? Low temperature and high extracellular potassium compromise muscle function in Locusta migratoria. J. Exp. Biol. 2014, 217, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, H.A.; Williams, C.M.; Staples, J.F.; Sinclair, B.J. Reestablishment of ion homeostasis during chill-coma recovery in the cricket Gryllus pennsylvanicus. Proc. Natl. Acad. Sci. USA 2012, 109, 20750–20755. [Google Scholar] [CrossRef]
- Wilches, D.M.; Laird, R.A.; Floate, K.D.; Fields, P.G. A review of diapause and tolerance to extreme temperatures in dermestids (Coleoptera). J. Stored Prod. Res. 2016, 68, 50–62. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Ameel, J.J.; Waldbauer, G.P. A Method for Sexing Living Pupal and Adult Yellow Mealworms. Ann. Entomol. Soc. Am. 1970, 63, 1783. [Google Scholar] [CrossRef]
- Scharf, I.; Sbilordo, S.H.; Martin, O.Y. Cold tolerance in flour beetle species differing in body size and selection temperature. Physiol. Entomol. 2014, 39, 80–87. [Google Scholar] [CrossRef]
- Berrigan, D.; Dagher, H.; Hoffmann, A.A. Comparing different measures of heat resistance in selected lines of Drosophila melanogaster. J. Insect Physiol. 1997, 43, 393–405. [Google Scholar] [CrossRef]
- Huey, R.B.; Crill, W.D.; Kingsolver, J.G.; Weber, K.E. A method for rapid measurement of heat or cold resistance of small insects. Funct. Ecol. 1992, 6, 489–494. [Google Scholar] [CrossRef]
- Lachenicht, M.W.; Clusella-Trullas, S.; Boardman, L.; Le Roux, C.; Terblanche, J.S. Effects of acclimation temperature on thermal tolerance, locomotion performance and respiratory metabolism in Acheta domesticus L. (Orthoptera: Gryllidae). J. Insect Physiol. 2010, 56, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Terblanche, J.S.; Chown, S.L. The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae). J. Exp. Biol. 2006, 209, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Renault, D.; Salin, C.; Vannier, G.; Vernon, P. Survival at low temperatures in insects: What is the ecological significance of the supercooling point? CryoLetters 2002, 23, 217–228. [Google Scholar] [PubMed]
- Jensen, D.; Overgaard, J.; Sørensen, J.G. The influence of developmental stage on cold shock resistance and ability to coldharden in Drosophila melanogaster. J. Insect Physiol. 2007, 53, 186–197. [Google Scholar] [CrossRef]
- Zeilstra, I.; Fischer, K. Cold tolerance in relation to developmental and adult temperature in a butterfly. Physiol. Entomol. 2005, 30, 92–95. [Google Scholar] [CrossRef]
- Scharf, I.; Segal, D.; Bar, A.; Gottlieb, D. Negative effects of fluctuating temperatures around the optimal temperature on reproduction and survival of the red flour beetle. J. Therm. Biol. 2022, 103, 103165. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, H.; Guo, J.; Zhou, Z. Effects of Fluctuating Thermal Regimes on Life History Parameters and Body Size of Ophraella communa. Insects 2022, 13, 821. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podlesnik, J. Pupal Development and Adult Acclimation Temperatures Influence the Cold and Heat Tolerance in Tenebrio molitor (Coleoptera: Tenebrionidae). Insects 2025, 16, 402. https://doi.org/10.3390/insects16040402
Podlesnik J. Pupal Development and Adult Acclimation Temperatures Influence the Cold and Heat Tolerance in Tenebrio molitor (Coleoptera: Tenebrionidae). Insects. 2025; 16(4):402. https://doi.org/10.3390/insects16040402
Chicago/Turabian StylePodlesnik, Jan. 2025. "Pupal Development and Adult Acclimation Temperatures Influence the Cold and Heat Tolerance in Tenebrio molitor (Coleoptera: Tenebrionidae)" Insects 16, no. 4: 402. https://doi.org/10.3390/insects16040402
APA StylePodlesnik, J. (2025). Pupal Development and Adult Acclimation Temperatures Influence the Cold and Heat Tolerance in Tenebrio molitor (Coleoptera: Tenebrionidae). Insects, 16(4), 402. https://doi.org/10.3390/insects16040402





