Wing Variability in Some Andean Brown Lacewing Insects as an Adaptive Survival Strategy (Insecta, Neuropterida, Neuroptera: Hemerobiidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cott, H.B. Adaptative Coloration in Animals; Methuen: London, UK, 1940. [Google Scholar]
- Wickler, W. Mimicry in Plants and Animals; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Endler, J.A. A predator’s view of animal colour patterns. J. Evol. Biol. 1978, 11, 319–364. [Google Scholar]
- Endler, J.A. Progressive background matching in moths, and a quantitative measure of crypsis. Biol. J. Linn. Soc. 1984, 22, 187–231. [Google Scholar] [CrossRef]
- Brower, L.P. (Ed.) Mimicry and the Evolutionary Process; University of Chicago Press: Chicago, Chicago, IL, USA, 1988. [Google Scholar]
- Bradbury, J.W.; Verhrencamp, S.L. Principles of Animal Communication; Sinauer Associates: Sunderland, UK, 1998. [Google Scholar]
- Bond, A.B.; Kamil, A.C. Visual predators select for crypticity and polymorphism in virtual prey. Nature 2002, 415, 609–613. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Sherratt, T.N.; Speed, M.P. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry; Oxford University Press: London, UK, 2004. [Google Scholar]
- Quicke, D.L.J. Mimicry, Crypsis, Masquerade and Other Adaptive Resemblances; Wiley-Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- Thenius, E. Lebende Fossilien. Oldtimer der Tier- und Pflanzenwelt—Zeugen der Vorzeit; Verlag Friedrich Pfeil: Munich, Germany, 2000. [Google Scholar]
- Schlüter, T. The Fossil Planipennia—A Review. In Recent Research in Neuropterology (Proceedings of the 2nd International Symposium on Neuropterology, Hamburg, Germany, 21–23 August 1984; Gepp, J., Aspöck, H., Hölzel, H., Eds.; Privately Printed: Graz, Austria, 1986; pp. 103–111. [Google Scholar]
- New, T.R. A Review of the Biology of Neuroptera Planipennia. Neuroptera Int. Suppl. 1986, 1, 1–58. [Google Scholar]
- New, T.R. Planipennia: Lacewings; Handbuch der Zoologie; Walter de Gruyter: Berlin, Germany, 1989; Volume IV, Pt 30. [Google Scholar]
- Grimaldi, D.A.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2005; xv-755p. [Google Scholar]
- Jepson, J.E.; Penney, D. Neuropteran (Insecta) palaeodiversity with predictions for the Cretaceous fauna of the Wealden. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 248, 109–118. [Google Scholar] [CrossRef]
- Nicholson, A.J. A new theory of mimicry in insects. Aust. Zool. 1927, 5, 10–104. [Google Scholar]
- Hardouin, R. Le Mimetisme Animal; Presses Universitaires de France: Paris, France, 1946; 219p. [Google Scholar]
- Brakefield, P.M. Crypsis. In Encyclopedia of Insects; Resh, V., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 269–273. [Google Scholar]
- Fang, S.-W.; Zhang, X.; Yang, Q.; Guan, X.-Y.; Gao, T.-P.; Ren, D. Mimicry and extinction mechanism of kalligrammatid lacewings during Mesozoic (Neuroptera, Kalligrammatidae). Acta Zootaxonomica Sin. 2010, 35, 165–172. [Google Scholar]
- Gao, T.-P.; Shih, C.-K.; Ren, D.; Zhao, Y.-Y. Mimicry. In Silent Stories—Insect Fossil Treasures from Dinosaur Era of the Northeastern China; Ren, D., Shih, C.-K., Gao, T.-P., Yao, Y.-Z., Zhao, Y.-Y., Eds.; Science Press: Beijing, China, 2010; pp. 311–322. [Google Scholar]
- Wang, Y.-J.; Liu, Z.-Q.; Wang, X.; Shih, C.-K.; Zhao, Y.-Y.; Engel, M.S.; Ren, D. Ancient pinnate leaf mimesis among lacewings. Proc. Natl. Acad. Sci. 2010, 107, 16212–16215. [Google Scholar] [CrossRef]
- McLachlan, R. A remarkable new mimetic species of Mantispa from Borneo. Entomol. Mon. Mag. 1900, 36, 127–129. [Google Scholar]
- Shelford, R. [W.C.] Observations on some mimetic insects and spiders from Borneo and Singapore, with appendices containing descriptions of new species by R. Shelford, Dr. Karl Jordan, C. J. Gahan, the Rev. H. S. Gorham, and Dr. A. Senna. Proc. Zool. Soc. Lond. 1902, 2, 230–284. [Google Scholar]
- Brach, V. Brachynemurus nebulosus (Neuroptera: Myrmeleontidae), a possible Batesian mimic of Florida mutillid wasps (Hymenoptera: Mutillidae). Entomol. News 1978, 89, 153–156. [Google Scholar]
- Opler, P.A. Polymorphic mimicry of polistine wasps by a neotropical Neuropteran. Biotropica 1981, 13, 165–176. [Google Scholar] [CrossRef]
- Boyden, T.C. Mimicry, predation and potential pollination by the mantispid, Climaciella brunnea var. instabilis (Say) (Mantispidae: Neuroptera). J. N. Y. Entomol. Soc. 1983, 91, 508–511. [Google Scholar]
- Redborg, K.E. Biology of the Mantispidae. Annu. Rev. Entomol. 1998, 43, 175–194. [Google Scholar]
- Ohl, M. A new wasp-mimicking species of the genus Euclimacia from Thailand (Neuroptera, Mantispidae). Denisia 2004, 13, 193–196. [Google Scholar]
- Beck, J. Wasp-mimicking Mantispidae (Insecta: Neuroptera) from Sabah, Malaysia. Sepilok Bull. 2005, 3, 37–40. [Google Scholar]
- Monserrat, V.J. Estrategias de defensa visual en los Neuropterida ibéricos (Megaloptera, Raphidioptera, Neuroptera). Boletín de la Soc. Entomol. Aragonesa 2015, 57, 459–480. [Google Scholar]
- Rasmussen, C.; Ardila-Camacho, A. New host record for the enigmatic neotropical mantidfly genus Anchieta Navás, 1909 (Neuroptera, Mantispidae), a mimic of wasps and stingless bees. Papéis Avulsos Zool. 2021, 61, e20216155. [Google Scholar] [CrossRef]
- Morton, K.J. Life-History of Drepanepteryx phalaenoides, Linn. Entomol. Mon. Mag. 1910, 46, 54–62. [Google Scholar]
- Faúndez, E. Asociación Críptica de Gayomyia falcata (Blanchard, 1851) (Neuroptera: Hemerobiidae) con la zarzaparrilla Ribes magellanicum Poiret, 1812 (Saxifragaceae) en la región de Magallanes. An. Inst. Patagon. 2005, 33, 63–64. [Google Scholar]
- Zimmerman, E.C. Ephemeroptera-Neuroptera-Trichoptera and Supplement to Volumes 1 to 5; Insects of Hawaii. A Manual of the Insects of the Hawaiian Islands, Including an Enumeration of the Species and Notes on Their Origin, Distribution, Hosts, Parasites, etc.; University of Hawaii Press: Honolulu, HI, USA, 1957; Volume 6. [Google Scholar]
- New, T.R. A revision of the Australian Hemerobiidae (Insecta: Neuroptera). Invertebr. Taxon. 1988, 2, 339–411. [Google Scholar] [CrossRef]
- Oswald, J.D. Revision and cladistic analysis of the world genera of the family Hemerobiidae (Insecta: Neuroptera). J. N. Y. Entomol. Soc. 1993, 101, 143–299. [Google Scholar]
- Monserrat, V.J. Contribución al conocimiento de los hemeróbidos de patagonia y Tierra del Fuego (Insecta, Neuroptera: Hemerobiidae). Graellsia 2003, 59, 37–56. [Google Scholar] [CrossRef]
- Burrows, M.; Dorosenko, M. Jumping mechanisms in lacewings (Neuroptera, Chrysopidae and Hemerobiidae). J. Exp. Biol. 2014, 217, 4252–4261. [Google Scholar] [CrossRef]
- Perkins, R.C.L. Neuroptera. In Fauna Hawaiiensis; Sharp, D., Ed.; Cambridge University Press: London, UK, 1899; Volume 2, Pt 2, pp. 31–89. [Google Scholar]
- Perkins, R.C.L. Supplement to Neuroptera. In Fauna Hawaiiensis; Sharp, D., Ed.; Cambridge University Press: London, UK, 1910; Volume 2, pp. 691–696. [Google Scholar]
- Zimmerman, E.C. Studies of Hawaiian Neuroptera. Proc. Hawaii. Entomol. Soc. 1940, 10, 487–510. [Google Scholar]
- Zimmerman, E.C. A remarkable new Pseudopsectra from Maui (Neuroptera: Hemerobiidae). Proc. Hawaii. Entomol. Soc. 1946, 12, 659–660. [Google Scholar]
- Simon, C.M.; Gagné, W.C.; Howarth, F.G.; Radovsky, F.J. Hawai’i: A Natural Entomological Laboratory. Bull. Entomol. Soc. Am. 1984, 30, 9–17. [Google Scholar] [CrossRef]
- Monserrat, V.J. New data on the Afrotropical brown lacewings (Neuroptera: Hemerobiidae). J. Entomol. Soc. S. Afr. 1992, 55, 123–136. [Google Scholar]
- Monserrat, V.J. Nuevos datos sobre algunas especies de hemeróbidos (Insecta: Neuroptera: Hemerobiidae). Heteropterus Rev. Entomol. 2004, 4, 1–26. [Google Scholar] [CrossRef]
- González Olazo, E.V. El género Megalomus Rambur (Neurop.-Planipennia-Hemerobiidae) en Argentina y Chile. Acta Zool. Lilloana 1981, 36, 97–113. [Google Scholar]
- Monserrat, V.J. Nuevos datos sobre los hemeróbidos de América (Neuroptera, Hemerobiidae). J. Neuropterol. 1998, 1, 109–153. [Google Scholar]
- Tjeder, B. Neuroptera-Planipennia. The Lace-Wings of Southern Africa. 4. Family Hemerobiidae. In South African Animal Life; Hanström, B., Brinck, P., Rudebec, G., Eds.; Swedish Natural Science Research Council: Stockholm, Sweden, 1961; Volume 8, pp. 296–408. [Google Scholar]
- Monserrat, V.J. Revisión del género Megalomus de Latinoamérica (Neuroptera, Hemerobiidae). Fragm. Entomol. 1997, 29, 123–206. [Google Scholar]
- Shannon, C.E. A Mathematical Theory of Communication 1. Bell Syst Tech J 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication 2. Bell Syst Tech J 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Brakefield, P.M.; Larsen, T.B. The evolutionary significance of dry and wet season forms in some tropical butterflies. Biol. J. Linn. Soc. 1984, 22, 1–12. [Google Scholar] [CrossRef]
- Brakefield, P.M. Tropical dry and wet season polyphemism in the butterfly Melanitis leda (Satyrinae), phenotypic plasticity and climatic correlates. Biol. J. Linn. Soc. 1989, 31, 175–191. [Google Scholar] [CrossRef]
- Moran, N.A. The evolutionary maintenance of alternative phenotypes. Am. Nat 1992, 139, 971–989. [Google Scholar] [CrossRef]
- Roskam, J.C.; Brakefield, P.M. Seasonal polyphenism in Bicyclus (Lepidoptera: Satyridae) butterflies: Different climates need different cues. Biol. J. Linn. Soc. 1999, 66, 345–356. [Google Scholar] [CrossRef]
- Scheiner, S.M. Selection experiments and the study of phenotypic plasticity. J. Evol. Biol. 2002, 15, 889–898. [Google Scholar] [CrossRef]
- Wijngaarden, P.J.; Koch, P.B.; Brakefield, P.M. Artificial selection on the shape of reaction norms for eyespot size in the butterfly Bicyclus anynana: Direct and correlated responses. J. Evol. Biol. 2002, 15, 290–300. [Google Scholar] [CrossRef]
- Schlichting, C.D.; Smith, H. Phenotypic plasticity: Linking molecular mechanism with evolutionary outcomes. Evol. Ecol. 2002, 16, 189–211. [Google Scholar] [CrossRef]
- Canard, M.; Séméria, Y.; New, T.R. Biology of Chrysopidae; Dr. W. Junk Publishers: The Hague, The Netherlands, 1984. [Google Scholar]
- Duelli, P. Body coloration and colour change in green lacewings (Insecta: Neuroptera: Chrysopidae). In Current Research in Neuropterology. Proceedings of the Fourth International Symposium on Neuropterology, Bagnères-de-Luchon, Haute-Garonne, France, 24–27 June 1991; Canard, M., Aspöck, H., Mansell, M.W., Eds.; Privately Printed: Toulouse, France, 1992; pp. 119–123. [Google Scholar]
- Duelli, P.; Johnson, J.B.; Waldburger, M.; Henry, C.S. A new look at adaptive body coloration and color change in “common green lacewings” of the genus Chrysoperla (Neuroptera: Chrysopidae). Ann. Entomol. Soc. Am. 2014, 107, 382–388. [Google Scholar] [CrossRef]
- Arndt, C. Über Vererbung der Binderverietäten bei H. nemoralis. Arch. Ver. Freunde Nat Mecklen 1878, 31, 120–124. [Google Scholar]
- Cain, A.J. The uniqueness of the polymorphism of Cepaea (Pulmonata: Helicidae) in Western Europe. J. Conch 1977, 29, 129–136. [Google Scholar] [CrossRef]
- Cook, L.M. A two-stage model for Cepaea polymorphism. Philos. Trans. Biol. Sci. 1998, 353, 1577–1593. [Google Scholar] [CrossRef]
- Wisse, K.A.J. Re-evaluation of three species of australian Hemerobiidae (Insecta: Neuroptera). N. Z. Entomol. 2000, 22, 15–21. [Google Scholar] [CrossRef]
- Faúndez, E.I. Asociación críptica entre Sinopla perpunctatus Signoret, 1863 (Acanthosomatidae: Hemiptera) y el ñirre Nothofagus antarctica (G. Forster) Oersted (Fagaceae) en la Región de Magallanes (Chile). Boletín Soc. Entomol. Aragonesa 2007, 40, 563–564. [Google Scholar]







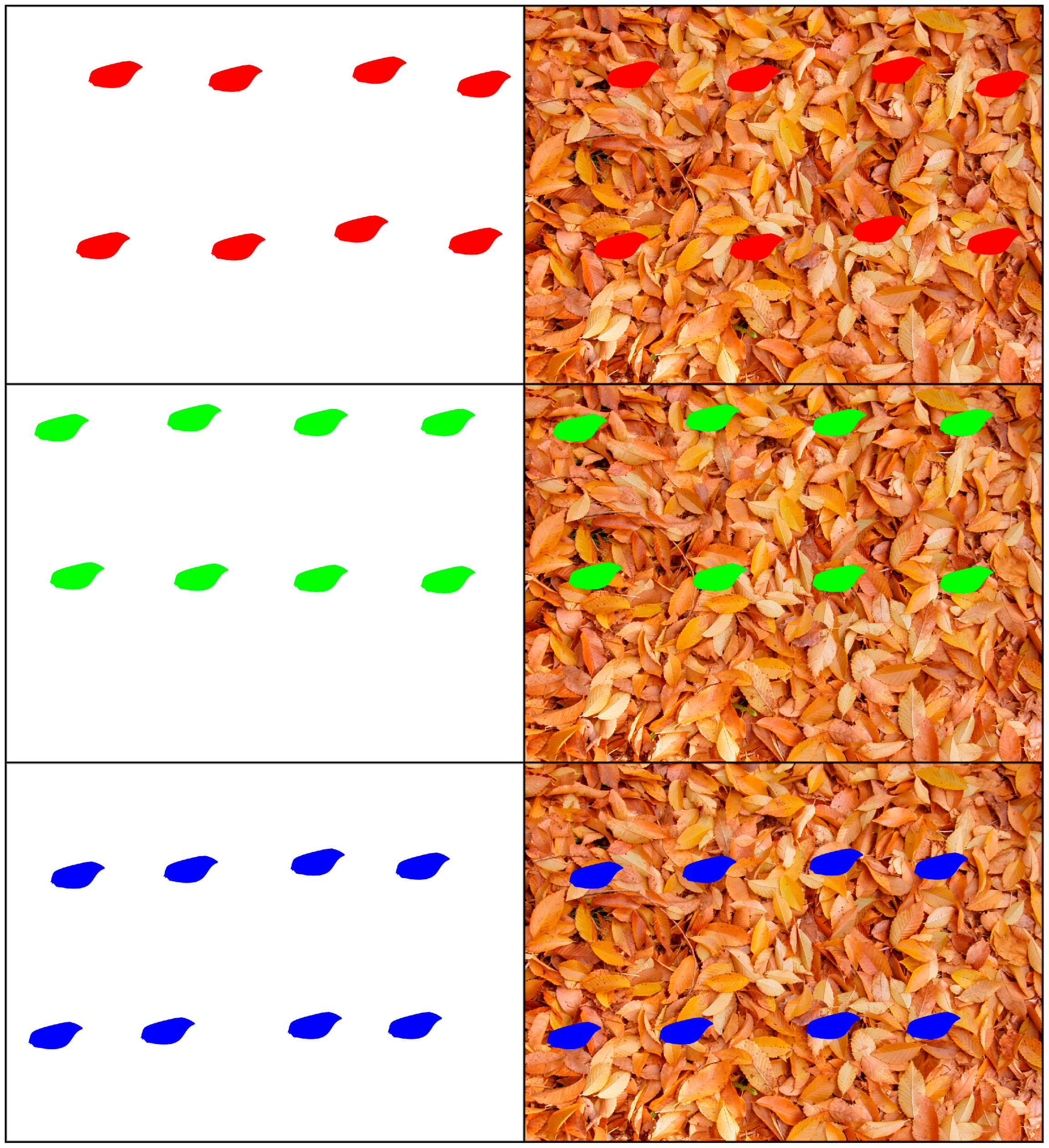
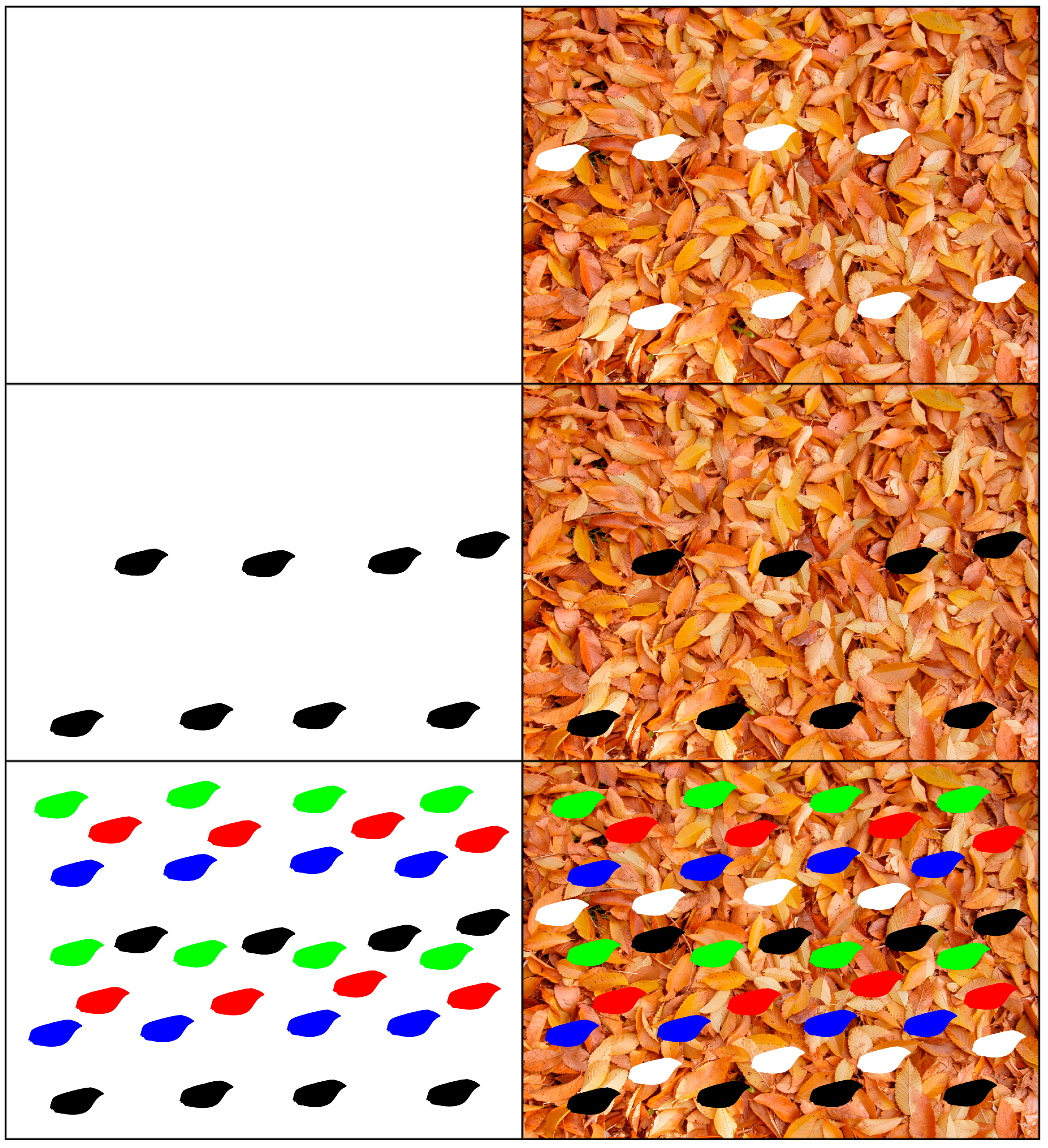
| White Background | Red | Green | Blue | Color | Grayscale |
|---|---|---|---|---|---|
| Background | 0.0000 (0.00%) | 0.0000 (0.00%) | 0.0000 (0.00%) | 0.0000 (0.00%) | 0.0000 (0.00%) |
| Colored wings | |||||
| Red | 0.2512 (3.14%) | 0.2977 (3.72%) | 0.3029 (3.79%) | 0.8517 (3.55%) | 0.3170 (3.96%) |
| Green | 0.2987 (3.73%) | 0.0345 (0.43%) | 0.3106 (3.88%) | 0.6438 (2.68%) | 0.2486 (3.11%) |
| Blue | 0.2992 (3.74%) | 0.2948 (3.69%) | 0.2630 (3.29%) | 0.8570 (3.57%) | 0.3177 (3.97%) |
| White | 0.0000 (0.00%) | 0.0000 (0.00%) | 0.0000 (0.00%) | 0.0000 (0.00%) | 0.0000 (0.00%) |
| Black | 0.2838 (3.55%) | 0.2838 (3.55%) | 0.2838 (3.55%) | 0.8515 (3.55%) | 0.2838 (3.55%) |
| Colored wing mix | 0.8980 (11.22%) | 0.6957 (8.70%) | 1.0029 (12.54%) | 2.5965 (10.82%) | 0.8875 (11.09%) |
| Gayomyia falcata | |||||
| GAY-1 | 0.6075 (7.59%) | 0.6231 (7.79%) | 0.6227 (7.78%) | 1.8534 (7.72%) | 0.6262 (7.83%) |
| GAY-2 | 0.5777 (7.22%) | 0.5812 (7.27%) | 0.5493 (6.87%) | 1.7083 (7.12%) | 0.5952 (7.44%) |
| GAY-3 | 0.5515 (6.89%) | 0.5508 (6.89%) | 0.5498 (6.87%) | 1.6522 (6.88%) | 0.5607 (7.01%) |
| GAY-4 | 0.6515 (8.14%) | 0.6234 (7.79%) | 0.5928 (7.41%) | 1.8677 (7.78%) | 0.6422 (8.03%) |
| GAY-5 | 0.5594 (6.99%) | 0.5589 (6.99%) | 0.5590 (6.99%) | 1.6772 (6.99%) | 0.5652 (7.06%) |
| GAY-6 | 0.6125 (7.66%) | 0.6011 (7.51%) | 0.5942 (7.43%) | 1.8079 (7.53%) | 0.6112 (7.64%) |
| GAY-mix | 2.8884 (36.10%) | 2.8638 (35.80%) | 2.8182 (35.23%) | 8.5704 (35.71%) | 2.9101 (36.38%) |
| Megalomus stangei | |||||
| MEG-1 | 0.3469 (4.34%) | 0.3508 (4.38%) | 0.3544 (4.43%) | 1.0521 (4.38%) | 0.3581 (4.48%) |
| MEG-2 | 0.3017 (3.77%) | 0.3065 (3.83%) | 0.3118 (3.90%) | 0.9199 (3.83%) | 0.3099 (3.87%) |
| MEG-3 | 0.4648 (5.81%) | 0.4623 (5.78%) | 0.4610 (5.76%) | 1.3881 (5.78%) | 0.4781 (5.98%) |
| MEG-mix | 0.9936 (12.42%) | 1.0119 (12.65%) | 1.0338 (12.92%) | 3.0393 (12.66%) | 1.0298 (12.87%) |
| Leaf Background | Red | Green | Blue | Color | Grayscale |
|---|---|---|---|---|---|
| Background | 7.1780 (89.72%) | 7.5146 (93.93%) | 7.2896 (91.12%) | 21.9822 (91.59%) | 7.8523 (98.15%) |
| Colored wings | |||||
| Red | 7.1458 (89.32%) | 7.4737 (93.42%) | 7.2214 (90.27%) | 21.8409 (91.00%) | 7.8176 (97.72%) |
| Green | 7.1514 (89.39%) | 7.4722 (93.40%) | 7.2440 (90.55%) | 21.8677 (91.12%) | 7.8343 (97.93%) |
| Blue | 7.1617 (89.52%) | 7.4715 (93.39%) | 7.2620 (90.78%) | 21.8952 (91.23%) | 7.8179 (97.72%) |
| White | 7.1404 (89.26%) | 7.4749 (93.44%) | 7.2663 (90.83%) | 21.8816 (91.17%) | 7.7934 (97.42%) |
| Black | 7.1467 (89.33%) | 7.4651 (93.31%) | 7.2188 (90.24%) | 21.8306 (90.96%) | 7.7777 (97.22%) |
| Colored wing mix | 6.8097 (85.12%) | 7.0149 (87.69%) | 6.9454 (86.82%) | 20.7699 (86.54%) | 7.3597 (92.00%) |
| Gayomyia falcata | |||||
| GAY-1 | 7.2185 (90.23%) | 7.5451 (94.31%) | 7.3188 (91.48%) | 22.0824 (92.01%) | 7.8597 (98.25%) |
| GAY-2 | 7.2001 (90.00%) | 7.5258 (94.07%) | 7.2904 (91.13%) | 22.0163 (91.73%) | 7.8623 (98.28%) |
| GAY-3 | 7.2057 (90.07%) | 7.5160 (93.95%) | 7.2996 (91.24%) | 22.0213 (91.76%) | 7.8498 (98.12%) |
| GAY-4 | 7.2742 (90.93%) | 7.5299 (94.12%) | 7.2690 (90.86%) | 22.0730 (91.97%) | 7.8539 (98.17%) |
| GAY-5 | 7.2313 (90.39%) | 7.5174 (93.97%) | 7.2951 (91.19%) | 22.0438 (91.85%) | 7.8510 (98.14%) |
| GAY-6 | 7.2530 (90.66%) | 7.5208 (94.01%) | 7.2781 (90.98%) | 22.0518 (91.88%) | 7.8520 (98.15%) |
| GAY-mix | 7.4489 (93.11%) | 7.5739 (94.67%) | 7.2972 (91.22%) | 22.3200 (93.00%) | 7.8611 (98.26%) |
| Megalomus stangei | |||||
| MEG-1 | 7.1846 (89.81%) | 7.5270 (94.09%) | 7.3183 (91.48%) | 22.0299 (91.79%) | 7.8551 (98.19%) |
| MEG-2 | 7.1832 (89.79%) | 7.5393 (94.24%) | 7.3374 (91.72%) | 22.0599 (91.92%) | 7.8568 (98.21%) |
| MEG-3 | 7.1924 (89.90%) | 7.5116 (93.89%) | 7.2853 (91.07%) | 21.9892 (91.62%) | 7.8515 (98.14%) |
| MEG-mix | 7.2022 (90.03%) | 7.5468 (94.33%) | 7.3570 (91.96%) | 22.1059 (92.11%) | 7.8574 (98.22%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monserrat, V.J.; Gavira, Ó. Wing Variability in Some Andean Brown Lacewing Insects as an Adaptive Survival Strategy (Insecta, Neuropterida, Neuroptera: Hemerobiidae). Insects 2025, 16, 401. https://doi.org/10.3390/insects16040401
Monserrat VJ, Gavira Ó. Wing Variability in Some Andean Brown Lacewing Insects as an Adaptive Survival Strategy (Insecta, Neuropterida, Neuroptera: Hemerobiidae). Insects. 2025; 16(4):401. https://doi.org/10.3390/insects16040401
Chicago/Turabian StyleMonserrat, Víctor J., and Óscar Gavira. 2025. "Wing Variability in Some Andean Brown Lacewing Insects as an Adaptive Survival Strategy (Insecta, Neuropterida, Neuroptera: Hemerobiidae)" Insects 16, no. 4: 401. https://doi.org/10.3390/insects16040401
APA StyleMonserrat, V. J., & Gavira, Ó. (2025). Wing Variability in Some Andean Brown Lacewing Insects as an Adaptive Survival Strategy (Insecta, Neuropterida, Neuroptera: Hemerobiidae). Insects, 16(4), 401. https://doi.org/10.3390/insects16040401





