An Overview of the Firefly Genus Pygoluciola Wittmer, a Phylogeny of the Luciolinae Using Mitochondrial Genomes, a Description of Six New Species, and an Assessment of a Copulation Clamp in This Genus (Coleoptera: Lampyridae: Luciolinae) †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphology and Taxonomic Characters
2.2. Sample Collection for Molecular Analysis
2.3. DNA Extraction Sequencing, Assembly, and Annotation of Mitogenomes
2.4. Phylogenetic Analysis
2.5. Data Availability
3. Results
3.1. Outcomes
3.2. Phylogeny
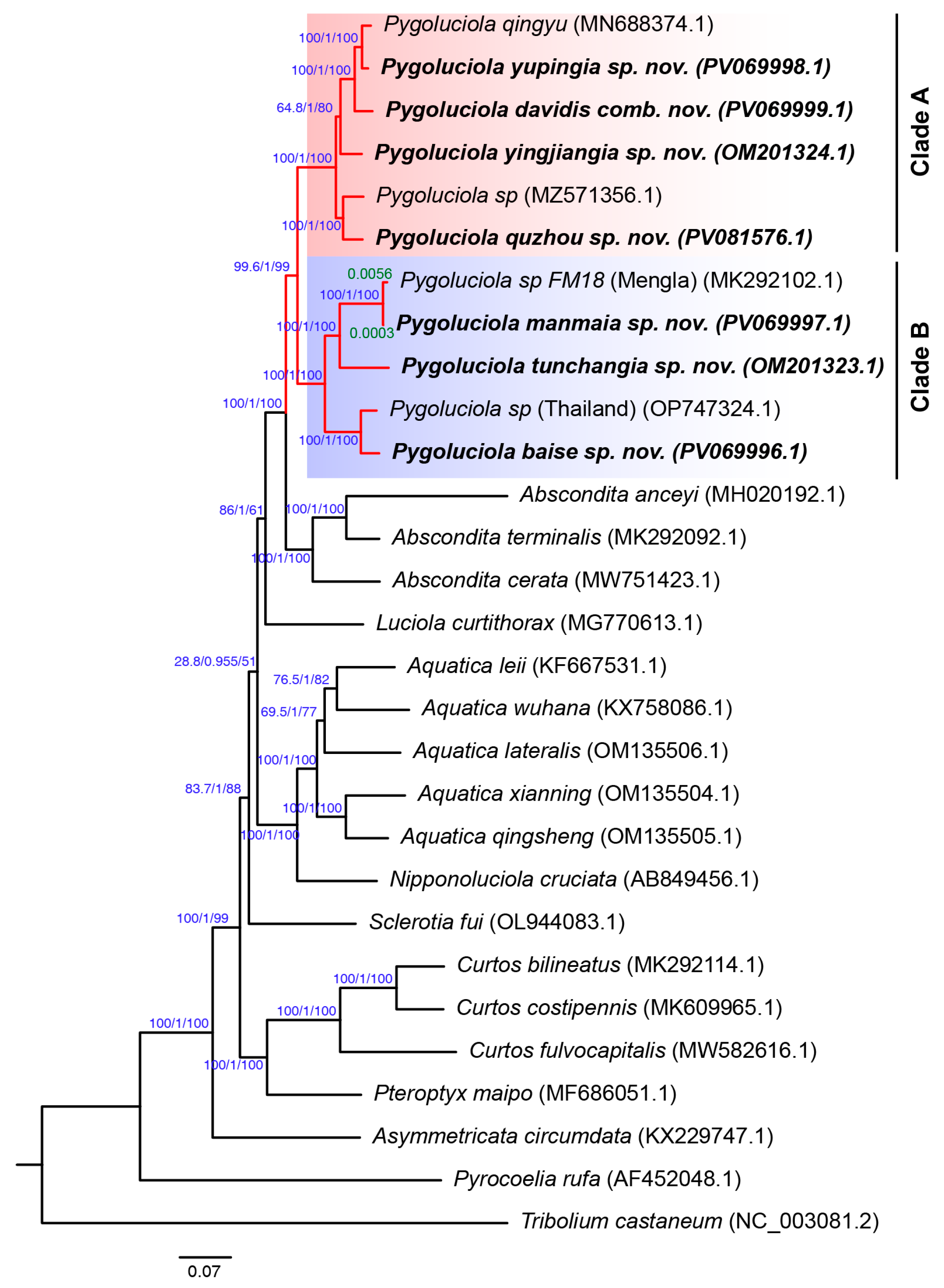
3.3. Taxonomy
3.3.1. Generic Description
3.3.2. Key to Species of Pygoluciola Using Males
- Median posterior margin of both V7 and T8 narrowed, prolonged, and often curving, V7 dorsally and T8 ventrally, and at least partially engulfing each other [2] (figures 41, 42), ref. [35] (figures 18–26) with apices of both often emarginated (Group 1)………………………………………………………………………………………………………………………………………………….2Median posterior margin of T8 not narrowed nor curving ventrally; median posterior margin of V7 either narrowed and elongated (Group 2) or median posterior margin of V7 rounded (may have short, slightly developed MPP) (Figure 3b, Figure 4c, Figure 5b, Figure 6d, Figure 7b, Figure 8b, Figure 9d and Figure 10d)………………………………………………………………7
- All tibiae curved; lateral margins of elytra tapering posteriorly along their length…………………………………………………...3
- Apex of median posterior projection of T8 no wider than rest and rounded, not emarginate; lateral margins of pronotum slightly sinuate [35] (figures 1, 6, 21)…………………………………………………………………………………....guigliae BallantyneApex of median posterior projection of T8 wider than rest and medianly emarginate; lateral margins of pronotum not slightly sinuate [35] (figures 4, 20, 23–25)………………………………………………………………………………………........stylifer Wittmer
- Median posterior projection of V7 bifurcate at apex [35] (figures 18, 26)……...……………………………………….…………........5Median posterior projection of V7 not bifurcate at apex …………………………………………………………………………............6
- Apex of median posterior projection of V7 deeply emarginate, laterally ensheathing the downturned apex of T8 and projecting laterally beside it…………………………..………………………………………………………………..…………..wittmeri (Ballantyne)Apex of median posterior projection of V7 shallowly emarginate, not laterally ensheathing the downturned apex of T8 nor projecting beside it [20] (figures 5, 10) ………………………………………………………………………...........kinabalua (Ballantyne)
- MPP of V7 elongate slender, longer than wide, ventral surface shallowly depressed along its length, bearing on its median dorsal surface two slender teeth; posterior apex of MPP not shallowly depressed; lateral margins of T8 downturned ……………………………………………………………………………………………………………………………….......satoi BallantyneMPP of V7 shorter, about as wide as long, ventral surface not shallowly depressed along its length, not bearing dorsal teeth; posterior apex (face) of MPP shallowly depressed; lateral margins T8 not downturned [2] (figures 41, 42); ………………………………………………………………………………………………………………………………..hamulata (Olivier)
- Median posterior margin of V7 prolonged (longer than wide), broad, subparallel-sided, ventral surface shallow, apex slightly emarginated; LO retracted from lateral and posterior margins; T8 with triangular posterior outline [2] (figures 452–455); aedeagal sheath sternite expanding gradually along its length (Group 2)……………………………………………………………...8Median posterior margin of V7 not usually prolonged, if slightly prolonged, often wider than long, and ventral surface not shallow, apex rounded; LO retracted from lateral and posterior margins or not; T8 without triangular outline, usually subparallel-sided, and posterior margin rounded or truncate………………………………………………………………………........9
- Posterior margin of LO in V7 rounded, without emargination; lateral margins of V7 and MPP pale yellow [7] (figures 2–4,10,11,14)……………………………………………………………………………………………………………………......dunguna NadaPosterior margin of LO in V7 with median emargination; lateral margins of V7 and MPP dark brown [2] (figures 449–455)………………………………………………………………………………………………………….……………………..tamarat Jusoh
- Posterior margin of T8 about as wide as preceding area of T8 and narrowly downturned [2] (figures 439, 440) (Group 3)……………………………………………………………………………………………………………………………………..………….10Posterior margin of T8 usually narrower than preceding area of T8 and not downturned……………………………..………......15
- V7 with short apically rounded MPP; LO in V7 subparallel-sided, retracted at sides from lateral margins of V7; elytra pale brownish yellow with white apices (underlying fat bodies), pronotum yellowish brown without darker median markings; ventrobasal portion of paired T8 arms with small pointed projections; aedeagus with elongate LL extending well beyond the ML apex; BP narrow; ref. [2] (figures 437–448)……………………………………………………………………………...phupan BallantyneMedian posterior margin of V7 rounded, without MPP, or with scarce or ill-defined MPP; LO in V7 with rounded margins occupying almost all of V7; elytra either very dark brown to black, sometimes with paler margins, or semitransparent pale brown with darker brown markings at base; ventrobasal portions of paired T8 arms without projections; aedeagus with short LL often interned at apices, not extending well beyond the ML apex; BP wide, well sclerotised …………………………………………………………………………………………………………………………….………………..……11
- Elytra black, with or without paler margins; pronotum with paired, well separated, ovoid, brown to black markings; if markings approach closely, then not extending to posterior margin……………………………..…………………………………....12Elytra pale, semitransparent, brown with darker brown markings at base; pronotum with paired median dark brown markings closely approaching in mid-line, with lateral margins divergent and extending to posterior margin (Figure 6) ……………………………………………………………………………………………………………………………….yingjiangia sp. nov.
- Elytra black with pale, semitransparent suture, apex, and lateral margins; MS and MN pale yellow, semitransparent; pronotum with small, paired, ovoid, dark brown well separated markings (Figure 3)………….……………......davidis comb. nov.Elytra black without any paler margins; pronotum either marked as above or with extensive median brown markings closely approaching in median line and with divergent lateral margins; MS and MN sometimes with pink fat body undertones……………………………………………………………….……………………………………………………………….........13
- T8 with paired arms expanded in vertical plane; lateral margins slightly convergent in posterior ¼; LL inturned at their apices; dark markings on pronotum closely approach along midline, with divergent lateral margins and slightly produced at posterolateral corners (Figure 7)…………………………………………………………………………………………...yupingia sp. nov.T8 subparallel-sided with paired arms narrow not expanded; LL not inturned at their apices; dark pronotal markings restricted to ovoid well separated areas ……….…………………….…………………….……………………………………………....14
- LO scarcely retracted from lateral and posterior margins of V7; MPP scarce; metaventrite black; basal abdominal ventrites black (Figure 4)……………………………………………………………………………………………………....qingyu Fu & BallantyneLO in V7 retracted from lateral and posterior margin, with short semitransparent MPP; metaventrite pale yellow with scarce brown median markings; basal abdominal ventrites pale brown, posterior margin of V6 narrowly mid-brown (Figure 5)………………………………………………………………………………………………………………………………....quzhou sp. nov.
- Aedeagal sheath sternite terminated by hairy curved (boomerang shaped) projection; aedeagus wider across middle than elsewhere, with membranous apical portion of LL wider at bases and tapering to apices [2] (figures 359–362, 377–379, 401–403, 458–462); (Group 4) …………………………………………………………………………………………………………………………..16Aedeagal sheath sternite not terminated by hairy curved (boomerang shaped) projection, except in P. nitescens, where the aedeagus is not wider across middle than elsewhere [2] (figures 422–436); aedeagus subparallel-sided, not wider across middle than elsewhere; membranous apical portions of the LL as wide at their base as at their apex [2] (figures 370–373, 389–393, 413–417, 428, 430–432); (Group 5) ..…………………………………………………………………………………………………....19
- Elytra dark brown, with brown base, and narrowly orange lateral and sutural margins, which may extend around apex……………………………………………………………………………………………………………………………………………..17Elytra mid-brown, always with base of elytron narrowly to widely paler orange, and narrowly orange lateral and sutural margins, which may extend around apex………………………………………………………..…………………………………….......18
- Elytron with narrowly pale apex; all abdominal tergites dark brown; LO in V6,7 retracted from lateral and posterior margin of V7, with narrow dark brown margins [2] (figures 374–381)……………………………………………………..bangladeshi BallantyneElytron with apex dark brown; T8 paler brown than rest; LO in V6,7 not retracted along lateral or posterior margins, without narrow dark brown margins [2] (figures 456–462)……………………………………….……………………...………….....vitalisi (Pic)
- All abdominal tergites very dark brown; LO in V6,7 not retracted from margins; margins not narrowly dark brown; known only from the Andaman Islands [2] (figures 394–404)…………………………………………………...……………..insularis (Olivier)T8 paler brown than rest, semitransparent; LO in V6 narrowly retracted along lateral margins, and along lateral and posterior margins in V7; margins narrowly dark brown; known only from Myanmar [2] (figures 356–363) ….....……...abscondita (Olivier)
- Elytra yellow without darker brown markings, or yellow with apices black......……………………..……..………………………..20Elytra not as above, either dark or pale brown …………………………………………………….………………….……..…………...24
- Sri Lankan; elytra yellow with no darker markings; aedeagal sheath sternite expanding along its length either in a regular fashion or with lateral margins arcuate…………………………………….…………….…………..……….………….......….....….......21Not known from Sri Lanka; found in mainland China; elytra yellow to orange yellow with some darker markings, especially at apex; aedeagal sheath sternite narrow, subparallel-sided along its length…………………………………………….....……......22
- Dorsal body yellow, without any underlying pinkish fat body; LO fills V7, except for narrow apical margin; aedeagal sheath sternite apically truncate with small, narrow median emargination; LL of aedeagus not expanded in horizontal plane [11] (figures 2, 3, 6–19)…………………………………………….………………….…………….……………..rammale Wijekoon & De SilvaDorsal body yellow with areas of underlying pinkish fat body; LO restricted to median area in V7 and retracted from lateral and posterior margins, sides of LO tapering slightly; aedeagal sheath sternite apically rounded without median emargination; LL of aedeagus wide, expanded in horizontal plane [11] (figures 25, 26, 29–42)………………………………………….………………….………………….…………..……………….....ruhuna Wijekoon & De Silva
- LO in V7 with posterior margin emarginated (Figure 8)………………………………….……….………………….….....baise sp. nov.Posterior margin of V7 LO not emarginated………………………………………….………………………….……....….……...….…23
- Basal abdominal ventrites black (Figure 10)…………………………………….….………….………………….…...tunchangia sp. nov.Basal abdominal ventrites yellow (Figure 9)………………….…………………….…….………..………………….....manmaia sp. nov.
- Elytra dark brown to black without paler margins………………………………………….……………….………………….…….....25Elytra never dark brown without pale margins; either mid-brown with all margins orange yellow, or mid-brown with lateral margin paler brown, or elytra brown with only suture paler brown………………..………………………………..………………..26
- 12.0 mm long; heavy bodied; LO in V7 retracted from posterior margin; MPP of V7 short, apex broadly rounded; T8 parallel-sided; LL broadly expanded in horizontal plane, not much longer than ML [2] (figures 422–436)……..…….....nitescens (Olivier)9–10 mm long; slender bodied; LO in V7 parallel-sided, retracted from lateral and posterior margins; MPP of V7 broad, apically truncate; T8 not parallel-sided; LL not broadly expanded in horizontal plane, elongate slender, about 2 X length of ML [2] (figures 382–393)…………………………………....……………..………….……...…..………...……………..…calceata (Olivier)
- Indonesian (Java); elytra mid-brown with lateral, sutural, and apical margins orange; V7 without reflexed margins [2] (figures 364–373)……………………………………………………………………...………....……..………………..……...……….ambita (Olivier)Either from Australia or the Philippines; with lateral margin only paler than rest, or elytra brown with suture paler than rest……………………………….………………………………………………..………..………………...………………………....…..….27
- Australian (Northern Territory around Darwin); large exposed head with posterior eye emarginations; pronotum with subparallel sides, wide median dark marking reaching to both anterior and posterior margins; elytra mid-brown with paler lateral margins; margins of V7 are not reflexed……………………..………..……………..……………………......cowleyi (Blackburn)Philippines; head not large, exposed, nor with emarginations; pronotal margins not subparallel-sided; elytra yellowish–mid-brown; reflexed margins of V7 envelop T8 at sides [2] (figures 405–421)………..…………………………......matalangao Ballantyne
3.3.3. Key to Females of Pygoluciola
- All tibiae curved………………………………………………………………………………....………………………guigliae (Ballantyne)No tibiae curved………………………………………………………………………………....…………………………………………….2
- Dorsal colour yellow, without darker brown markings; bursa hooks minute……………………………..…………………………. 3Dorsal colour always with some darker brown to black markings, on pronotum, elytra; bursa hooks, if observed, large, well defined……………………………………………………………………………………………………………………………………..…....4
- V2 light brown; V3 dark brown; V4,5 black; no underlying pink fat body evident [11] (figures 4, 5)………………………………………………………………………………………..………………………rammale Wijekoon & De SilvaV2−4 dark brown, 5 black; pink fat body visible beneath cuticle of pronotum andelytral apices [11] (figures 27, 28)………………………….…………………………..……….…..………..ruhuna Wijekoon & De Silva
- Elytra black, with or without paler margins …………………………………………………………………………….…………........…5Elytra never uniformly black; pale brown with apices black, or pale brown with darker brown at base...….....…….…....…….…6
- Black metaventrite, and abdominal V2-5; posterior emargination of V7 reaching beneath the posterior margin of V6 (Figure 4d–m)…………………………………………... ………………………………………….………………………....qingyu Fu & BallantynePale brown metaventrite and abdominal V2-5; posterior emargination of V7 not reaching beneath the posterior margin of V6 (Figure 5c,d,k–n)…….…………………………………………………………………………………..……………………..quzhou sp. nov.
- Dorsal surface pale, semitransparent, light brown, or yellow, with dark brown to black elytral apices………………………………………….……….…………………………………………………………………...…………………....7Dorsal colouration never as above……………………………………..……………………………………....……………………….…..10
- Dorsal surface very pale brown, semitransparent, with brown elytral apices; ventral body pale yellowish brown, with narrow dark brown band across posterior margin of V5; each bursa hook in two elongate curved sections [7] (figures 25–37)………………………………………………………………………………...………………….…………………………...dunguna NadaDorsal surface yellow with black elytral apices; ventral body either pale yellow with minimal darker markings or V2-5 very dark brown; bursa plates not detected (but absence not assumed)……………………………..………………………………………..8
- V2-5 and metaventrite yellow with no dark brown markings; anterior corners of T7 broadly rounded (Figure 9e,f,r–u)……………………………….………………………………………..…………….....…………………….......…………manmaia sp. nov.V2-5 and metaventrite dark brown to black; anterior corners of narrowed, acute………………………….……….……………....…9
- Posterior emargination of V7 not projecting beneath LO in V6; posterior corners of V7 rounded; anterior corners of T7 narrowed acute; anterior apodeme of V8 separate from posterior area (Figure 10e,f,r–u)……………..………..tunchangia sp. nov.Posterior margin of V7 deep, projecting beneath LO in V7; posterior corners of V7 acute; anterior corners T7 elongate slender, acute, slightly divergent; anterior apodeme of V8 continuous with posterior area (Figure 8e–h,p–s)………………...baise sp. nov.
- Posterior margin of V7 deeply emarginate; bearing a small ridge anterior to median area of deepest emargination (possibility this ridge is an artefact, having been seen only on dried pinned specimens); V8 with anteromedian prolongation not any more sclerotised than remainder of V8; T7 with anteromedian area rounded and elevated, lateral areas not flattened [20] (figures 16, 17); (Figure 2)…………………………………………………………………………....……………………………...kinabalua (Ballantyne)Posterior margin of V7 with or without an anteromedian ridge; V8 with anteromedian prolongation well sclerotised and visibly separated from remainder of V7; T7 without a rounded and elevated anteromedian area, with lateral areas flattened……………………………………………….………………………………………………………………………………………..11
- V2-5 very dark brown to black; V7 with anteromedian ridge narrowly edged in black; elytra pale, semitransparent, light brown with dark brown markings across base (Figure 6e,f)…………………………….…….…………………….yingjiangia sp. nov.V2-5 yellowish to mid-brown, never black; V7 without anteromedian ridge; elytra mid-brown, with suture or suture and lateral margin semitransparent and paler than rest; without darker brown markings at elytral base……………………..…………..………..………………..………………..……………………………..………………..………………12
- Pronotum with extensive median dark brown markings reaching to posterior margin; elytra mid-brown with suture narrowly yellow; posterolateral areas of V7 irregularly expanded, with aggregation of fat body beneath ……………………………………………………………………….………………..………………..…………..…....……satoi (Ballantyne)Pronotum with paired ovoid brown markings not attaining posterior margin; elytra mid-brown semitransparent, with both suture and lateral margin paler brown than rest; posterolateral areas of V7 not expanded, extent of fat body distribution not determined in original examination………………………………..………………..………………..…………...…wittmeri (Ballantyne)
3.3.4. Overview of Species of Pygoluciola Including Descriptions of New Species
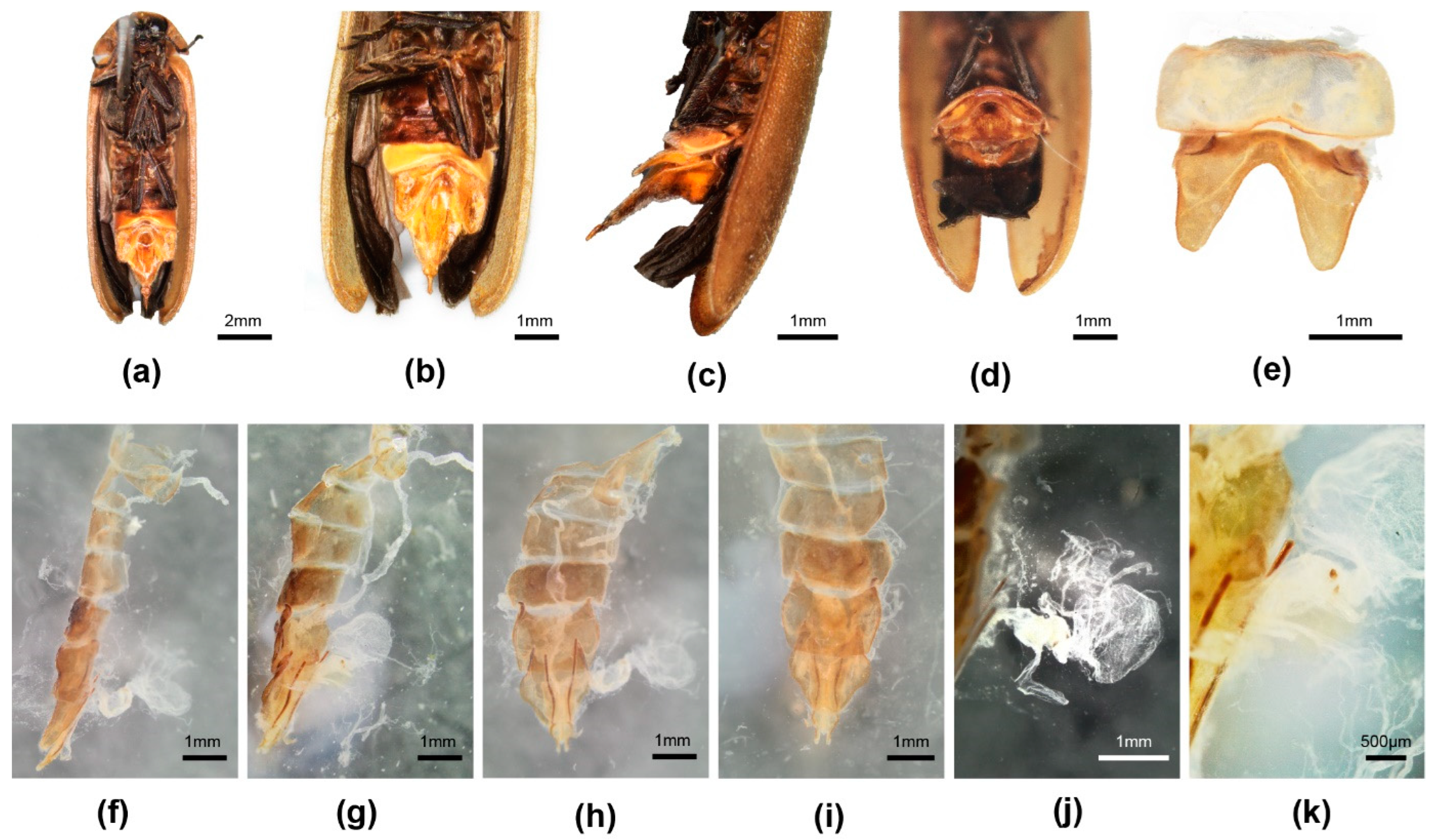
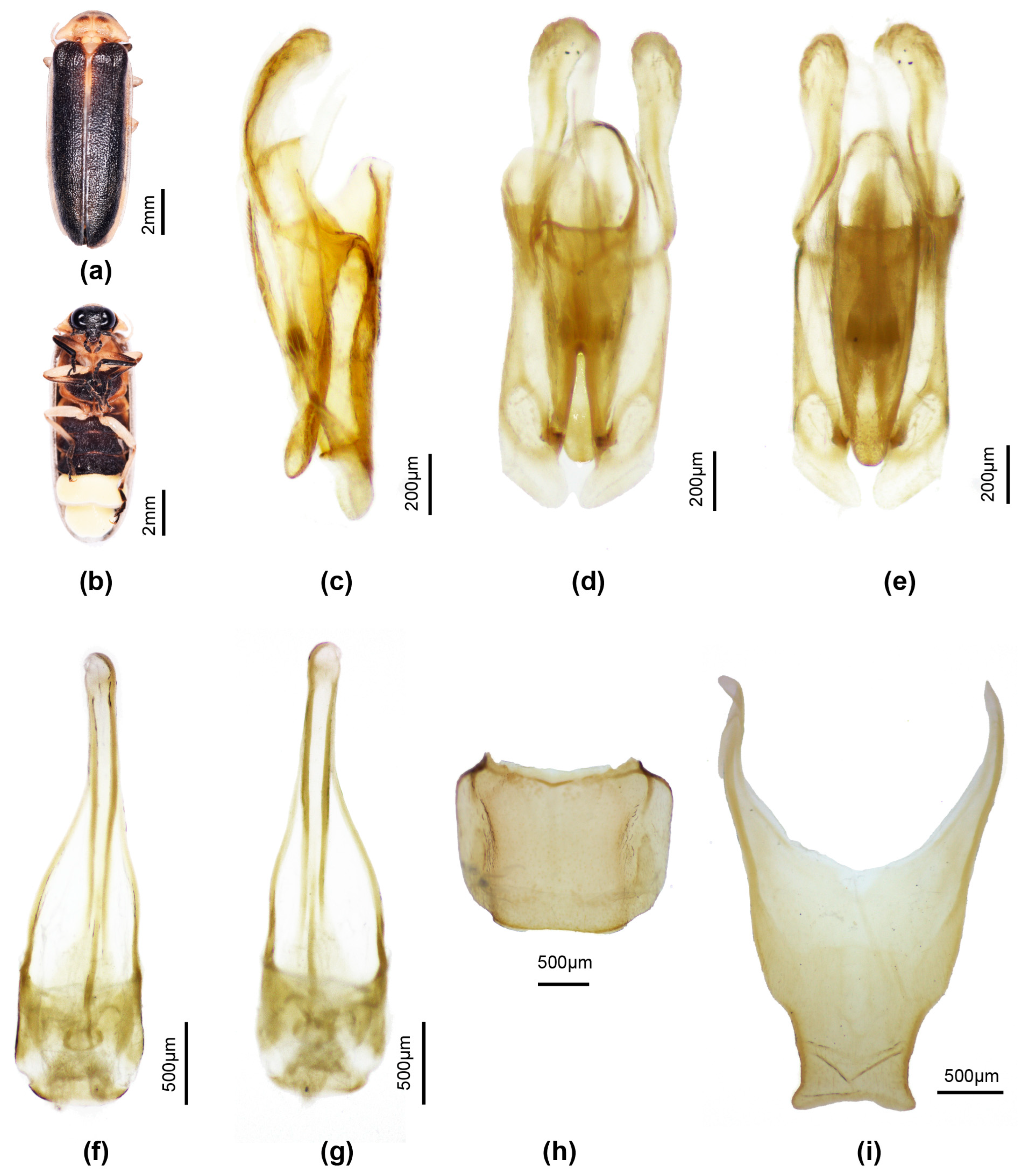
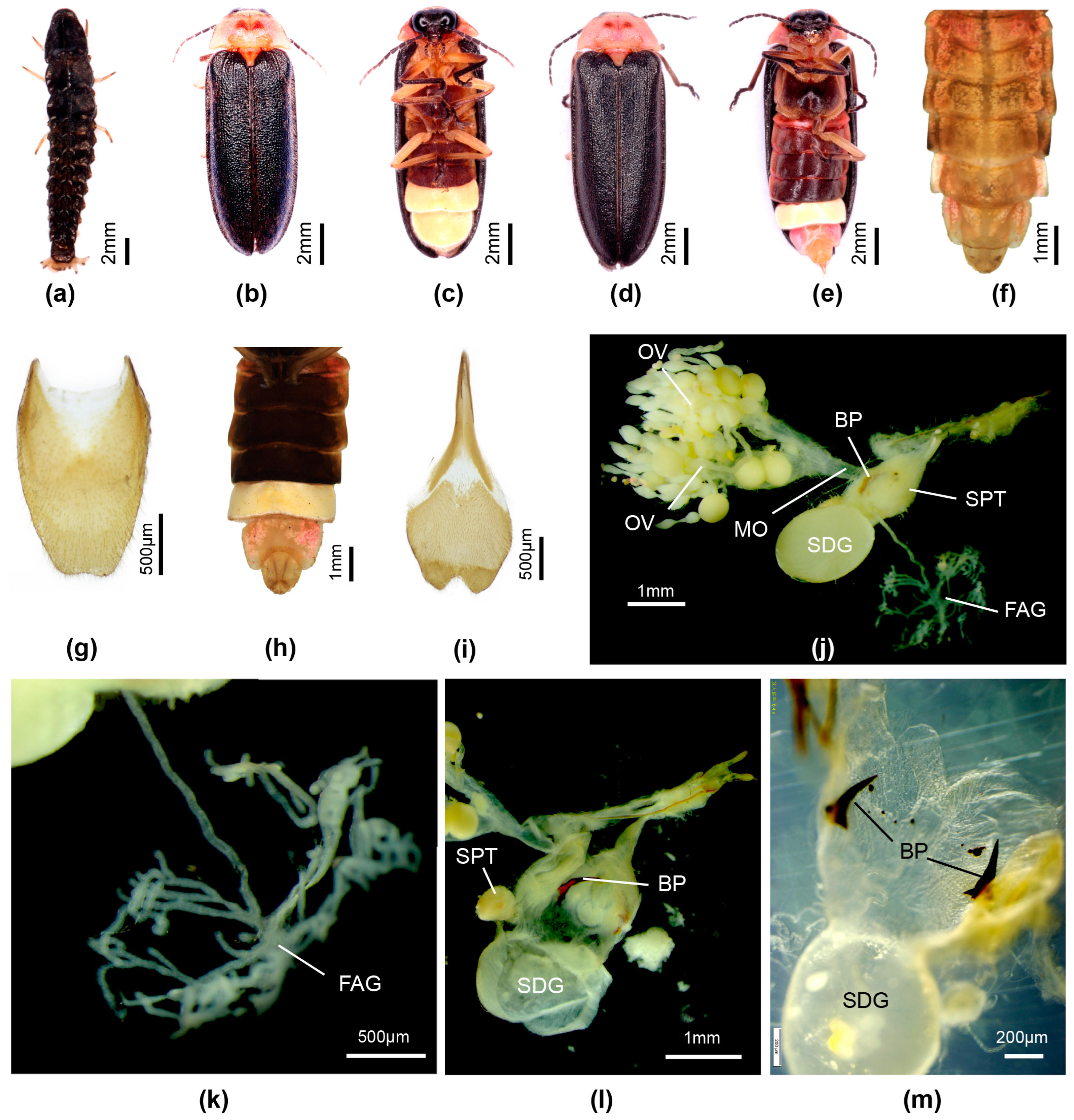
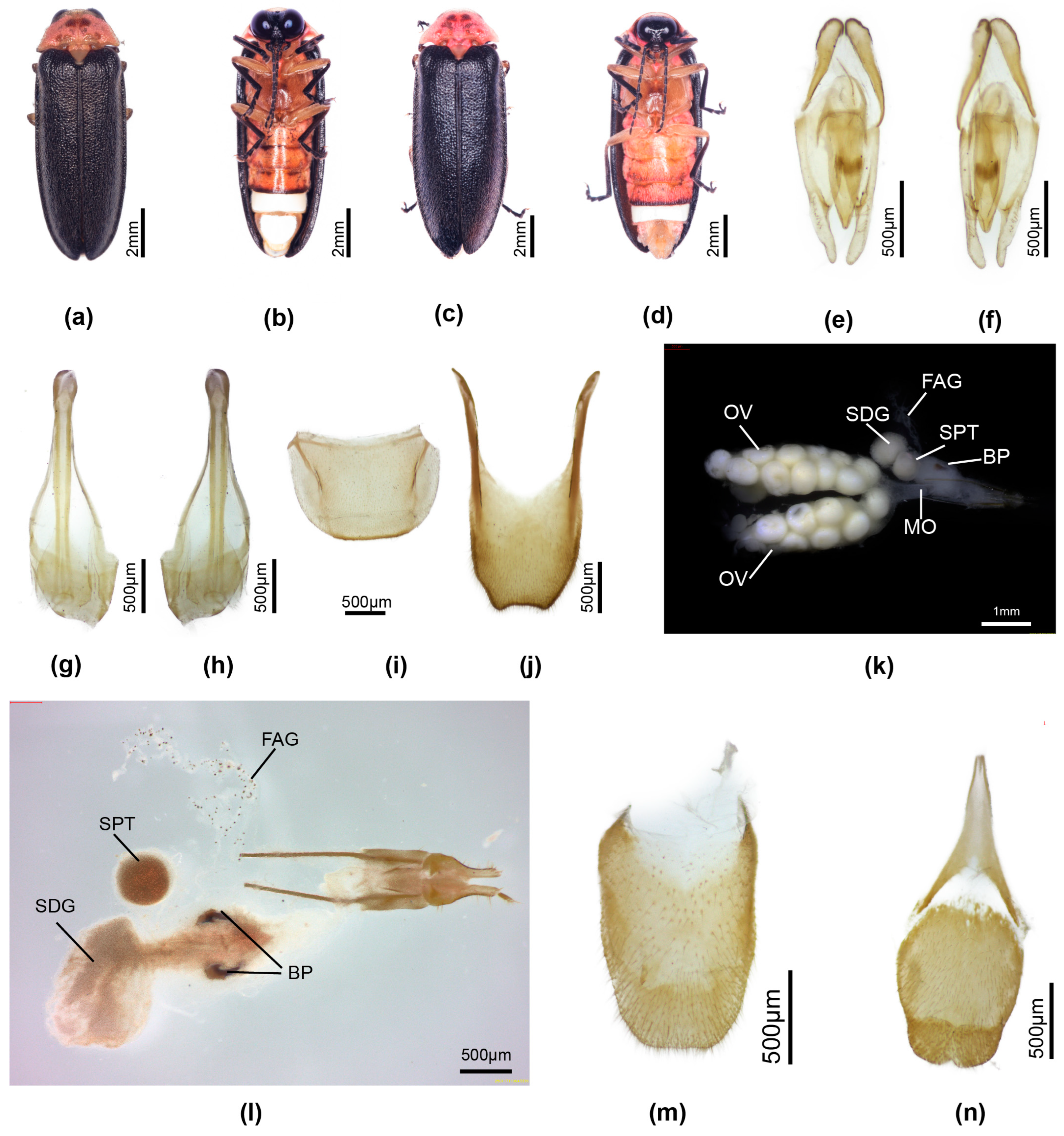
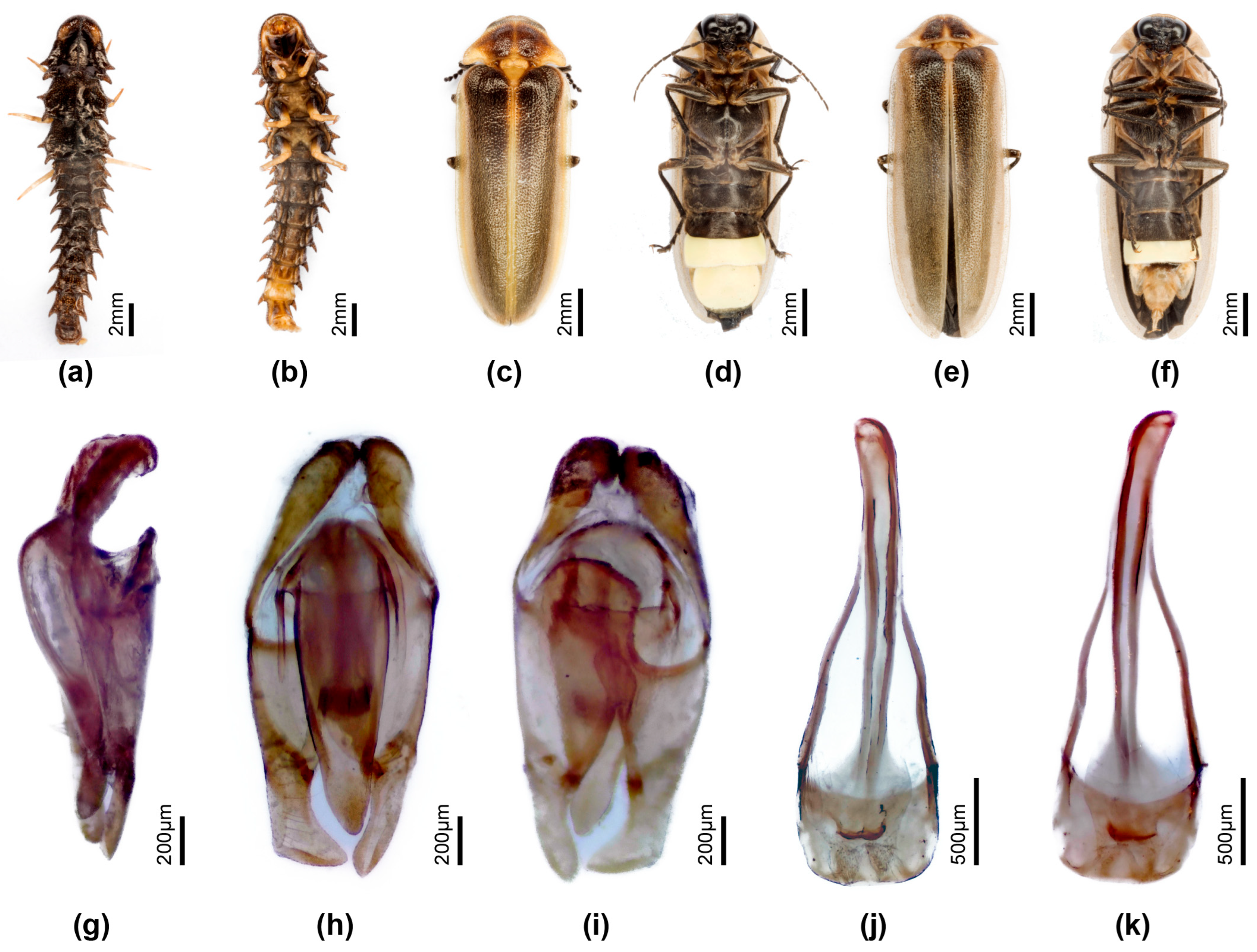
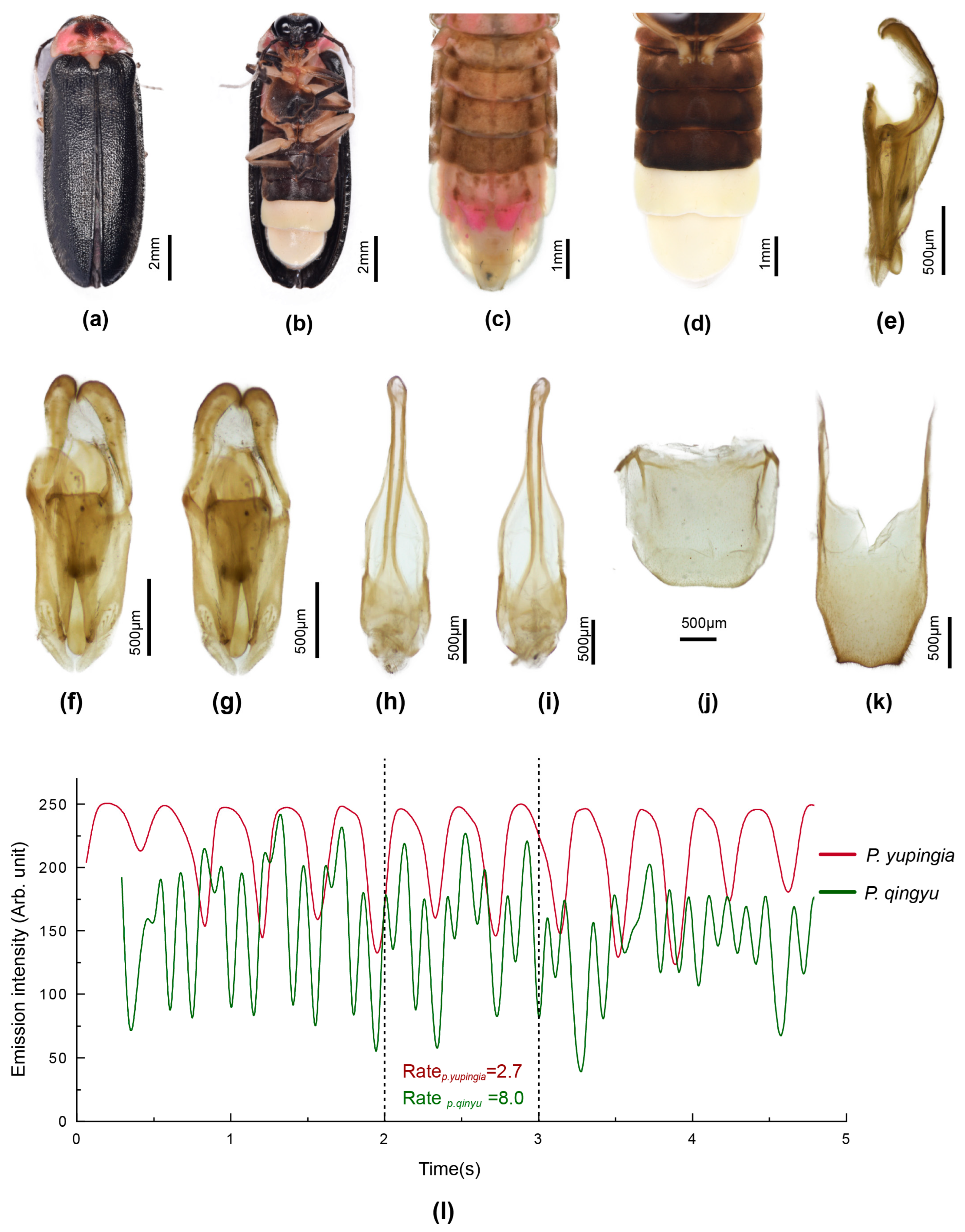
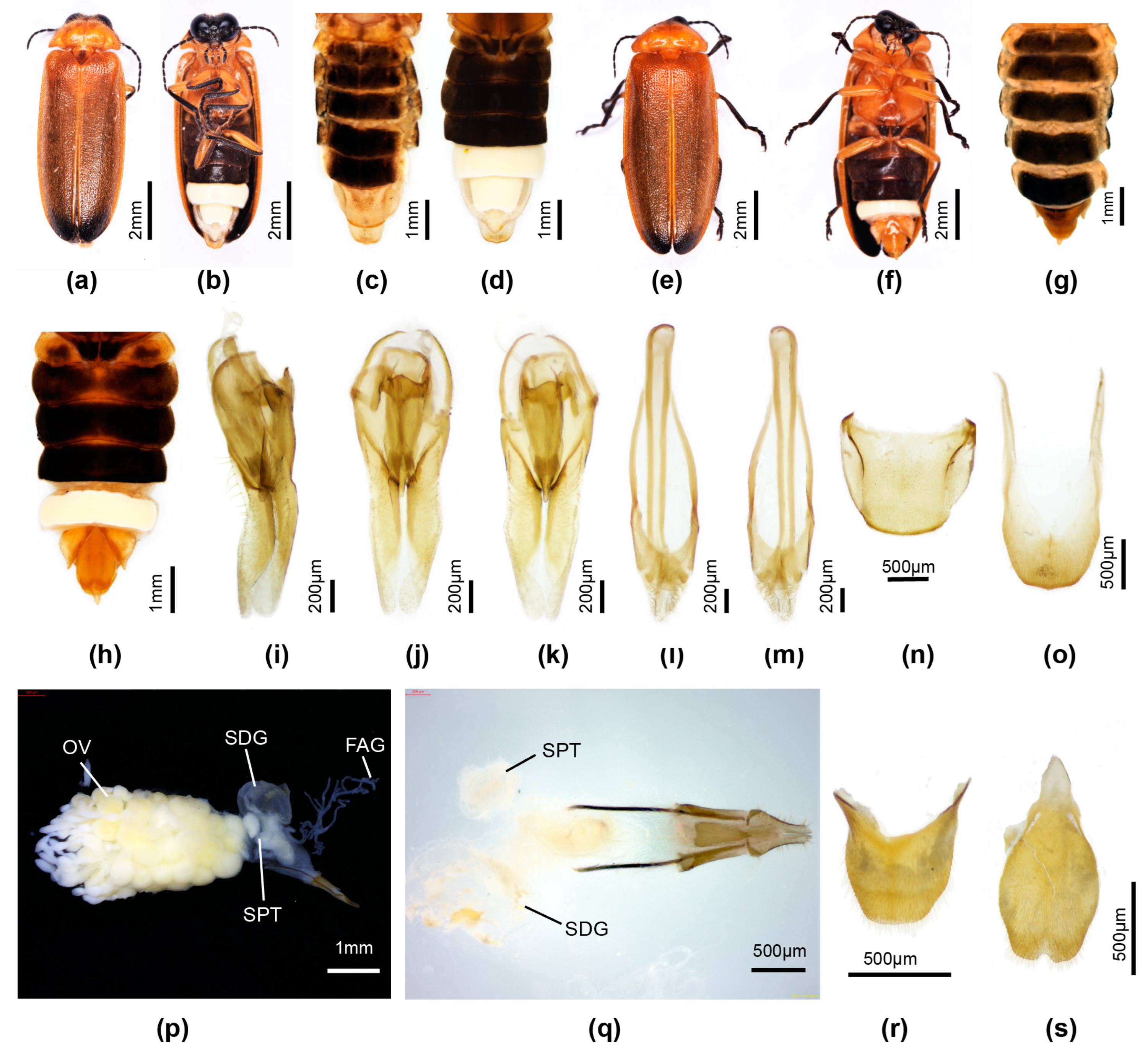
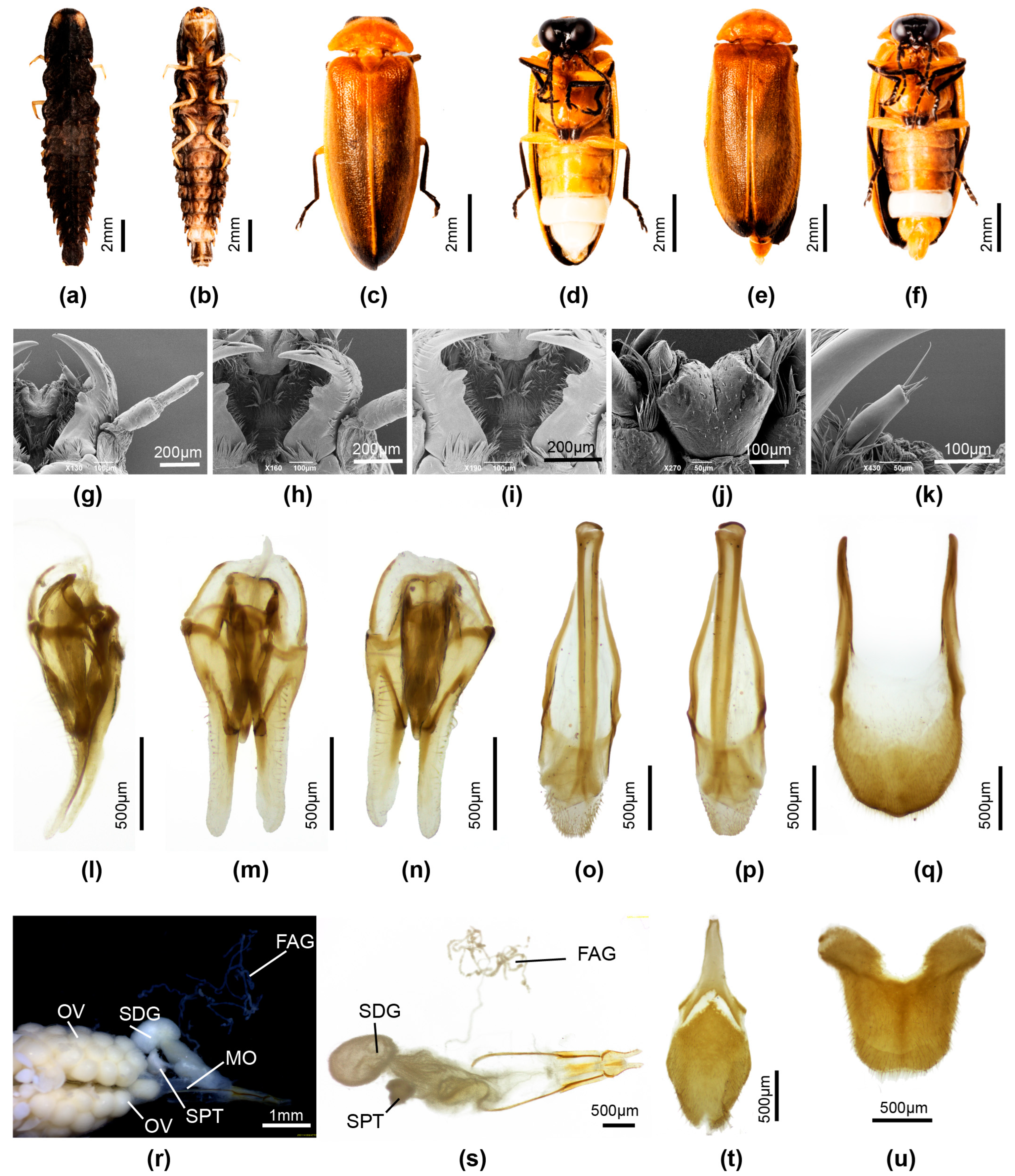
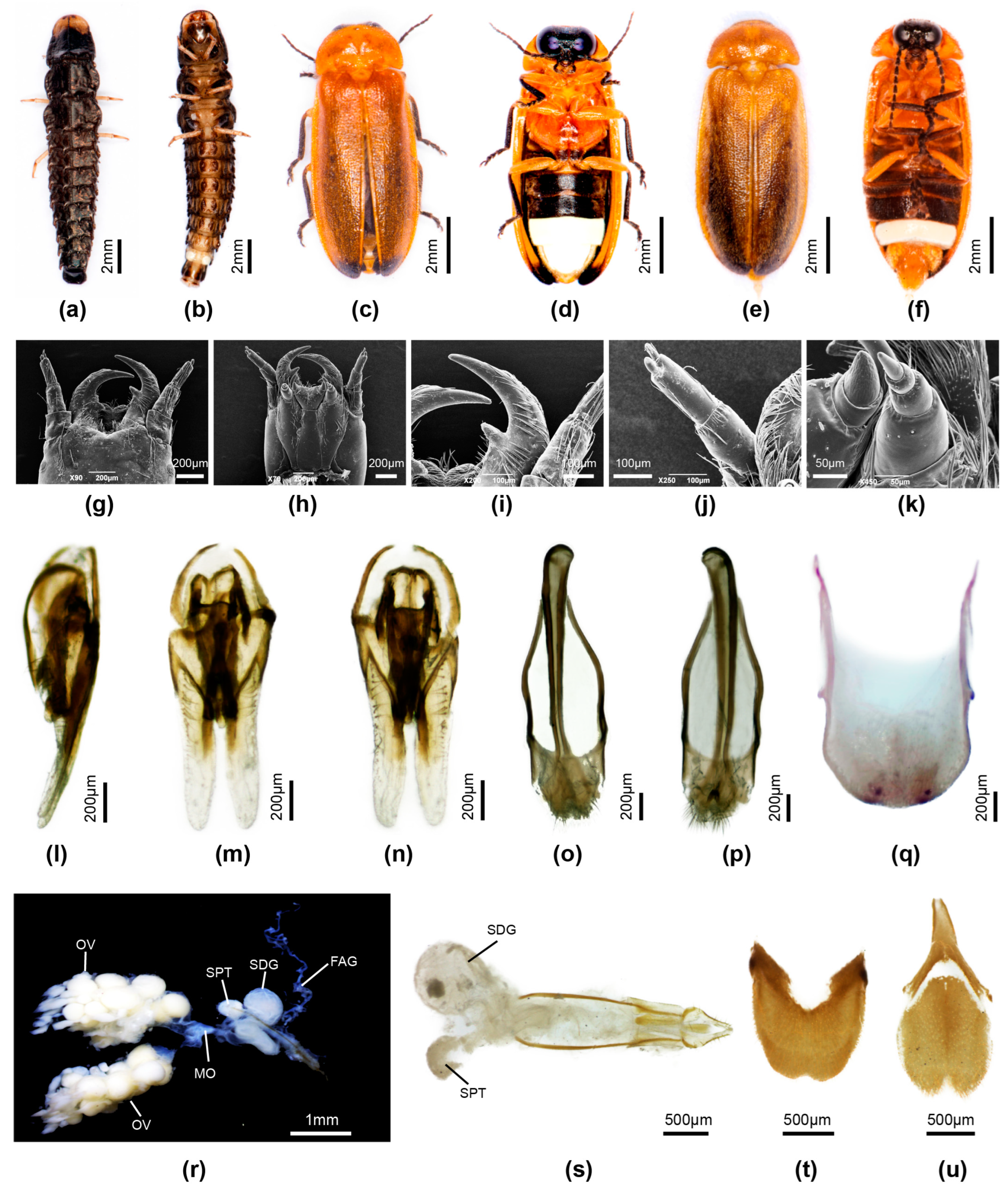
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations for taxonomic characters | |
| A | pronotal dimension measured from above; width across anterior third |
| ASD | distance between antennal sockets |
| ASW | antennal socket greatest horizontal diameter |
| B | pronotal dimension measured from above; width across middle |
| BL | body length taken as median length of pronotum plus length of elytron |
| C | pronotal dimension measured from above; width across posterior third |
| EL | length of elytron |
| FAG | female accessory gland |
| FS | antennal flagellar segments |
| GHW | greatest head width (across eyes, measured parallel to ASD) |
| L | length |
| LA | length aedeagus, measured from below, specimen horizontal, anterior margin of BP to tip of LL or tip of ML, whichever is longer |
| Legs 1, 2 etc. | legs and parts of legs are referred to by their thoracic segment number, e.g., legs 1 = prothoracic legs; tarsi 2 = mesothoracic tarsi; femora 3 = metathoracic femora |
| LL | lateral lobes, aedeagus |
| LO | light organ |
| ML | median lobe of the aedeagus |
| MN | mesonotal plates |
| MS | mesoscutellum |
| SIW | smallest interocular width (measured horizontally, may be on the same level as ASD, ASW above it if the eyes are closer there) |
| T7, 8 etc. | abdominal tergites |
| V6, 7 etc. | abdominal ventrites, referred to by actual, not visible number |
| W | width |
| W/L | width/length |
| X | times |
| Abbreviations for depositories of specimens | |
| ANIC | Australian National Insect Collection, Canberra |
| BPBM | Bernice Bishop Museum, Honolulu, Hawaii |
| DZURSL | Department of Zoology, University of Ryuhuna, Sri Lanka |
| HZMAU | Natural History Museum Huazhong Agricultural University, Wuhan |
| MCSN | Museo civico di storia naturale, Genoa |
| MNHN | Musée d’histoire naturelle, Paris |
| MZUM | Museum of Zoology, University of Malaya, Malaysia |
| NHML | Natural History Museum, London |
| BASEL | Natural History Museum, Basel |
| RMNH | National Natural History Museum, Leiden |
| ZRC | Raffles Museum, Singapore |
References
- Wittmer, W. Fauna Buruana. Coleoptera 7. Beitrag zur Kenntnis der indo- malayischen Malacodermata. Treubia 1939, 17, 21–24+127. [Google Scholar]
- Ballantyne, L.; Lambkin, C.; Ho, J.-Z.; Jusoh, W.; Nada, B.; Nak-Eiam, S.; Thancharoen, A.; Wattanachaiyingcharoen, W.; Yiu, V. The Luciolinae of SE Asia and the Australopacific region: A revisionary checklist (Coleoptera: Lampyridae) including description of three new genera and 13 new species. Zootaxa 2019, 4687, 1–174. [Google Scholar]
- Mobilim, V.; Dawood, M.M. Solitary fireflies of Kangkawat Research Station, Imbak Canyon, Sabah. J. Trop. Biol. Conserv. (JTBC) 2020, 17, 131–147. [Google Scholar] [CrossRef]
- Mobilim, V.; Dawood, M.M. What’s flashing in Kadamaian? A Note on Fireflies (Coleoptera: Lampyridae) in Kadamaian, Sabah. J. Trop. Biol. Conserv. (JTBC) 2021, 18, 9–20. [Google Scholar] [CrossRef]
- Chey, V. Fireflies of Kionsom. Sepilok Bull. 2008, 8, 15–22. [Google Scholar]
- Ghosh, S.; Sarkar, S.K.; Chakraborty, S.K. Two new records of the subfamily Luciolinae Lacordaire, 1857 (Coleoptera: Lampyridae) with a checklist of genus Abscondita from India. J. Asia-Pac. Biodivers. 2021, 14, 53–59. [Google Scholar] [CrossRef]
- Nada, B.; Ballantyne, L. A new species of Pygoluciola Wittmer with unusual abdominal configuration, from lowland dipterocarp forest in peninsular Malaysia (Coleoptera Lampyridae: Luciolinae). Zootaxa 2018, 4455, 343–362. [Google Scholar] [CrossRef]
- Fu, X.H.; Ballantyne, L. Taxonomy and behaviour of lucioline fireflies (Coleoptera: Lampyridae: Luciolinae) with redefinition and new species of Pygoluciola Wittmer from mainland China and review of Luciola LaPorte. Zootaxa 2008, 1733, 1–44. [Google Scholar] [CrossRef]
- Lewis, S.M.; Jusoh, W.F.; Walker, A.C.; Fallon, C.E.; Joyce, R.; Yiu, V. Illuminating Firefly Diversity: Trends, Threats and Conservation Strategies. Insects 2024, 15, 71. [Google Scholar] [CrossRef]
- McDermott, F.A. The taxonomy of the Lampyridae (Coleoptera). Trans. Am. Entomol. Soc. 1964, 90, 1–72. [Google Scholar]
- Wijekoon, W.; Ballantyne, L.; de Silva, D.; Wegiriya, H.; Madushanka, A. First record of the genus Pygoluciola Wittmer (Coleoptera: Lampyridae: Luciolinae) from Sri Lanka with two new species, P. rammale and P. ruhuna. Zootaxa 2024, 5428, 393–412. [Google Scholar] [CrossRef]
- Ballantyne, L.; Fu, X.H.; Lambkin, C.; Jeng, M.-L.; Faust, L.; Wijekoon, W.M.C.D.; Li, D.; Zhu, T. Studies on South-east Asian fireflies: Abscondita, a new genus with details of life history, flashing patterns and behaviour of Abs. chinensis (L.) and Abs. terminalis (Olivier)(Coleoptera: Lampyridae: Luciolinae). Zootaxa 2013, 3721, 1–48. [Google Scholar] [CrossRef]
- Liu, G.C.; Dong, Z.W.; He, J.W.; Zhao, R.P.; Wang, W.; Li, X.Y. Genome size of 14 species of fireflies (Insecta, Coleoptera, Lampyridae). Zool. Res. 2017, 38, 449. [Google Scholar] [PubMed]
- Chen, X.; Dong, Z.; Liu, G.; He, J.; Zhao, R.; Wang, W.; Peng, Y.; Li, X. Phylogenetic analysis provides insights into the evolution of Asian fireflies and adult bioluminescence. Mol. Phylogenetics Evol. 2019, 140, 106600. [Google Scholar] [CrossRef]
- Ge, X.; Yuan, L.; Kang, Y.; Liu, T.; Liu, H.; Yang, Y. Characterization of the first complete mitochondrial genome of Cyphonocerinae (Coleoptera: Lampyridae) with implications for phylogeny and evolution of fireflies. Insects 2021, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, W.F.; Ballantyne, L.; Chan, S.H.; Wong, T.W.; Yeo, D.; Nada, B.; Chan, K.O. Molecular systematics of the firefly genus Luciola (Coleoptera: Lampyridae: Luciolinae) with the description of a new species from Singapore. Animals 2021, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fu, X. The genetic variations in the mitochondrial genomes of three Luciolinae fireflies. Mitochondrial DNA Part B 2020, 5, 3210–3214. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, J.S.; Kim, I. Complete mitochondrial genome of the Korean endemic firefly, Luciola unmunsana (Coleoptera: Lampyridae). Mitochondrial DNA Part B 2020, 5, 3165–3167. [Google Scholar] [CrossRef]
- Martin, G.J.; Branham, M.A.; Whiting, M.F.; Bybee, S.M. Total evidence phylogeny and the evolution of adult bioluminescence in fireflies (Coleoptera: Lampyridae). Mol. Phylogenetics Evol. 2017, 107, 564–575. [Google Scholar] [CrossRef]
- Ballantyne, L.A.; Lambkin, C. A new firefly, Luciola (Pygoluciola) kinabalua, new species (Coleoptera: Lampyridae), from Malaysia, with observations on a possible copulation clamp. Raffles Bull. Zool. 2001, 49, 363–378. [Google Scholar]
- Mora, R.; Retana, A.; Espinoza, A. External morphology of Tagosodes orizicolus (Homoptera: Delphacidae) revealed by scanning electron microscopy. Ann. Entomol. Soc. Am. 2001, 94, 438–448. [Google Scholar] [CrossRef]
- Branham, M.A.; Greenfield, M.D. Flashing males win mate success. Nature 1996, 381, 745–746. [Google Scholar] [CrossRef]
- Shahjahan, R.; Hughes, K.; Leopold, R.; Devault, J. Lower incubation temperatures increase yield of insect genomic DNA isolated by the CTAB method. Biotechniques 1995, 19, 332–334. [Google Scholar]
- Donath, A.; Jühling, F.; Al-Arab, M.; Bernhart, S.H.; Reinhardt, F.; Stadler, P.F.; Middendorf, M.; Bernt, M. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 2019, 47, 10543–10552. [Google Scholar] [CrossRef]
- Chan, P.P.; Lin, B.Y.; Mak, A.J.; Lowe, T.M. tRNAscan-SE 2.0: Improved detection and functional classification of transfer RNA genes. Nucleic Acids Res. 2021, 49, 9077–9096. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Ranwez, V.; Douzery, E.J.; Cambon, C.; Chantret, N.; Delsuc, F. MACSE v2: Toolkit for the alignment of coding sequences accounting for frameshifts and stop codons. Mol. Biol. Evol. 2018, 35, 2582–2584. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Ballantyne, L.; Lambkin, C. A phylogenetic reassessment of the rare SE Asian firefly genus Pygoluciola Wittmer (Coleoptera: Lampyridae: Luciolinae). Raffles Bull. Zool. 2006, 54, 21–48. [Google Scholar]
- Ballantyne, L.A. Revisional Studies of Australian and Indomalayan Luciolini (Coleoptera, Lampyridae, Luciolinae). Univ. Qld. Pap. 1968, II, 103–139. [Google Scholar]
- Ballantyne, L. Pygoluciola satoi, a new species of the rare Southeast Asian firefly genus Pygoluciola Wittmer (Coleoptera: Lampyridae: Luciolinae) from the Philippines. Raffles Bull. Zool. 2008, 56, 1–9. [Google Scholar]
- Jusoh, W.F.; Ballantyne, L. A catalogue and redescription of type specimens of fireflies (Coleoptera, Lampyridae, Luciolinae) deposited in Naturalis Biodiversity Center, Leiden. Contrib. Entomol. 2024, 74, 63–80. [Google Scholar] [CrossRef]
- Olivier, E. Deux espèces nouvelles de Lucioles. Bull. Société Entomol. De Fr. 1895, 1, 148–149. [Google Scholar]
- Olivier, E. Catalogue des espèces de ‘Luciola’ et genres voisins décrits jusqu’à ce jour. Rev. Sci. Bourbonnaiset Cent. Fr. 1902, 15, 69–88. [Google Scholar]
- Olivier, E. Viaggio di Leonardo Fea in Birmanie e Regioni vicine. XXXV. Lampyrides rapportés de Birmanie par M. L.Fea avec descriptions des espèces nouvelles. Ann. Mus. Civ. Stor. Nat. Genova 1891, 30, 595–604. [Google Scholar]
- Gorham, H. On Coleoptera collected in India by MM. HE and HL Andrews. Families: Malacodermata, Erotylidae, Endomycidae and Coccinellidae. Ann. Société Entomol. Belg. 1903, 47, 323–347. [Google Scholar]
- Pic, M. Notes diverses, descriptions et diagnoses. L’Échange Rev. Linnéenne 1914, 30, 49–56. [Google Scholar]
- Olivier, E. Descriptions de nouvelles espèces de Lampyrides du Musée de Tring. Novit. Zool. 1896, III, 1–3. [Google Scholar]
- Ballantyne, L.A.; Lambkin, C.L.; Luan, X.; Boontop, Y.; Nak-Eiam, S. Further studies on south eastern Asian Luciolinae: 1. Sclerotia Ballantyne, a new genus of fireflies with back swimming larvae 2. Triangulara Pimpasalee, a new genus from Thailand (Coleoptera: Lampyridae). Zootaxa 2016, 4170, 201–249. [Google Scholar] [CrossRef]
- Olivier, E. Descriptions de Lampyrides nouveaux. Ann. Soc. Entomol. Belg. XLIX 1905, 49, 206–209. [Google Scholar] [CrossRef]
- Olivier, E. Les Lampyrides de l’Indo-Chine. Rev. Sci. Bourbon. Cent. Fr. 1912, 25, 88–92. [Google Scholar]
- Olivier, E. Coleoptera, Lampyridae. Genera Insectorum 1907, 53, 55. [Google Scholar]
- Blackburn, T. Further notes on Australian Coleoptera, with descriptions of new genera and species. Trans. Proc. R. Soc. South Aust. 1897, 21, 28–39. [Google Scholar]
- Lea, A.M. Revision of the Australian and Tasmanian Malacodermidae. Trans. Entomol. Soc. Lond. 1909, 45–251, [Lampyridae, 106–111]. [Google Scholar] [CrossRef]
- Calder, A.A. Coleoptera: Elateroidea in Wells. In Zoological catalogue of Australia 29.6; Wells, A., Ed.; CSIRO Publishing: Clayton, Australia, 1998; pp. 1–248. [Google Scholar]
- Ballantyne, L.; Lambkin, C. Lampyridae of Australia (Coleoptera: Lampyridae: Luciolinae: Luciolini). Mem. Qld. Mus. 2000, 46, 15–93. [Google Scholar]
- Ballantyne, L.A.; Lambkin, C. Systematics of Indo-Pacific fireflies with a redefinition of Australasian Atyphella Olliff, Madagascan Photuroluciola Pic, and description of seven new genera from the Luciolinae (Coleoptera: Lampyridae). Zootaxa 2009, 1997, 1–188. [Google Scholar] [CrossRef]
- Pic, M. Lampyridae, Drilidae, Cantharidae, Malachiidae, und Prionoceridae. in Handschin, E., Studienreise auf den Sundainsein und in Nord Australien 1930–32. Entomol. Ber. 1938, 10, 3. [Google Scholar]
- Ballantyne, L.A.; Lambkin, C.L. Systematics and phylogenetics of Indo-Pacific Luciolinae fireflies (Coleoptera: Lampyridae) and the description of new genera. Zootaxa 2013, 3653, 1–162. [Google Scholar] [CrossRef] [PubMed]
- Olivier, E. Coléoptères Lampyrides capturés é Dardjilling par M.le Dr. Harmand. Bull. Mus. D’histoire Nat. Paris 1903, 9, 19–20. [Google Scholar]
- Olivier, E. The Lampyridae of Borneo. Sarawak Mus. J. 1913, 3, 55–60. [Google Scholar]
- Olivier, E. Contribution à l’étude de la faune entomologique de Sumatra. XI. Lampyrides détermines et décrits. Société R. D’entomologie Belg. Ann. 1900, 44, 234–238. [Google Scholar]
- Fu, X.; Ballantyne, L. An Overview of Aquatica Fu et al., a Phylogeny of Aquatic Fireflies Using Mitochondrial Genomes, a Description of Two New Species, and a New Record of Aquatic Fireflies in China. Insects 2024, 15, 31. [Google Scholar] [CrossRef]
- Ballantyne, L.; Fu, X.H.; Shih, C.H.; Cheng, C.Y.; Yiu, V. Pteroptyx maipo Ballantyne, a new species of bent-winged firefly (Coleoptera: Lampyridae) from Hong Kong, and its relevance to firefly biology and conservation. Zootaxa 2011, 2931, 8–34. [Google Scholar] [CrossRef]
- Ballantyne, L.A. Lucioline morphology, taxonomy and behaviour: A reappraisal (Coleoptera, Llampyridae). Trans. Am. Entomol. Soc. 1987, 113, 171–188. [Google Scholar]
- Wattanachaiyingcharoen, W.; Nak-Eiam, S. First record of the firefly genus Wittmer (Coleoptera: Lampyridae: Luciolinae) in Thailand. Lampyrid 2012, 2, 24–29. [Google Scholar]
- Wing, S.; Lloyd, J.E.; Hongtrakul, T. Male competition in Pteroptyx fireflies: Wing-cover clamps, female anatomy, and mating plugs. Fla. Entomol. 1983, 66, 86–91. [Google Scholar] [CrossRef]
- Silveira, L.; Souto, P.; Khattar, G.; Taklya, D.M.; Nunes, V.; Mermudes, J.R.M.; Monteiro, R.; Macedo, M. Unlocking the evolution of abdominal specialization in Luciuranus fireflies (Coleoptera: Lampyridae). Zool. Scr. 2022, 51, 708–723. [Google Scholar] [CrossRef]
- Silveira, L.F.L.d.; Lima, W.; Fonseca, C.R.V.d.; McHugh, J. Haplocauda, a New Genus of Fireflies Endemic to the Amazon Rainforest (Coleoptera: Lampyridae). Insects 2022, 13, 58. [Google Scholar] [CrossRef]
| Direction | Abdominal Segment | Male | Female |
|---|---|---|---|
| ventral | basal | Ventrite (V) 2 | V2 |
| antepenultimate | V5 | V6 light organ | |
| penultimate | V6 light organ | V7 | |
| terminal | V7 light organ | V8 | |
| dorsal | antepenultimate | Tergite (T) 6 | T6 |
| penultimate | T7 | T7 | |
| terminal | T8 | T8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Ballantyne, L. An Overview of the Firefly Genus Pygoluciola Wittmer, a Phylogeny of the Luciolinae Using Mitochondrial Genomes, a Description of Six New Species, and an Assessment of a Copulation Clamp in This Genus (Coleoptera: Lampyridae: Luciolinae). Insects 2025, 16, 394. https://doi.org/10.3390/insects16040394
Fu X, Ballantyne L. An Overview of the Firefly Genus Pygoluciola Wittmer, a Phylogeny of the Luciolinae Using Mitochondrial Genomes, a Description of Six New Species, and an Assessment of a Copulation Clamp in This Genus (Coleoptera: Lampyridae: Luciolinae). Insects. 2025; 16(4):394. https://doi.org/10.3390/insects16040394
Chicago/Turabian StyleFu, Xinhua, and Lesley Ballantyne. 2025. "An Overview of the Firefly Genus Pygoluciola Wittmer, a Phylogeny of the Luciolinae Using Mitochondrial Genomes, a Description of Six New Species, and an Assessment of a Copulation Clamp in This Genus (Coleoptera: Lampyridae: Luciolinae)" Insects 16, no. 4: 394. https://doi.org/10.3390/insects16040394
APA StyleFu, X., & Ballantyne, L. (2025). An Overview of the Firefly Genus Pygoluciola Wittmer, a Phylogeny of the Luciolinae Using Mitochondrial Genomes, a Description of Six New Species, and an Assessment of a Copulation Clamp in This Genus (Coleoptera: Lampyridae: Luciolinae). Insects, 16(4), 394. https://doi.org/10.3390/insects16040394






