Species Composition, Ecological Preferences, and Chromosomal Polymorphism of Malaria Mosquitoes of the Crimean Peninsula and the Black Sea Coast of the Caucasus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Collection and Material Preservation
2.2. Karyotyping
2.3. Genotyping by RLFP-RCR
2.4. Genotyping by Sequencing
2.5. Map Production
2.6. Bioinformatic and Statistical Analysis
3. Results
3.1. Species Composition, Geographical Distribution, and Ecological Preferences
3.2. Chromosomal Inversion Polymorphism
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BRFFR | the Belarusian Republican Foundation for Basic Research |
| ITS2 | the second internal transcribed spacer |
| PCR | polymerase chain reaction |
| RFLP | restriction fragment length polymorphism |
| RSF | the Russian Science Foundation |
| TBE | Tris–Borate–EDTA buffer |
References
- Mironova, L.P. Socio-ecological problems of Eastern Crimea in the past and present: Causes of emergence, ways of solution. Hist. Mod. 2017, 1, 79–106. [Google Scholar]
- Kudaktin, A.N. Ecological threats to the resorts of the south of Russia. Fundam. Res. 2006, 10, 56–58. [Google Scholar]
- Rossati, A.; Bargiacchi, O.; Kroumova, V.; Zaramella, M.; Caputo, A.; Garavelli, P.L. Climate, environment and transmission of malaria. Infez. Med. 2016, 24, 93–104. [Google Scholar]
- Fischer, L.; Gultekin, N.; Kaelin, M.B.; Fehr, J.; Schlagenhauf, P. Rising temperature and its impact on receptivity to malaria transmission in Europe: A systematic review. Travel. Med. Infect. Dis. 2020, 36, 101815. [Google Scholar] [CrossRef]
- Regional Strategy: From Malaria Control to Elimination in the WHO European Region 2006–2015; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2006; p. 40. Available online: https://iris.who.int/bitstream/handle/10665/107760/E88840.pdf?sequence=1&isAllowed=y (accessed on 20 September 2024).
- Ejov, M.; Sergiev, V.; Baranova, A.; Kurdova-Mintcheva, R.; Emiroglu, N.; Gasimov, E. Malaria in the WHO European Region on the Road to Elimination 2000–2015: Summary; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2018; p. 40. Available online: https://iris.who.int/bitstream/handle/10665/342148/9789289053112-eng.pdf?sequence=1&isAllowed=y (accessed on 20 September 2024).
- Lysenko, A.J.; Kondrashin, A.V. Malariology; World Health Organization: Geneva, Switzerland, 1999; p. 248. [Google Scholar]
- Morenets, T.M.; Isaeva, E.B.; Gorodin, V.N.; Avdeeva, M.G.; Grechanaya, T.V. Clinical and epidemiologic aspects of malaria in Krasnodar Krai. Epidemiol. Infect. Dis. 2016, 21, 253–261. [Google Scholar] [CrossRef]
- Karimov, I.Z.; Los’-Yatsenko, N.G.; Midikari, A.S.; Gorovenko, M.V.; Arshinov, P.S. Clinical and epidemiological features of imported malaria in the Republic of Crimea for a twenty-year period (1994–2014). Kazan. Med. J. 2014, 95, 916–920. [Google Scholar] [CrossRef]
- Baranova, A.M.; Sergiev, V.P.; Guzeeva, T.M.; Tokmalaev, A.K. Clinical suspicion to imported malaria: Transfusion cases and deaths in Russia. Infect. Dis. News Opin. Train. 2018, 7, 97–101. [Google Scholar] [CrossRef]
- Gornostaeva, R.M. A checklist of the mosquitoes (fam. Culicidae) in the European part of Russia. Parazitologiia 2000, 34, 428–434. [Google Scholar]
- Gutsevich, A.V.; Dubitskiy, A.M. New species of mosquitoes in the fauna of the USSR. Parazitol. Sbornic 1981, 30, 97–165. [Google Scholar]
- Kitzmiller, J.B.; Frizzi, G.; Baker, R. Evolution and speciation within the Maculipennis complex of the genus Anopheles. In Genetics of Insect Vectors of Disease; Wright, J.W., Ed.; Elsevier Publishing Company: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 1967; pp. 151–210. [Google Scholar]
- Coluzzi, M. Sibling species in Anopheles and their importance in malariology. Misc. Publ. Entomol. Soc. Am. 1970, 7, 63–77. [Google Scholar] [CrossRef]
- Knight, K.L.; Stone, A. A Catalog of the Mosquitoes of the World (Diptera, Culicidae), 2nd ed.; Thomas Say Found; Entomological Society of America: College Park, MD, USA, 1977; pp. 1–611. [Google Scholar]
- White, G.B. The place of morphological studies in the investigation of Anopheles species complexes. Mosq. Syst. 1977, 9, 1–24. [Google Scholar]
- White, G.B. Systematic reappraisal of the Anopheles maculipennis complex. Mosq. Syst. 1978, 10, 13–44. [Google Scholar]
- Stegniy, V.N. Population Genetics and Evolution of Malaria Mosquitoes; Tomsk State University Publisher: Tomsk, Russia, 1991; pp. 1–137. ISBN 5-7511-0073-5. [Google Scholar]
- Frizzi, G. Salivary gland chromosomes of Anopheles. Nature 1947, 160, 226–227. [Google Scholar] [CrossRef]
- Frizzi, G. Nuovi contributi e prospetti di ricerca nel gruppo Anopheles maculipennis in base allo studio del dimorfismo cromosomico. Symp. Genet. 1952, 3, 231–265. [Google Scholar]
- Frizzi, G. Etude cytogénétique d’Anopheles maculipennis en Italie. Bull. World Health Organ. 1953, 9, 335–344. [Google Scholar]
- Kiknadze, I.I. Chromosomes of Diptera. Evolutionary and practical significance. Genetika 1967, 7, 145–165. [Google Scholar]
- Kabanova, V.M.; Kartashova, N.N.; Stegnii, V.N. Karyological study of natural populations of malarial mosquitoes in the Middle Ob river. I. Characteristics of the karyotype of Anopheles maculipennis messeae. Tsitologiia 1972, 14, 630–636. [Google Scholar]
- Stegnii, V.N.; Kabanova, V.M. Cytoecological study of natural populations of malaria mosquitoes on the USSR territory. 1. Isolation of a new species of Anopheles in Maculipennis complex by the cytodiagnostic method. Med. Parazitol. (Mosk) 1976, 45, 192–198. [Google Scholar] [PubMed]
- Stegniy, V.N. Detection of chromosomal races in the malaria mosquito Anopheles sacharovi. Tsitologiia 1976, 18, 1039–1041. [Google Scholar]
- Collins, F.H.; Paskewitz, S.M. A review of the use of ribosomal DNA (rDNA) to differentiate among cryptic Anopheles species. Insect Mol. Biol. 1996, 5, 1–9. [Google Scholar] [CrossRef]
- Harbach, R.E. The classification of genus Anopheles (Diptera: Culicidae): A working hypothesis of phylogenetic relationships. Bull. Entomol. Res. 2004, 94, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Harbach, R.E. Review of the internal classification of the genus Anopheles (Diptera: Culicidae): The foundation for comparative systematics and phylogenetic research. Bull. Entomol. Res. 1994, 84, 331–342. [Google Scholar] [CrossRef]
- Fedoroff, N.V. On spacers. Cell 1979, 16, 697–710. [Google Scholar] [CrossRef]
- Sedaghat, M.M.; Linton, Y.-M.; Oshaghu, M.A.; Vatandoost, H.; Harbach, R.E. The Anopheles maculipennis complex in Iran: Molecular characterization and recognition of a new species. Bull. Entomol. Res. 2003, 93, 527–535. [Google Scholar] [CrossRef]
- Sedaghat, M.M.; Howard, T.; Harbach, R.E. Morphological study and description of Anopheles (Anopheles) persiensis, a member of the Maculipennis Group (Diptera: Culicidae: Anophelinae) in Iran. J. Entomol. Soc. Iran. 2009, 28, 25–35. [Google Scholar]
- Nicolescu, G.; Linton, Y.-M.; Vladimirescu, A.; Howard, T.M.; Harbach, R.E. Mosquitoes of the Anopheles maculipennis group (Diptera: Culicidae) in Romania, with the discovery and formal recognition of a new species based on molecular and morphological evidence. Bull. Entomol. Res. 2004, 94, 525–535. [Google Scholar] [CrossRef]
- Gordeev, M.I.; Zvantsov, A.B.; Goriacheva, I.I.; Shaĭkevich, E.V.; Ezhov, M.N. Description of the new species Anopheles artemievi sp.n. (Diptera, Culicidae). Med. Parazitol. (Mosk) 2005, 2, 4–5. [Google Scholar]
- Linton, Y.-M.; Smith, L.; Harbach, R.E. Observations on the taxonomic status of Anopheles subalpinus Hackett & Lewis and An. melanoon Hackett. Eur. Mosq. Bull. 2002, 13, 1–7. [Google Scholar]
- Marinucci, M.; Romi, R.; Mancini, P.; Di Luca, M.; Severini, C. Phylogenetic relationships of seven palearctic members of the maculipennis complex inferred from ITS2 sequence analysis. Insect Mol. Biol. 1999, 8, 469–480. [Google Scholar] [CrossRef]
- Proft, J.; Maier, W.A.; Kampen, H. Identification of six sibling species of the Anopheles maculipennis complex (Diptera: Culicidae) by a polymerase chain reaction assay. Parasitol. Res. 1999, 85, 837–843. [Google Scholar] [CrossRef]
- Harbach, R.E. The phylogeny and classification of Anopheles. In Anopheles Mosquitoes—New Insights into Malaria Vectors; Manguin, S., Ed.; InTech: Rijeka, Croatia, 2013; Chapter 1; pp. 3–55. [Google Scholar] [CrossRef]
- Hodge, J.M.; Yurchenko, A.A.; Karagodin, D.A.; Masri, R.A.; Smith, R.C.; Gordeev, M.I.; Sharakhova, M.V. The new Internal Transcribed Spacer 2 diagnostic tool clarifies the taxonomic position and geographic distribution of the North American malaria vector Anopheles punctipennis. Malar. J. 2021, 20, 141. [Google Scholar] [PubMed]
- Naumenko, A.N.; Karagodin, D.A.; Yurchenko, A.A.; Moskaev, A.V.; Martin, O.I.; Baricheva, E.M.; Sharakhov, I.V.; Gordeev, M.I.; Sharakhova, M.V. Chromosome and Genome Divergence between the Cryptic Eurasian Malaria Vector-Species Anopheles messeae and Anopheles daciae. Genes 2020, 11, 165. [Google Scholar] [CrossRef]
- Yurchenko, A.A.; Naumenko, A.N.; Artemov, G.N.; Karagodin, D.A.; Hodge, J.M.; Velichevskaya, A.I.; Kokhanenko, A.A.; Bondarenko, S.M.; Abai, M.R.; Kamali, M.; et al. Phylogenomics revealed migration routes and adaptive radiation timing of Holarctic malaria mosquito species of the Maculipennis Group. BMC Biol. 2023, 21, 63. [Google Scholar] [CrossRef]
- Khalin, A.V.; Gornostaeva, R.M. On the taxonomic composition of mosquitoes (Diptera: Culicidae) of the world and Russian fauna (critical review). Parazitologiia 2008, 42, 360–381. [Google Scholar]
- Shlenova, M.F. Observations on the biology of malarial mosquitoes in the vicinity of Sochi. Med. Parazitol. (Mosk) 1938, 7, 514–529. [Google Scholar]
- Lomeiko, E.I. Detection of Anopheles algariensis Theob. in the Adygean Autonomous Region. Med. Parazitol. (Mosk) 1942, 11, 131. [Google Scholar]
- Sigida, S.I. Blood-sucking mosquitoes (Diptera, Culicidae) of landscape provinces of the Stavropol Krai—Malaria vectors. Sci. Innov. Technol. 2021, 1, 53–64. [Google Scholar] [CrossRef]
- Gordeev, M.I.; Moskaev, A.V.; Perevozkin, V.P. Analysis of species and chromosomal composition of malaria mosquitoes of the Republic of Adygea. Bull. Mosc. State Reg. Univ. (Nat. Sci.) Bull. 2010, 3, 64–71. [Google Scholar]
- Gordeev, M.I.; Moskaev, A.V. Species composition and ecological characteristics of larval biotopes of malarial mosquitoes in the south of European Russia. Med. Parazitol. (Mosk) 2016, 4, 31–35. [Google Scholar]
- Razumeiko, V.N.; Ivashov, A.V.; Oberemok, V.V. Seasonal activity and density dynamics of blood-sucking mosquitoes (Diptera, Culicidae) in water bodies of the Southern Coast of Crimea. Sci. Notes V.I. Vernadsky Tauride Natl. Univ. Biol. Chem. 2010, 23, 114–128. [Google Scholar]
- Razumeiko, V.N.; Ivashov, A.V. Peculiarities of distribution of blood-sucking mosquitoes of Anopheles complex in the Salgir River basin. Ecosyst. Their Optim. Prot. 2011, 4, 78–83. [Google Scholar]
- Artova, M.A.; Razumeiko, V.N.; Simchuk, A.P. To the question of genetic polymorphism of sibling species of malaria mosquitoes Anopheles maculipennis complex in the Crimea. Sci. Notes V.I. Vernadsky Crime. Fed. Univ. Biol. Chem. 2014, 27, 3–11. [Google Scholar]
- Gutsevich, A.V. Blood-sucking mosquitoes of the Crimea. In Proceedings of the Crimean Branch of the Academy of Sciences of the USSR; Crimean Branch of the Academy of Sciences of the USSR: Simferopol, USSR, 1953; Volume 3, pp. 57–69. [Google Scholar]
- Alekseev, E.V. Biodiversity of blood-sucking mosquitoes (Diptera, Culicidae) of Crimea, its origin and epidemiological significance. Issues Crime. Dev. 2003, 15, 111–131. [Google Scholar]
- Alekseev, E.V. On the influence of anthropogenic transformations on the fauna of blood-sucking mosquitoes (Diptera, Culicidae) of the flat Crimea. Med. Parazitol. (Mosk) 1979, 5, 67–71. [Google Scholar]
- Gornostaeva, R.M. Analysis of modern data on the fauna and ranges of malaria mosquitoes (Diptera: Culicidae: Anopheles) on the territory of Russia. Parazitologiia 2003, 37, 298–305. [Google Scholar] [PubMed]
- Gornostaeva, R.M.; Danilov, A.V. On ranges of the malaria mosquitoes (Diptera: Culicidae: Anopheles) of the Maculipennis complex on the territory of Russia. Parazitologiia 2002, 36, 33–47. [Google Scholar]
- Benedict, M.; Dotson, E. Methods in Anopheles Research; Malaria Research and Reference Reagent Resource Center: Atlanta, GA, USA, 2015. [Google Scholar]
- Gutsevich, A.V.; Monchadskii, A.S.; Shtakelberg, A.A. Fauna of the USSR. Diptera. Mosquitoes; Family Culicidae; Zoological Institute, USSR Academy of Science: Leningrad, Russia, 1971; p. 407. [Google Scholar] [CrossRef]
- Fedorova, M.V.; Sycheva, K.A. Blood-Sucking Mosquitoes (Diptera:Culicidae) of the Krasnodar Territory and the Crimean Peninsula: An Identifier; Akimkin, V.G., Ed.; FBIS Central Research Institute of Epidemiology: Moscow, Russia, 2024; p. 220. [Google Scholar]
- Moskaev, A.V.; Gordeev, M.I.; Kuzmin, O.V. Chromosomal composition of populations of malaria mosquito Anopheles messeae in the centre and on the periphery of the species range. Bull. Mosc. State Reg. Univ. Nat. Sci. 2015, 1, 29–36. [Google Scholar]
- Stegniy, V.N.; Kabanova, V.M. Chromosomal analysis of malaria mosquitoes Anopheles atroparvus and A. maculipennis (Diptera, Culicidae). Zool. Zhurnal 1978, 57, 613–619. [Google Scholar]
- Artemov, G.N.; Fedorova, V.S.; Karagodin, D.A.; Brusentsov, I.I.; Baricheva, E.M.; Sharakhov, I.V.; Gordeev, M.I.; Sharakhova, M.V. New cytogenetic photomap and molecular diagnostics for the cryptic species of the malaria mosquitoes Anopheles messeae and Anopheles daciae from Eurasia. Insects 2021, 12, 835. [Google Scholar] [CrossRef]
- Corder, G.W.; Foreman, D.I. Nonparametric Statistics: A Step-By-Step Approach, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; p. 288. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Purification of nucleic acids by extraction with phenol:chloroform. CSH Protoc. 2006, 1, pdb-prot4455. [Google Scholar]
- Brusentsov, I.I.; Gordeev, M.I.; Yurchenko, A.A.; Karagodin, D.A.; Moskaev, A.V.; Hodge, J.M.; Burlak, V.A.; Artemov, G.N.; Sibataev, A.K.; Becker, N.; et al. Patterns of genetic differentiation imply distinct phylogeographic history of the mosquito species Anopheles messeae and Anopheles daciae in Eurasia. Mol. Ecol. 2023, 32, 5609–5625. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H.J. Primer 3 on the WWW for general users and biologist programmers. In Methods in Molecular Biology: Bioinformatics Methods and Protocols; Misener, S., Krawetz, S., Eds.; Humana Press: Totowa, NJ, USA, 2000; Volume 132, pp. 365–386. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, 115. [Google Scholar] [CrossRef]
- Gisinfo. Available online: https://gisinfo.net/download/download.htm#2 (accessed on 24 March 2025).
- Adobe. Available online: https://blog.adobe.com/en/publish/2019/11/04/adobe-illustrator-2020 (accessed on 24 March 2025).
- Technelysium. Available online: https://technelysium.com.au/wp/chromaspro/ (accessed on 24 March 2025).
- NCBI. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 24 March 2025).
- Statsoft. Available online: https://www.statsoft.de/en/discover-statistica/ (accessed on 24 March 2025).
- Moskaev, A.V.; Bega, A.G.; Panov, V.I.; Perevozkin, V.P.; Gordeev, M.I. Chromosomal polymorphism of malaria mosquitoes of Karelia and expansion of northern boundaries of species ranges. Russ. J. Genet. 2024, 60, 754–762. [Google Scholar] [CrossRef]
- Gordeev, M.I.; Moskaev, A.V.; Bezzhonova, O.V. Chromosomal polymorphism in the populations of malaria vector mosquito Anopheles messeae at the south of Russian plain. Russ. J. Genet. 2012, 48, 962–965. [Google Scholar] [CrossRef]
- Beklemishev, V.N. Ecology of the Malaria Mosquito; Medgiz: Moscow, Russia, 1944; pp. 1–299. [Google Scholar]
- Jetten, T.H.; Takken, W. Anophelism without Malaria in Europe: A Review of the Ecology and Distribution of the Genus Anopheles in Europe; Agric Univ Pap.: Wageningen, The Netherlands, 1994; Volume 94, pp. 1–69. [Google Scholar]
- Bertola, M.; Mazzucato, M.; Pombi, M.; Montarsi, F. Updated occurrence and bionomics of potential malaria vectors in Europe: A systematic review (2000–2021). Parasit. Vectors 2022, 15, 88. [Google Scholar] [CrossRef]
- Yanchilina, A.G.; Ryan, W.B.F.; McManus, J.F.; Dimitrov, P.; Dimitrov, D.; Slavova, K.; Filipova-Marinova, M. Compilation of geophysical, geochronological, and geochemical evidence indicates a rapid Mediterranean-derived submergence of the Black Sea’s shelf and subsequent substantial salinification in the early Holocene. Mar. Geol. 2017, 383, 14–34. [Google Scholar] [CrossRef]
- Gordeev, M.I.; Temnikov, A.A.; Panov, V.I.; Klimov, K.S.; Lee, E.Y.; Moskaev, A.V. Chromosomal variability in populations of malaria mosquitoes in different landscape zones of Eastern Europe and the Southern Urals. Bull. Mosc. State Reg. Univ. (Geogr. Environ. Living Syst.) 2022, 4, 48–66. [Google Scholar] [CrossRef]
- Alekseev, E.V.; Razumeiko, V.N. Blood-sucking mosquitoes (Diptera, Culicidae) of anthropogenic landscapes of flat Crimea. Ecosyst. Their Optim. Prot. 2005, 16, 120–129. [Google Scholar]
- Hubenov, Z. Species Composition and Distribution of the Dipterans (Insecta: Diptera) in Bulgaria. Advanced Books; National Museum of Natural History: Sofia, Bulgaria, 2021. [Google Scholar] [CrossRef]
- Șuleșco, T.; Sauer, F.G.; Lühken, R. Update on the distribution of Anopheles maculipennis s. l. members in the Republic of Moldova with the first record of An. daciae. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Bezzhonova, O.V.; Babuadze, G.A.; Gordeev, M.I.; Goriacheva, I.I.; Zvantsov, A.B.; Ezhov, M.N.; Imnadze, P.; Iosava, M.; Kurtsikashvili, G. Malaria mosquitoes of the Anopheles maculipennis (Diptera, Culicidae) complex in Georgia. Med. Parazitol. (Mosk) 2008, 3, 32–36. [Google Scholar]
- Akiner, M.M.; Cağlar, S.S. Identification of Anopheles maculipennis group species using polymerase chain reaction (PCR) in the regions of Birecik, Beyşehir and Cankiri. Turk. Parazitol. Derg. 2010, 34, 50–54. [Google Scholar]
- Simsek, F.M.; Ulger, C.; Akiner, M.M.; Tuncay, S.S.; Kiremit, F.; Bardakci, F. Molecular identification and distribution of Anopheles maculipennis complex in the Mediterranean region of Turkey. Biochem. Syst. Ecol. 2011, 39, 258–265. [Google Scholar] [CrossRef]
- Sharakhova, M.V.; Stegnii, V.N.; Braginets, O.P. Interspecies differences in the ovarian trophocyte precentromere heterochromatin structure and evolution of the malaria mosquito complex Anopheles maculipennis. Genetika 1997, 33, 1640–1648. (In Russian) [Google Scholar] [PubMed]
- Stegnii, V.N. Structure reorganization of the interphase nuclei during ontogenesis and phylogenesis of malaria mosquitoes. Dokl. Akad. Nauk. SSSR 1979, 249, 1231–1234. [Google Scholar] [PubMed]
- Stegnii, V.N. Systemic reorganization of the architectonics of polytene chromosomes in the onto- and phylogenesis of malaria mosquitoes. Genetika 1987, 23, 821–827. [Google Scholar]
- Stegnii, V.N.; Sharakhova, M.V. Structural features regional of chromosomal adhesion to the nuclear membrane. Genetika 1991, 27, 828–835. [Google Scholar]
- Kadamov, D.S.; Zvantseva, A.B.; Karimov, S.S.; Gordeev, M.I.; Goriacheva, I.I.; Ezhov, M.N.; Tadzhiboev, A. Malaria mosquitoes (Diptera, Culicidae, Anopheles) of North Tajikistan, their ecology, and role in the transmission of malaria pathogens. Med. Parazitol. (Mosk) 2012, 3, 30–34. [Google Scholar]
- Perevozkin, V.P. Chromosomal polymorphism of malarial mosquitoes (Diptera, Culicidae) of Primorsky Krai. Tomsk. State Pedagog. Univ. Bull. 2009, 11, 181–185. [Google Scholar]
- Khrabrova, N.V.; Andreeva, Y.V.; Sibataev, A.K.; Alekseeva, S.S.; Esenbekova, P.A. Mosquitoes of Anopheles hyrcanus (Diptera, Culicidae) group: Species diagnostic and phylogenetic relationships. Am. J. Trop. Med. Hyg. 2015, 93, 619–622. [Google Scholar] [CrossRef]
- Agarkova-Lyakh, I.V. Natural complexes of the coastal zone of the Southern coast of Crimea. Sci. Notes V.I. Vernadsky Crime. Fed. Univ. Geol. 2015, 1, 42–58. [Google Scholar]
- Gordeev, M.I.; Zvantsov, A.B.; Goriacheva, I.I.; Shaĭkevich, E.V.; Ezhov, M.N.; Usenbaev, N.T.; Shapieva, Z.Z.; Zhakhongirov, S.M. Anopheles mosquitoes (Diptera, Culicidae) of the Tien Shan: Morphological, cytogenetic, and molecular genetic analysis. Med. Parazitol. (Mosk) 2008, 3, 25–32. [Google Scholar]
- Service, M.W. 1968. Observations on feeding and oviposition in some British mosquitoes. Entomol. Exp. Appl. 1968, 11, 277–285. [Google Scholar] [CrossRef]
- Schaffner, F.; Thiéry, I.; Kaufmann, C.; Zettor, A.; Lengeler, C.; Mathis, A.; Bourgouin, C. Anopheles plumbeus (Diptera: Culicidae) in Europe: A mere nuisance mosquito or potential malaria vector? Malar. J. 2012, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Heym, E.C.; Kampen, H.; Fahle, M.; Hohenbrink, T.L.; Schäfer, M.; Scheuch, D.E.; Walther, D. Anopheles plumbeus (Diptera: Culicidae) in Germany: Updated geographic distribution and public health impact of a nuisance and vector mosquito. Trop. Med. Int. Health 2017, 22, 103–112. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–577. [Google Scholar] [CrossRef]
- Bueno-Marí, R.; Jiménez-Peydró, R. Anopheles plumbeus Stephens, 1828: A neglected malaria vector in Europe. Malar. Rep. 2011, 1, 2. [Google Scholar] [CrossRef]
- Dekoninck, W.; Hendrickx, F.; Vasn Bortel, W.; Versteirt, V.; Coosemans, M.; Damiens, D.; Hance, T.; De Clercq, E.M.; Hendrickx, G.; Schaffner, F.; et al. Human-induced expanded distribution of Anopheles plumbeus, experimental vector of West Nile virus and a potential vector of human malaria in Belgium. J. Med. Entomol. 2011, 48, 924–928. [Google Scholar] [CrossRef]
- Bega, A.G.; Moskaev, A.V.; Gordeev, M.I. Ecology and distribution of the invasive mosquito species Aedes albopictus (Skuse, 1895) in the south of the European Part of Russia. Russ. J. Biol. Invasions 2021, 12, 148–156. [Google Scholar] [CrossRef]
- Marchant, P.; Eling, W.; van Gemert, G.J.; Leake, C.J.; Curtis, C.F. Could british mosquitoes transmit falciparum malaria? Parasitol. Today 1998, 14, 344–345. [Google Scholar] [CrossRef]
- Bueno-Marí, R.; Jiménez-Peydró, R. Study of the malariogenic potential of Eastern Spain. Trop. Biomed. 2012, 29, 39–50. [Google Scholar]
- Krüger, A.; Rech, A.; Su, X.Z.; Tannich, E. Two cases of autochthonous Plasmodium falciparum malaria in Germany with evidence for local transmission by indigenous Anopheles plumbeus. Trop. Med. Int. Health 2001, 6, 983–985. [Google Scholar] [CrossRef]
- Medlock, J.M.; Snow, K.R.; Leach, S. Potential transmission of West Nile virus in the British Isles: An ecological review of candidate mosquito bridge vectors. Med. Vet. Entomol. 2005, 19, 2–21. [Google Scholar] [CrossRef]
- Medlock, J.M.; Snow, K.R.; Leach, S. Possible ecology and epidemiology of medically important mosquito-borne arboviruses in Great Britain. Epidemiol. Infect. 2007, 135, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Shcherbina, V.P. Materials on the fauna of blood-sucking mosquitoes (Diptera, Culicidae) of the Lower Don and North Caucasus. Parasitol. Sb. ZIN AS USSR 1974, 26, 205–217. [Google Scholar]
- Enikolopov, S.K. Biology of Anopheles algeriensis Theo. Med. Parazitol. (Mosk) 1944, 13, 68–69. [Google Scholar]
- Enikolopov, S.K. On the ecology of Anopheles algeriensis Theo. 1903. Med. Parazitol. (Mosk) 1937, 6, 354–359. [Google Scholar]
- Savitskiĭ, B.P. Blood-sucking mosquitoes (Culicidae) attacking man in the region of the Eastern Manych (Kalmyk ASSR). Parazitologiia 1982, 16, 163–165. [Google Scholar] [PubMed]
- Tippelt, L.; Walther, D.; Scheuch, D.E.; Schäfer, M.; Kampen, H. Further reports of Anopheles algeriensis Theobald, 1903 (Diptera: Culicidae) in Germany, with evidence of local mass development. Parasitol. Res. 2018, 117, 2689–2696. [Google Scholar] [CrossRef]
- Perevozkin, V.P.; Bondarchuk, S.S.; Gordeev, M.I. The population-and-species-specific structure of malaria (Diptera, Culicidae) mosquitoes in the Caspian Lowland and Kuma-Manych Hollow. Med. Parazitol. (Mosk). 2012, 1, 12–17. [Google Scholar]
- Martens, W.J.M.; Kovats, R.S.; Nijhof, S.; deVries, P.; Livermore, M.J.T.; Mc Michael, A.J.; Bradley, D.; Cox, J. Climate change and future populations at risk of malaria. Glob. Environ. Change 1999, 9, S89–S107. [Google Scholar] [CrossRef]
- Yasjukevich, V.V. Malaria in Russia and its immediate geographical environment: Analysis of the situation in connection with the expected climate change. Probl. Ecol. Monit. Model. Ecosyst. 2002, 18, 142–157. [Google Scholar]
- Yasjukevich, V.V.; Titkina, S.N.; Popov, I.O.; Davidovich, E.A.; Yasjukevich, N.V. Climate-dependent diseases and arthropod vectors: Possible impact of climate change observed in Russia. Probl. Ecol. Monit. Model. Ecosyst. 2013, 25, 314–359. [Google Scholar]

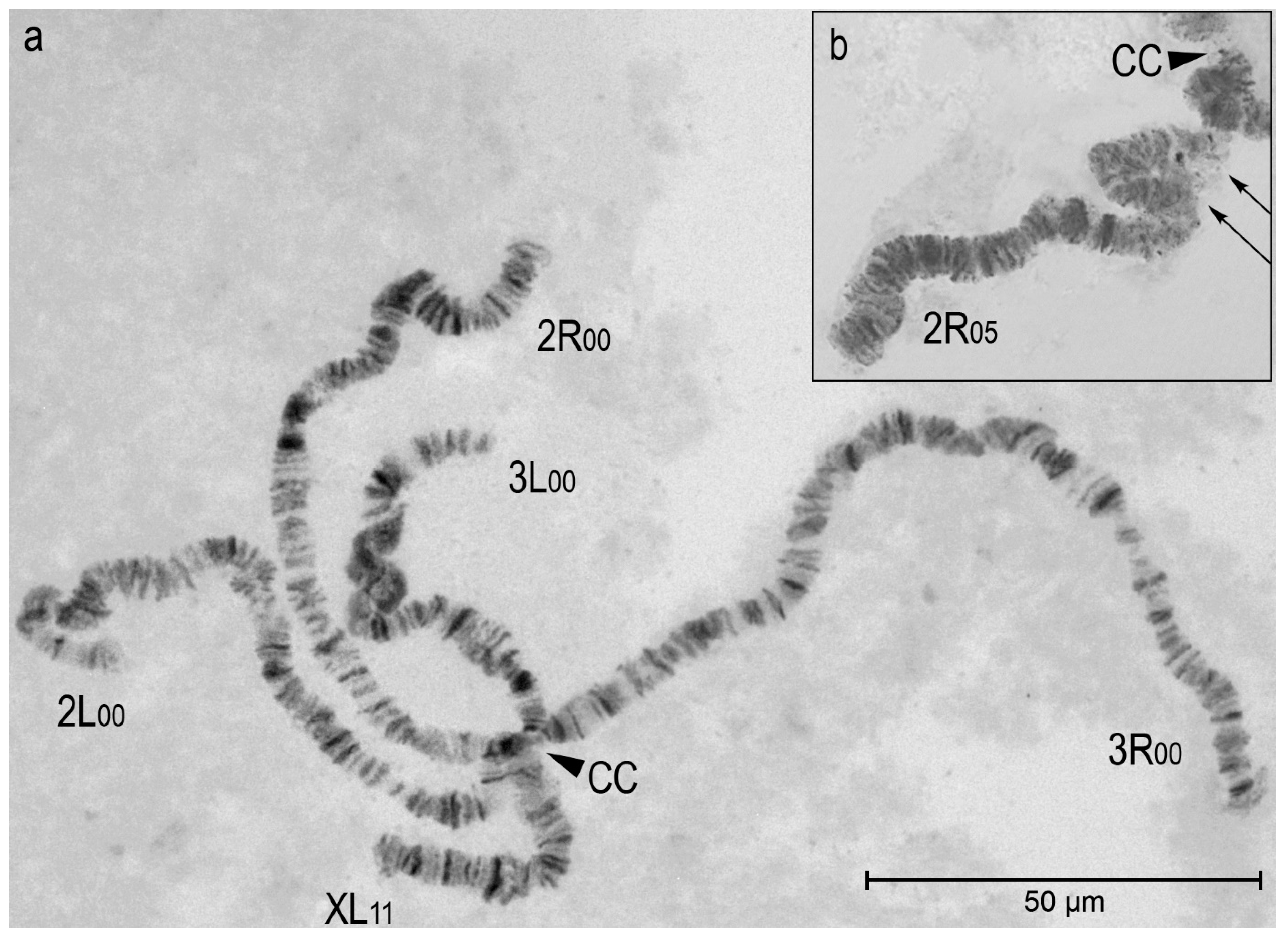
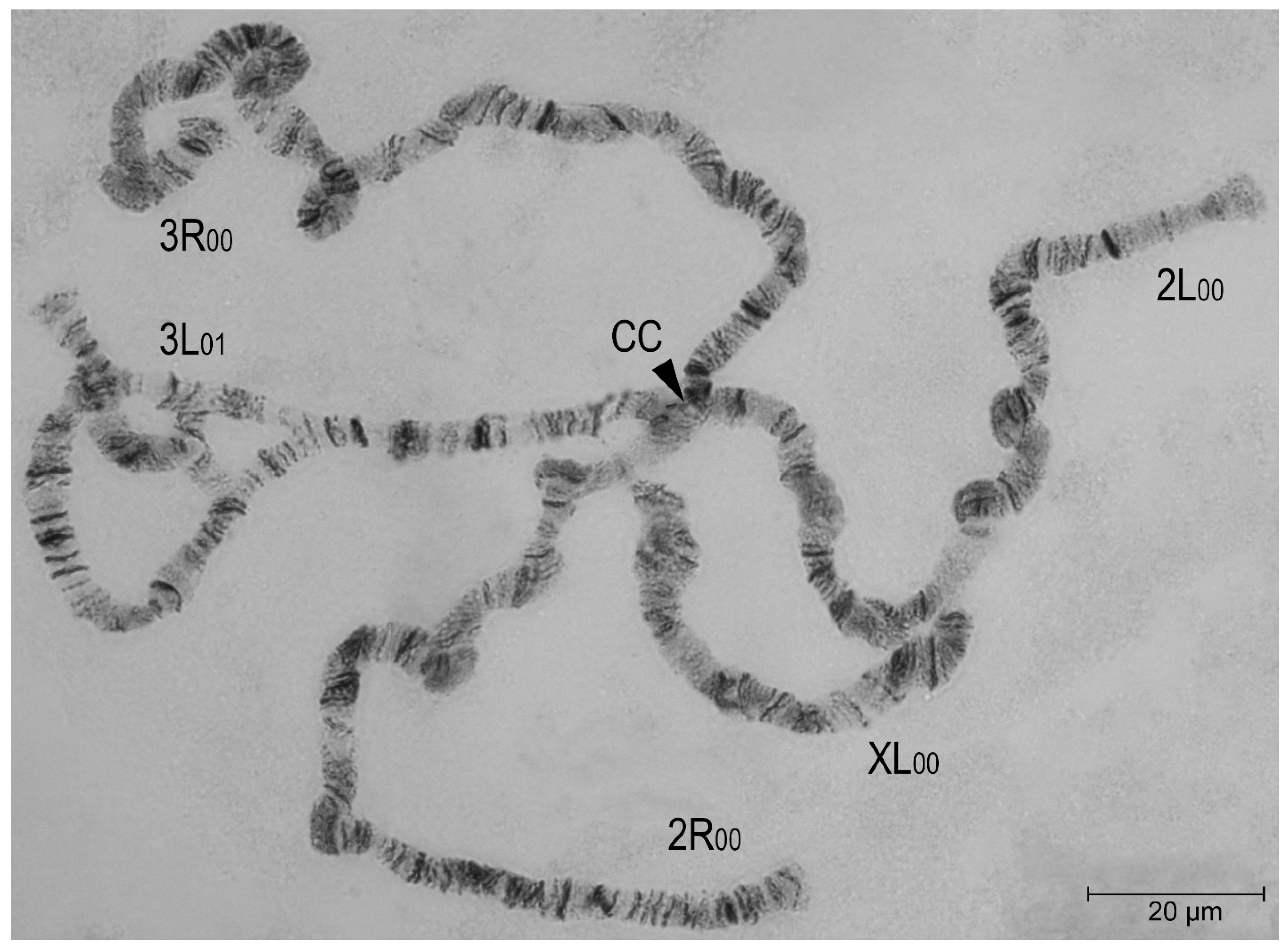
| No. | Location/Breeding Habitat Type | Latitude | Longitude | Date of Sampling | Number (%) of Mosquitoes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | AT | CL | HY | MA | DA | PL | ML | |||||
| Crimean Peninsula | ||||||||||||
| 1 | Pirogovka village, Nakhimov district of Sevastopol/ water storage | 44.685296 | 33.739026 | 11.09.2016 | 18 | - | - | - | 18 (100) | - | - | - |
| 2 | Bakhchisaray town/pond | 44.763889 | 33.853056 | 10.09.2016 | 12 | 3 (25.0) | - | - | 9 (75.0) | - | - | - |
| 3 | Bakhchisaray town/ dried-up creek | 44.763889 | 33.853611 | 10.09.2016 | 10 | - | - | - | 10 (100) | - | - | - |
| 4 | Simferopol city, botanical garden/pond | 44.939167 | 34.133056 | 10.09.2016 | 15 | - | - | - | 15 (100) | - | - | - |
| 5 *1 | Mazanka village, Simferopol district/pond | 45.014861 | 34.235861 | 12.07.2016 | 57 | - | - | - | 57 (100) | - | - | - |
| 6 | Konstantinovka village, Simferopol district/lake | 44.856389 | 34.123333 | 20.08.2016 | 3 | - | - | - | 3 (100) | - | - | - |
| 7 | Mramornoye village, Simferopol district/lake | 44.813889 | 34.237222 | 20.06.2016 | 9 | - | - | - | 7 (77.8) | - | 2 (22.2) | - |
| 8 | Mezhgorye village, Belogorsky district/river | 44.970556 | 34.416111 | 20.06.2016 | 9 | - | 5 (55.6) | - | 4 (44.4) | - | - | - |
| 9 | Krasnosyolovka village, Belogorsky district/river spill | 44.917778 | 34.633333 | 20.06.2016 | 2 | - | - | - | - | - | 2 (100) | - |
| 10A | Tylovoye village, Balaklava district of Sevastopol/pond | 44.441389 | 33.728056 | 13.09.2016 | 128 | 2 (1.6) | - | - | 60 (46.9) | 66 (51.5) | - | - |
| 10B *2 | 44.443570 | 33.739879 | 12.08.2017 | 21 | - | - | - | 12 (57.1) | 9 (42.9) | - | - | |
| 10C | 84 | 1 (1.2) | - | - | 83 (98.8) | - | - | - | ||||
| 10D *3 | 44.441740 | 33.727469 | 08.08.2019 | 53 | - | - | - | 23 (43.4) | 30 (56.6) | - | - | |
| 10E | 95 | - | - | - | 55 (57.9) | 40 (42.1) | - | - | ||||
| 11 | Rodnikovoye village, Balaklava district of Sevastopol/puddle | 44.453611 | 33.862222 | 20.07.2016 | 3 | - | - | - | - | - | 3 (100) | - |
| 12 | Rodnikovoye village, Balaklava district of Sevastopol/tree hollow | 44.457222 | 33.872778 | 20.07.2016 | 7 | - | - | - | - | - | 7 (100) | - |
| 13 | Simeiz Settlement, Yalta district/mountain puddle | 44.403611 | 33.991667 | 20.06.2016 | 5 | - | - | - | - | - | 5 (100) | - |
| 14 | Yalta district/forest puddle | 44.516389 | 34.143889 | 20.07.2016 | 37 | - | - | - | 37 (100) | - | - | - |
| 15 | Gaspra settlement, Yalta district/water in rock cracks | 44.433611 | 34.130000 | 20.06.2016 | 3 | - | - | - | - | - | 3 (100) | - |
| 16 | Gaspra settlement, Yalta district/tree hollow | 44.445278 | 34.118889 | 20.06.2016 | 2 | - | - | - | - | - | 2 (100) | - |
| 17 | Voskhod settlement, Yalta district/lake | 44.517417 | 34.219796 | 20.08.2016 | 8 | - | 8 (100) | - | - | - | - | - |
| 18 | Nikitsky Botanical Gardens, Yalta district/pond | 44.508831 | 34.233093 | 13.09.2016 | 7 | - | - | - | 1 (14.3) | - | 6 (85.7) | - |
| 19 | Krasnokamenka village, Yalta district/forest puddle | 44.577500 | 34.255556 | 20.06.2016 | 2 | - | - | - | - | - | 2 (100) | - |
| 20 | Zaprudnoye village, Alushta district/lake | 44.599444 | 34.305000 | 20.08.2016 | 14 | - | 14 (100) | - | - | - | - | - |
| 21 | Nizhnyaya Kutuzovka village, Alushta district/pond | 44.709842 | 34.377544 | 14.09.2016 | 31 | - | - | - | 31 (100) | - | - | - |
| 22 | Alushta district/pond | 44.814167 | 34.657778 | 14.09.2016 | 12 | - | - | - | 12 (100) | - | - | - |
| 23 | Gromovka village, Sudak district/pond | 44.857778 | 34.791389 | 20.06.2016 | 3 | - | 3 (100) | - | - | - | - | - |
| 24 | Voron village, Sudak district/spring | 44.892222 | 34.820278 | 20.08.2016 | 3 | - | 3 (100) | - | - | - | - | - |
| 25 | Veseloye village, Sudak district/water reserve | 44.849722 | 34.883611 | 15.09.2016 | 9 | - | - | - | 9 (100) | - | - | - |
| 26 | Sudak district/pond | 44.868611 | 34.900556 | 15.09.2016 | 3 | - | - | - | 3 (100) | - | - | - |
| 27 | Veseloye village, Sudak district/lake | 44.851944 | 34.883333 | 20.08.2016 | 2 | - | 2 (100) | - | - | - | - | - |
| 28 | Dachnoye village, Sudak district/river spill | 44.888889 | 34.990278 | 20.08.2016 | 1 | - | - | 1 (100) | - | - | - | - |
| 29 | Dachnoye village, Sudak district/lake | 44.897362 | 35.040173 | 20.08.2016 | 4 | - | - | 4 (100) | - | - | - | - |
| 30 | Mindalnoye village, Sudak district/lake | 44.831756 | 35.082243 | 20.07.2016 | 3 | - | - | 3 (100) | - | - | - | - |
| 31 | Grushevka village, Sudak district/lake | 45.010570 | 34.971796 | 15.09.2016 | 48 | - | - | - | 48 (100) | - | - | - |
| 32 | Feodosia city/pond | 45.063792 | 35.341071 | 16.09.2016 | 9 | 7 (77.8) | - | - | 2 (22.2) | - | - | - |
| Black Sea coast of the Caucasus | ||||||||||||
| 33 | Krasnogvardeyskoye village, Stavropol Krai/dried up river | 45.850476 | 41.482381 | 12.08.2015 | 27 | 27 (100) | - | - | - | - | - | - |
| 34 | Stavropol city/pond | 45.013332 | 41.974723 | 12.08.2015 | 30 | - | - | - | 26 (86.7) | 4 (13.3) | - | - |
| 35 | Malevanyi settlement, Krasnodar Krai/river | 45.531517 | 39.461591 | 21.08.2015 | 50 | - | - | - | 1 (2.0) | 49 (98.0) | - | - |
| 36 | Razdolnaya village, Korenovsky district, Krasnodar Krai/lake | 45.383469 | 39.537257 | 01.08.2024 | 100 | - | - | 18 (18.0) | 52 (52.0) | 30 (30.0) | - | - |
| 37 | Shengzhiy settlement, Republic of Adygeya/channel | 44.883810 | 39.075139 | 05.08.2009 | 54 | - | - | 1 (1.9) | 2 (3.7) | 51 (94.4) | - | - |
| 38 | Novonikolayevskaya village, Krasnodar Krai/pond | 45.581165 | 38.369233 | 04.08.2019 | 120 | - | - | - | - | 120 (100) | - | - |
| 39 | Tamanskoye Settlement, Temryuksky district, Krasnodar Krai/lake | 45.144803 | 36.700849 | 07.07.2016 | 35 | 35 (100) | - | - | - | - | - | - |
| 40 *4 | Abinsk town, Krasnodar Krai/pond | 44.862380 | 38.183556 | 14.08.2018 | 108 | - | - | - | - | 108 (100) | - | - |
| 41 | Gaiduk village, Novorossiysk district, Krasnodar Krai/pond | 44.781486 | 37.679653 | 03.08.2018 | 100 | - | - | - | - | 100 (100) | - | - |
| 42 | Novorossiysk district, Krasnodar Krai/water storage | 44.780000 | 37.815833 | 09.07.2016 | 108 | - | - | - | 1 (0.9) | 107 (99.1) | - | - |
| 43 | Pshada village, Gelendzhik district, Krasnodar Krai/river | 44.452257 | 38.346501 | 19.08.2015 | 106 | - | - | - | 70 (66.0) | 36 (34.0) | - | - |
| 44 | Community Zarya, Tuapse district, Krasnodar Krai/ car tire | 44.082778 | 39.131667 | 31.07.2021 | 36 | - | - | - | - | - | 36 (100) | - |
| 45 | Novomikhailovsky settlement, Tuapse district, Krasnodar Krai/drainage ditch | 44.247752 | 38.844524 | 18.08.2015 | 100 | - | 100 (100) | - | - | - | - | - |
| 46 | Agui-Shapsug village, Tuapse district, Krasnodar Krai/river | 44.174722 | 39.066944 | 11.07.2016 | 35 | - | - | - | 35 (100) | - | - | - |
| 47 | Zubova Shchel village, Sochi district, Krasnodar Krai/river | 43.837451 | 39.441109 | 13.07.2016 | 34 | - | - | - | 34 (100) | - | - | - |
| 48 | Sochi city, Krasnodar Krai/tree hollow | 43.675833 | 39.608889 | 30.07.2021 | 60 | - | - | - | - | - | 60 (100) | - |
| 49 | Adler town, Krasnodar Krai/ swamp | 43.432222 | 39.947222 | 17.07.2016 | 32 | - | - | - | 32 (100) | - | - | - |
| 50A | Verkhneveseloye village, Sochi district, Krasnodar Krai/drainage ditch | 43.426067 | 39.973288 | 04.08.2023 | 14 | - | 14 (100) | - | - | - | - | - |
| 50B *5 | 6 | - | 6 (100) | - | - | - | - | - | ||||
| 50C | 43.426306 | 39.973515 | 04.08.2024 | 3 | - | 3 (100) | - | - | - | - | - | |
| 51 *6 | Sochi city, Krasnodar Krai/stream | 43.410321 | 39.983947 | 07.08.2024 | 2 | - | - | - | - | - | - | 2 (100) |
| 52 | Sirius settlement, Krasnodar Krai/fire pond | 43.412778 | 39.937778 | 16.07.2016 | 156 | - | - | - | 156 (100) | - | - | - |
| 53 | Vesyoloye microdistrict, Sochi city, Krasnodar Krai/car tire | 43.409722 | 40.008330 | 11.08.2018 | 32 | - | - | - | - | - | 32 (100) | - |
| 54 | Krasnaya Polyana Resort, Sochi district, Krasnodar Krai/hollow tree | 43.711944 | 40.209167 | 25.07.2021 | 94 | - | - | - | - | - | 94 (100) | - |
| 55 | Rosa Khutor resort, Sochi district, Krasnodar Krai/ hollow tree | 43.638978 | 40.307983 | 29.07.2021 | 39 | - | - | - | - | - | 39 (100) | - |
| 56 | Ritsinsky National Park, Gudauta district, Abkhazia/ car tire | 43.473889 | 40.538056 | 24.07.2021 | 16 | - | - | - | - | - | 16 (100) | - |
| Total | 2229 | 75 | 158 | 27 | 908 | 750 | 309 | 2 | ||||
| No. | Location/Breeding Place | Date of Sampling | Density of Larvae (1–4 Instars/sq. m) | Ecological Characteristics of Habitats | ||||
|---|---|---|---|---|---|---|---|---|
| h (m) | pH | T (°C) | ppt | O2 (mg/L) | ||||
| Crimean Peninsula | ||||||||
| 1 | Pirogovka village, Nakhimov district of Sevastopol/water storage | 11.09.2016 | 45 | 63 | 8.05 | 30.0 | 0.34 | 6.0 |
| 2 | Bakhchisaray town/pond | 10.09.2016 | 28 | 160 | 8.05 | 18.0 | 0.59 | 7.7 |
| 3 | Bakhchisaray town/dried-up creek | 10.09.2016 | 160 | 160 | 7.70 | 20.7 | 0.70 | 4.5 |
| 4 | Simferopol city, botanical garden/pond | 10.09.2016 | 76 | 255 | 6.48 | 22.6 | 0.16 | 4.5 |
| 5 | Mazanka village, Simferopol district/pond | 12.07.2016 | - | 298 | 8.15 | 24.5 | 0.26 | 7.0 |
| 6 | Konstantinovka village, Simferopol district/lake | 20.08.2016 | 3 | 421 | 7.84 | 24.8 | 2.56 | - |
| 7 | Mramornoye village, Simferopol district/lake | 20.06.2016 | 7 | 493 | 7.62 | 16.5 | 2.14 | - |
| 8 | Mezhgorye village, Belogorsky district/river | 20.06.2016 | 9 | 385 | 7.22 | 21.6 | 1.21 | - |
| 9 | Krasnosyolovka village, Belogorsky district/river spill | 20.06.2016 | 2 | 401 | 7.62 | 18.3 | 1.94 | - |
| 10 | Tylovoye village, Balaklava district of Sevastopol/pond | 13.09.2016 | 15 | 295 | 8.15 | 22.1 | 0.22 | 7.8 |
| 11 | Rodnikovoye village, Balaklava district of Sevastopol/puddle | 20.07.2016 | 3 | 657 | 7.32 | 32.3 | 0.14 | - |
| 12 | Rodnikovoye village, Balaklava district of Sevastopol/tree hollow | 20.07.2016 | 7 | 434 | 7.14 | 24.7 | 0.04 | - |
| 13 | Simeiz Settlement, Yalta District/mountain puddle | 20.06.2016 | 5 | 149 | 8.32 | 18.0 | 0.17 | - |
| 14 | Yalta district/forest puddle | 20.07.2016 | 7 | 212 | 7.24 | 19.7 | 0.15 | - |
| 15 | Gaspra settlement, Yalta District/water in rock cracks | 20.06.2016 | 3 | 24 | 7.52 | 24.8 | 0.31 | - |
| 16 | Gaspra settlement, Yalta district/tree hollow | 20.06.2016 | 2 | 338 | 7.16 | 25.1 | 0.03 | - |
| 17 | Voskhod settlement, Yalta district/lake | 20.08.2016 | 8 | 330 | 7.30 | 21.5 | 0.24 | - |
| 18 | Nikitsky Botanical Gardens, Yalta district/pond | 13.09.2016 | 15 | 110 | 7.37 | 20.2 | 0.31 | 7.5 |
| 19 | Krasnokamenka village, Yalta district/forest puddle | 20.06.2016 | 2 | 770 | 7.23 | 14.9 | 0.11 | - |
| 20 | Zaprudnoye village, Alushta district/lake | 20.08.2016 | 14 | 614 | 7.14 | 17.5 | 0.21 | - |
| 21 | Nizhnyaya Kutuzovka village, Alushta district/pond | 14.09.2016 | 78 | 153 | 7.29 | 25.2 | 0.20 | 10.2 |
| 22 | Alushta district/pond | 14.09.2016 | 70 | 70 | 7.05 | 22.4 | 0.98 | 4.4 |
| 23 | Gromovka village, Sudak district/pond | 20.06.2016 | 3 | 157 | 7.52 | 16.5 | 2.08 | - |
| 24 | Voron village, Sudak district/spring | 20.08.2016 | 3 | 229 | 7.72 | 16.7 | 0.74 | - |
| 25 | Veseloye village, Sudak district/water reserve | 15.09.2016 | - | 131 | 7.28 | 23.2 | 0.81 | 2.3 |
| 26 | Sudak district/pond | 15.09.2016 | - | 125 | 8.31 | 22.6 | 0.25 | - |
| 27 | Veseloye village, Sudak district/lake | 20.08.2016 | 2 | 101 | 7.42 | 23.7 | 0.24 | - |
| 28 | Dachnoye village, Sudak district/river spill | 20.08.2016 | - | 76 | 7.47 | 22.4 | 3.02 | - |
| 29 | Dachnoye village, Sudak district/lake | 20.08.2016 | 4 | 239 | 7.54 | 23.8 | 0.92 | - |
| 30 | Mindalnoye village, Sudak district/lake | 20.07.2016 | 3 | 33 | 8.24 | 26.3 | 1.18 | - |
| 31 | Grushevka village, Sudak district/lake | 15.09.2016 | 29 | 223 | 6.93 | 22.0 | 0.27 | 8.0 |
| 32 | Feodosia city/pond | 16.09.2016 | 26 | 20 | 7.14 | 24.3 | 1.46 | 16.5 |
| Black Sea coast of the Caucasus | ||||||||
| 33 | Krasnogvardeyskoye village, Stavropol Krai/dried up river | 12.08.2015 | 1 | 60 | 9.10 | 28.0 | 5.99 | 11.3 |
| 34 | Stavropol city/pond | 12.08.2015 | - | 484 | 8.85 | 26.5 | 0.20 | 7.9 |
| 35 | Malevanyi settlement, Krasnodar Krai/river | 21.08.2015 | 1 | 40 | 8.00 | 24.0 | 1.58 | 6.9 |
| 36 | Razdolnaya village, Korenovsky district, Krasnodar Krai/lake | 01.08.2024 | 10 | 47 | 8.06 | 25.6 | 1.01 | 10.0 |
| 37 | Shengzhiy settlement, Republic of Adygeya/channel | 05.08.2009 | - | 44 | 7.00 | 33.3 | 0.81 | - |
| 38 | Novonikolayevskaya village, Krasnodar Krai/pond | 04.08.2019 | 29 | 2 | 8.50 | 25.5 | 0.19 | 8.0 |
| 39 | Tamanskoye Settlement, Temryuksky district, Krasnodar Region/lake | 07.07.2016 | 11 | 155 | 8.00 | 20.5 | - | 8.0 |
| 40 | Abinsk town, Krasnodar Krai/pond | 14.08.2018 | - | 29 | 7.60 | 30.0 | 0.27 | 5.0 |
| 41 | Gaiduk village, Novorossiysk district, Krasnodar Krai/pond | 03.08.2018 | 16 | 98 | 7.40 | 26.2 | 0.27 | 4.0 |
| 42 | Novorossiysk District, Krasnodar Krai/water storage | 09.07.2016 | 14 | 162 | 8.40 | 21.5 | - | 5.0 |
| 43 | Pshada village, Gelendzhik District, Krasnodar Krai/river | 19.08.2015 | 56 | 27 | 7.30 | 21.8 | 0.48 | 8.1 |
| 44 | Community Zarya, Tuapse district, Krasnodar Krai/car tire | 31.07.2021 | - | 172 | 5.50 | 26.5 | 1.45 | 2.5 |
| 45 | Novomikhailovsky settlement, Tuapse district, Krasnodar Krai/drainage ditch | 18.08.2015 | 32 | 5 | 7.54 | 23.4 | 0.45 | 2.5 |
| 46 | Agui-Shapsug village, Tuapse district, Krasnodar Krai/river | 11.07.2016 | 67 | 31 | 7.80 | 22.0 | - | 8.0 |
| 47 | Zubova Shchel village, Sochi district, Krasnodar Krai/river | 13.07.2016 | 72 | 175 | 7.80 | 24.0 | - | 8.0 |
| 48 | Sochi city, Krasnodar Krai/tree hollow | 30.07.2021 | - | 74 | 6.00 | 24.0 | 2.04 | 3.0 |
| 49 | Adler town, Krasnodar Krai/ swamp | 17.07.2016 | 1,5 | 45 | 8.20 | - | - | 6 |
| 50 | Verkhneveseloye village, Sochi district, Krasnodar Krai/drainage ditch | 04.08.2024 | 3 | 31 | 9.26 | 27.8 | 0.54 | 7.6 |
| 51 | Sochi city, Krasnodar Krai/stream | 07.08.2024 | - | 14 | 7.60 | - | - | - |
| 52 | Sirius settlement, Krasnodar Krai/fire pond | 16.07.2016 | 18 | 15 | 8.40 | 27.0 | - | 4 |
| 53 | Vesyoloye microdistrict, Sochi city, Krasnodar Krai/car tire | 11.08.2018 | - | 13 | 7.40 | 27.3 | 0.25 | - |
| 54 | Krasnaya Polyana Resort, Sochi district, Krasnodar Krai/hollow tree | 25.07.2021 | - | 1693 | 5.50 | 22.6 | 2.30 | 3.5 |
| 55 | Rosa Khutor resort, Sochi district, Krasnodar Krai/hollow tree | 29.07.2021 | - | 1708 | 5.20 | 21.5 | 1.50 | 3.0 |
| 56 | Ritsinsky National Park, Gudauta district, Abkhazia/car tire | 24.07.2021 | - | 1041 | 6.00 | 21.3 | 2.78 | 2.5 |
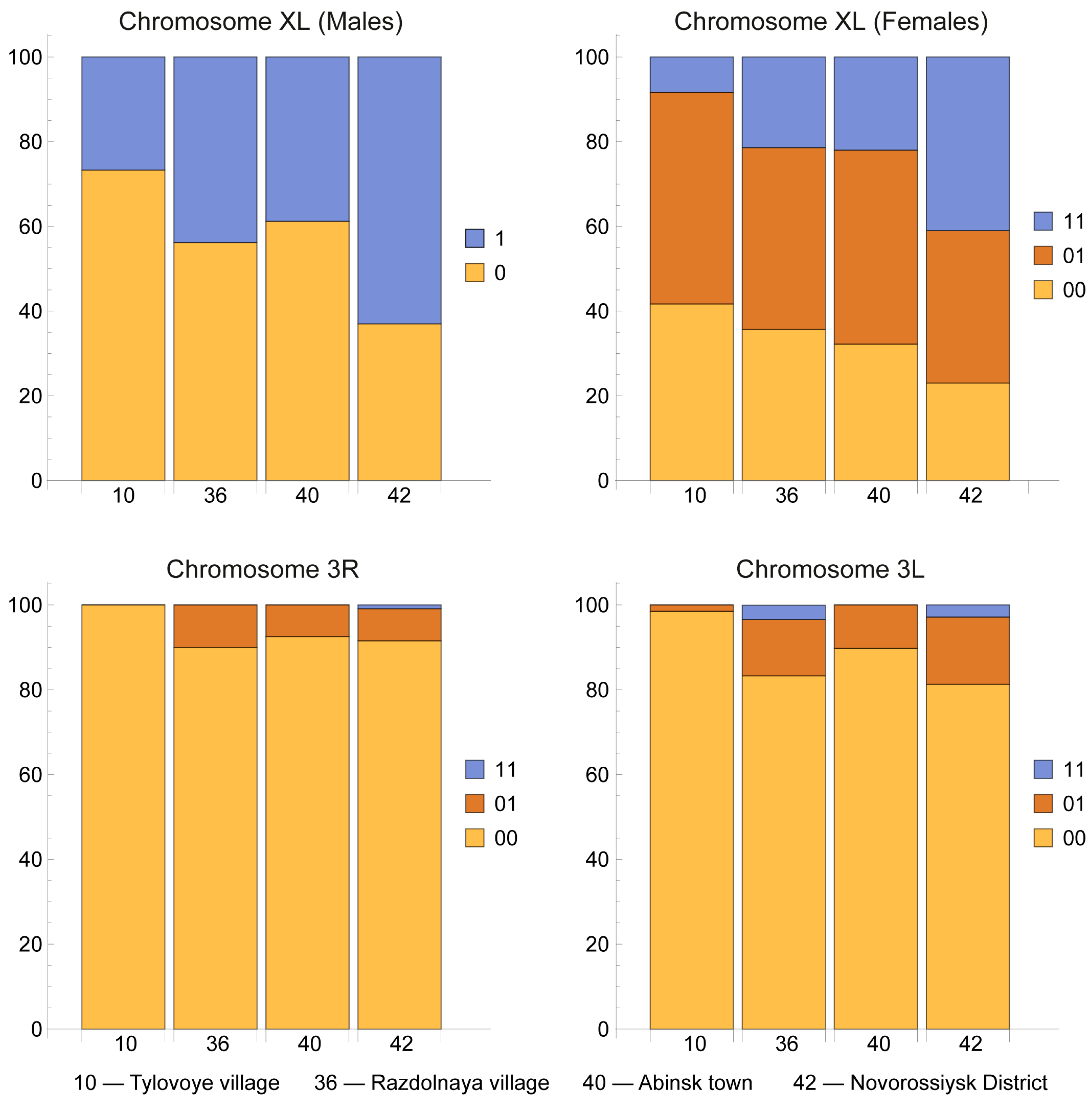
| Inversion Homo- and Heterozygotes | Frequencies of Chromosomal Variants, f ± Sf, % | |||
|---|---|---|---|---|
| Crimean Peninsula | Black Sea Coast of the Caucasus | |||
| Location 10 | Location 36 | Location 40 | Location 42 | |
| Males, n | 30 | 16 | 49 | 46 |
| XL0 | 73.3 ± 8.1 | 56.2 ± 12.4 | 61.2 ± 7.0 | 37.0 ± 7.1 |
| XL1 | 26.7 ± 8.1 | 43.8 ± 12.4 | 38.8 ± 7.0 | 63.0 ± 7.1 |
| Females, n | 36 | 14 | 59 | 61 |
| XL00 | 41.7 ± 8.2 | 35.7 ± 12.8 | 32.2 ± 6.1 | 23.0 ± 5.4 |
| XL01 | 50.0 ± 8.3 | 42.9 ± 13.2 | 45.8 ± 6.5 | 36.0 ± 6.1 |
| XL11 | 8.3 ± 4.6 | 21.4 ± 11.0 | 22.0 ± 5.4 | 41.0 ± 6.3 |
| Both sexes, n | 66 | 30 | 108 | 107 |
| 2R00 | 100 | 100 | 100 | 99.1 ± 0.9 |
| 2R05 | 0 | 0 | 0 | 0.9 ± 0.9 |
| 2L00 | 100 | 100 | 100 | 100 |
| 3R00 | 100 | 90.0 ± 5.5 | 92.6 ± 2.5 | 91.6 ± 2.7 |
| 3R01 | 0 | 10.0 ± 5.5 | 7.4 ± 2.5 | 7.5 ± 2.5 |
| 3R11 | 0 | 0 | 0 | 0.9 ± 0.9 |
| 3L00 | 98.5 ± 1.5 | 83.3 ± 6.8 | 89.8 ± 2.9 | 81.3 ± 3.8 |
| 3L01 | 1.5 ± 1.5 | 13.3 ± 6.2 | 10.2 ± 2.9 | 15.9 ± 3.5 |
| 3L11 | 0 | 3.3 ± 3.3 | 0 | 2.8 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskaev, A.V.; Bega, A.G.; Brusentsov, I.I.; Naumenko, A.N.; Karagodin, D.A.; Razumeiko, V.N.; Andrianov, B.V.; Goryacheva, I.I.; Lee, E.Y.; Panov, V.I.; et al. Species Composition, Ecological Preferences, and Chromosomal Polymorphism of Malaria Mosquitoes of the Crimean Peninsula and the Black Sea Coast of the Caucasus. Insects 2025, 16, 367. https://doi.org/10.3390/insects16040367
Moskaev AV, Bega AG, Brusentsov II, Naumenko AN, Karagodin DA, Razumeiko VN, Andrianov BV, Goryacheva II, Lee EY, Panov VI, et al. Species Composition, Ecological Preferences, and Chromosomal Polymorphism of Malaria Mosquitoes of the Crimean Peninsula and the Black Sea Coast of the Caucasus. Insects. 2025; 16(4):367. https://doi.org/10.3390/insects16040367
Chicago/Turabian StyleMoskaev, Anton V., Anna G. Bega, Ilya I. Brusentsov, Anastasia N. Naumenko, Dmitriy A. Karagodin, Vladimir N. Razumeiko, Boris V. Andrianov, Irina I. Goryacheva, Elizaveta Y. Lee, Vladimir I. Panov, and et al. 2025. "Species Composition, Ecological Preferences, and Chromosomal Polymorphism of Malaria Mosquitoes of the Crimean Peninsula and the Black Sea Coast of the Caucasus" Insects 16, no. 4: 367. https://doi.org/10.3390/insects16040367
APA StyleMoskaev, A. V., Bega, A. G., Brusentsov, I. I., Naumenko, A. N., Karagodin, D. A., Razumeiko, V. N., Andrianov, B. V., Goryacheva, I. I., Lee, E. Y., Panov, V. I., Sharakhov, I. V., Sharakhova, M. V., & Gordeev, M. I. (2025). Species Composition, Ecological Preferences, and Chromosomal Polymorphism of Malaria Mosquitoes of the Crimean Peninsula and the Black Sea Coast of the Caucasus. Insects, 16(4), 367. https://doi.org/10.3390/insects16040367









