A Novel Biosensor for the Early Detection of Aethina tumida via Kodamaea ohmeri in Honeybee Colonies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, DNA Extraction, and PCR Amplification of Kodamaea ohmeri and RT-PCR of Aethina tumida
2.2. Identification of a Peptide from Kodamaea ohmeri
2.3. Development of a Micro/Nanogravimetric Biosensor for Detecting Kodamaea ohmeri Contamination
2.4. Surface Immobilization Protocol
2.5. Peptide Detection
3. Results and Discussions
3.1. Detection of Kodamaea ohmeri and Aethina tumida in Hive Samples
3.2. Molecular Detection of Kodamaea ohmeri in Hive Samples
3.3. Peptide and Anti-Peptide Productions
3.4. Validation of Surface Immobilization
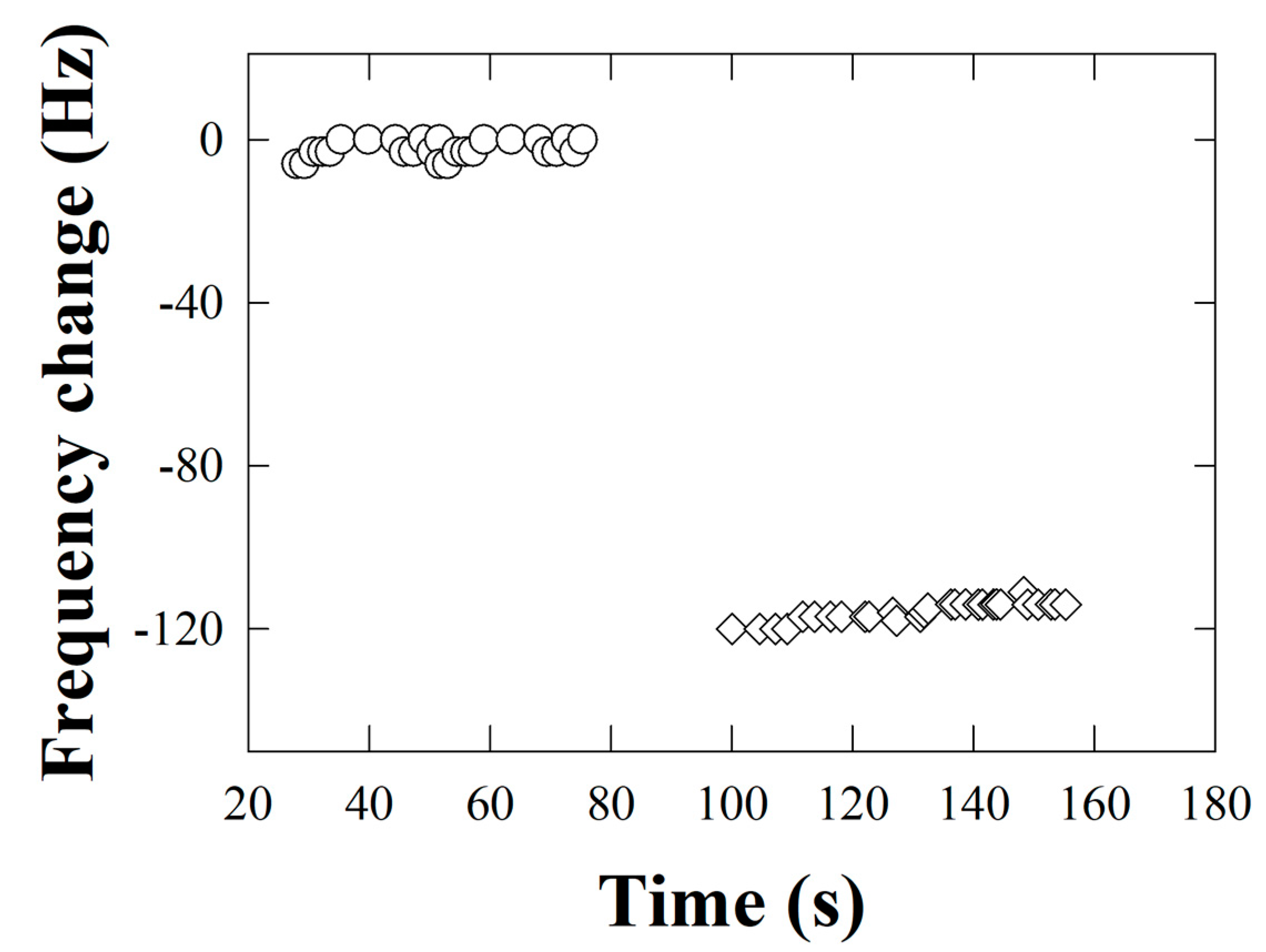
3.5. Detection of Kodamaea ohmeri from the Biosensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SHB | Small Hive Beetle, Aethina tumida |
| Ko | Kodamaea ohmeri |
| QCM | Quartz Crystal Microbalance |
| COI | Cytochrome Oxidase I |
| ITS2 | Internal Transcribed Spacer |
References
- Roth, M.A.; Wilson, J.M.; Gross, A.D. Biology and management of small hive beetles (Coleoptera: Nitidulidae): A pest of European honey bee (Hymenoptera: Apidae) colonies. J. Integr. Pest Manag. 2022, 13, 7. [Google Scholar] [CrossRef]
- Mutinelli, F.; Montarsi, F.; Federico, G.; Granato, A.; Ponti, A.M.; Grandinetti, G.; Ferrè, N.; Franco, S.; Duquesne, V.; Rivière, M.-P.; et al. Detection of Aethina tumida Murray (Coleoptera: Nitidulidae.) in Italy: Outbreaks and early reaction measures. J. Apic. Res. 2014, 53, 569–575. [Google Scholar] [CrossRef]
- Patel, V.; Pauli, N.; Biggs, E.; Barbour, L.; Boruff, B. Why bees are critical for achieving sustainable development. Ambio 2021, 50, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Torto, B.; Boucias, D.G.; Arbogast, R.T.; Tumlinson, J.H.; Teal, P.E.A. Multitrophic interaction facilitates parasite–host relationship between an invasive beetle and the honey bee. Proc. Natl. Acad. Sci. USA 2007, 104, 8374–8378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beaurepaire, A.; Rogers, C.W.; Lopez, D.; Evans, J.D.; Straub, L.; Neumann, P.; Cook, S.C.; Huang, Q. Gene expression and functional analyses of odorant receptors in small hive beetles (Aethina tumida). Int. J. Mol. Sci. 2020, 21, 4582. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.O.; Cardaio, I.; Cilia, G.; Cornelissen, B.; Crailsheim, K.; Formato, G.; Lawrence, A.K.; Le Conte, Y.; Mutinelli, F.; Nanetti, A.; et al. How to slow the global spread of small hive beetles, Aethina tumida. Biol. Invasions 2019, 21, 1451–1459. [Google Scholar] [CrossRef]
- Amos, B.A.; Leemon, D.L.; Hayes, R.A.; Cribb, B.W.; Furlong, M.J. Associations between the small hive beetle and the yeast Kodamaea ohmeri throughout the host life cycle. J. Econ. Entomol. 2018, 111, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.S.; Gifford, R. Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 2005, 20, 2388–2403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zheng, C.; Zhu, L.; Wang, J. A review on rapid detection of modified quartz crystal microbalance sensors for food: Contamination, flavour and adulteration. TrAC Trends Anal. Chem. 2022, 157, 116805. [Google Scholar] [CrossRef]
- Li, D.; Waite, D.W.; Fan, Q.H.; George, S.; Semeraro, L.; Blacket, M.J. Molecular detection of small hive beetle Aethina tumida Murray (Coleoptera: Nitidulidae): DNA barcoding and development of a real-time PCR assay. Sci. Rep. 2018, 8, 9623. [Google Scholar] [CrossRef] [PubMed]
- Santino, I.; Bono, S.; Borruso, L.; Bove, M.; Cialdi, E.; Martinelli, D.; Alari, A. Kodamaea ohmeri isolate from two immunocompromised patients: First report in Italy. Mycoses 2013, 56, 179–181. [Google Scholar] [PubMed]
- Ward, L.; Brown, M.; Neumann, P.; Wilkins, S.; Pettis, J.; Boonham, N. A DNA method for screening hive debris for the presence of small hive beetle (Aethina tumida). Apidologie 2007, 38, 272–280. [Google Scholar] [CrossRef]
- Ghisellini, P.; Caiazzo, M.; Alessandrini, A.; Eggenhöffner, R.; Vassalli, M.; Facci, P. Direct electrical control of IgG conformation and functional activity at surfaces. Sci. Rep. 2016, 6, 37779. [Google Scholar] [CrossRef] [PubMed]
- Pizzoni, D.; Mascini, M.; Lanzone, V.; Del Carlo, M.; Di Natale, C.; Compagnone, D. Selection of peptide ligands for piezoelectric peptide based gas sensors arrays using a virtual screening approach. Biosens. Bioelectron. 2014, 52, 247–254. [Google Scholar] [CrossRef] [PubMed]


| Ko Primers for PCR | Sequence |
|---|---|
| Ko Fw | 5′-TAATTTGAAGATTGCGTCTTG-3′ |
| Ko Rv | 5′-TACCCACACTGACAATCTGAC-3′ |
| SHB Primer/Probe name for RT-PCR | Sequence |
| Fw SBH207F | 5′-TCTAAATACTACTTTCTTCGACCCAT(A/G)-3′ |
| Rv SBH315R 5′ | 5′-TCCTGGTAGAATTAAAATATAAACTTCTGG-3′ |
| SBH245T PROBE | 5′-FAM-ATCCAATCCTATACCAACACTTATTTTGATTCTTCGGAC-3′-TAMRA |
| Sample ID | Real-Time PCR for Aethina tumida (Ct Value) | Qualitative PCR for Kodamaea ohmeri (Positive/Negative) |
|---|---|---|
| 1 | 31.23 | Positive |
| 2 | 32.01 | Positive |
| 3 | 31.55 | Positive |
| 4 | 30.47 | Positive |
| 5 | 29 | Positive |
| 6 | 31.4 | Positive |
| 7 | 30.72 | Positive |
| 8 | 31.95 | Positive |
| 9 | 31.22 | Positive |
| 10 | 0 | Positive |
| 11 | 35 | Positive |
| 12 | 33.92 | Positive |
| 13 | 32.49 | Positive |
| 14 | 0 | Positive |
| 15 | 27.86 | Positive |
| Sample ID | Query Cover (%) | Identity (%) |
|---|---|---|
| 1 | 99% | 97% |
| 2 | 96% | 99% |
| 3 | 96% | 99% |
| 4 | 97% | 99% |
| 5 | 99% | 98% |
| 7 | 96% | 99% |
| 8 | 94% | 99% |
| 9 | 97% | 99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghisellini, P.; Garbati, P.; Pietropaoli, M.; Cersini, A.; Pietrella, G.; Rando, C.; Giacomelli, L.; Ottoboni, S.; Formato, G.; Eggenhöffner, R. A Novel Biosensor for the Early Detection of Aethina tumida via Kodamaea ohmeri in Honeybee Colonies. Insects 2025, 16, 363. https://doi.org/10.3390/insects16040363
Ghisellini P, Garbati P, Pietropaoli M, Cersini A, Pietrella G, Rando C, Giacomelli L, Ottoboni S, Formato G, Eggenhöffner R. A Novel Biosensor for the Early Detection of Aethina tumida via Kodamaea ohmeri in Honeybee Colonies. Insects. 2025; 16(4):363. https://doi.org/10.3390/insects16040363
Chicago/Turabian StyleGhisellini, Paola, Patrizia Garbati, Marco Pietropaoli, Antonella Cersini, Gabriele Pietrella, Cristina Rando, Luca Giacomelli, Stefano Ottoboni, Giovanni Formato, and Roberto Eggenhöffner. 2025. "A Novel Biosensor for the Early Detection of Aethina tumida via Kodamaea ohmeri in Honeybee Colonies" Insects 16, no. 4: 363. https://doi.org/10.3390/insects16040363
APA StyleGhisellini, P., Garbati, P., Pietropaoli, M., Cersini, A., Pietrella, G., Rando, C., Giacomelli, L., Ottoboni, S., Formato, G., & Eggenhöffner, R. (2025). A Novel Biosensor for the Early Detection of Aethina tumida via Kodamaea ohmeri in Honeybee Colonies. Insects, 16(4), 363. https://doi.org/10.3390/insects16040363










