Fly in the Ointment: Host-Specificity Challenges for Botanophila turcica, a Candidate Agent for the Biological Control of Saffron Thistle in Australia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Characterization
2.2. Field Surveys
2.3. Host-Specificity Testing
2.3.1. Plant Material
2.3.2. No-Choice Tests
2.3.3. Choice Tests
2.4. Statistical Analyses
3. Results
3.1. Molecular Characterization
3.2. Field Surveys
3.3. Host-Specificity Testing
3.3.1. No-Choice Tests
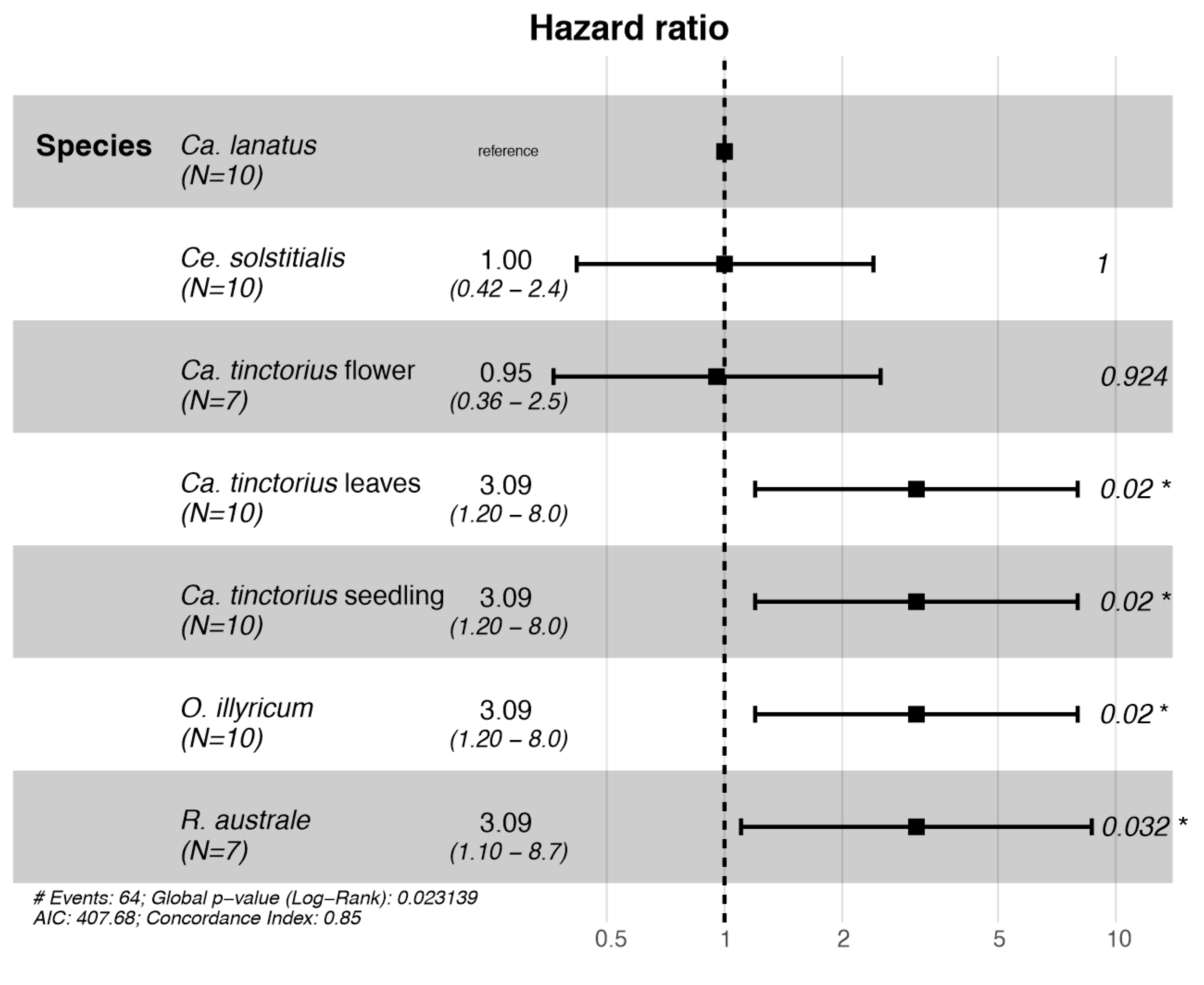
3.3.2. Choice Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarzländer, M.; Hinz, H.L.; Winston, R.; Day, M. Biological control of weeds: An analysis of introductions, rates of establishment and estimates of success, worldwide. BioControl 2018, 63, 319–331. [Google Scholar] [CrossRef]
- Cullen, J.; Sheppard, A.; Raghu, S. Effectiveness of classical weed biological control agents released in Australia. Biol. Control 2022, 166, 104835. [Google Scholar] [CrossRef]
- Hinz, H.L.; Winston, R.L.; Schwarzländer, M. A global review of target impact and direct nontarget effects of classical weed biological control. Curr. Opin. Insect Sci. 2020, 38, 48–54. [Google Scholar] [CrossRef]
- Page, A.; Lacey, K. Economic Impact Assessment of Australian Weed Biological Control; CRC for Australian Weed Management: Sydney, Australia, 2006. [Google Scholar]
- Morin, L.; Reid, A.M.; Sims-Chilton, N.; Buckley, Y.; Dhileepan, K.; Hastwell, G.T.; Nordblom, T.; Raghu, S. Review of approaches to evaluate the effectiveness of weed biological control agents. Biol. Control 2009, 51, 1–15. [Google Scholar] [CrossRef]
- Briese, D. Potential impact of the stem-boring weevil Lixus cardui on the growth and reproductive capacity of Onopordum thistles. Biocontrol Sci. Technol. 1996, 6, 251–262. [Google Scholar] [CrossRef]
- Briese, D.; Walker, A.; Pettit, W.; Sagliocco, J.-L. Host-specificity of candidate agents for the biological control of Onopordum spp. thistles in Australia: An assessment of testing procedures. Biocontrol Sci. Technol. 2002, 12, 149–163. [Google Scholar]
- Van Klinken, R.D.; Raghu, S. A scientific approach to agent selection. Aust. J. Entomol. 2006, 45, 253–258. [Google Scholar] [CrossRef]
- Paynter, Q.; Fowler, S.V.; Groenteman, R. Making weed biological control predictable, safer and more effective: Perspectives from New Zealand. BioControl 2018, 63, 427–436. [Google Scholar] [CrossRef]
- Sheppard, A.; Van Klinken, R.; Heard, T. Scientific advances in the analysis of direct risks of weed biological control agents to nontarget plants. Biol. Control 2005, 35, 215–226. [Google Scholar]
- Grace, B.S.; Sheppard, A.W.; Whalley, R.; Sindel, B.M. Recent news about saffron thistle (Carthamus lanatus L.). Plant Prot. Q. 2004, 19, 36–39. [Google Scholar]
- Grace, B.; Sheppard, A.; Whalley, R.; Sindel, B. Seedbanks and seedling emergence of saffron thistle (Carthamus lanatus) in eastern Australian pastures. Aust. J. Agric. Res. 2002, 53, 1327–1334. [Google Scholar] [CrossRef]
- Grace, B.; Whalley, R.; Sheppard, A.; Sindel, B. Managing saffron thistle in pastures with strategic grazing. Rangel. J. 2002, 24, 313–325. [Google Scholar] [CrossRef]
- Morin, L.; Sheppard, A.W. Carthamus lanatus L.—Saffron thistle. In Biological Control of Weeds in Australia; Cullen, J., Julien, M., McFadyen, R.C., Eds.; CSIRO Publishing: Melbourne, Australia, 2012; pp. 139–145. [Google Scholar]
- Aeschlimann, J.-P. Reappraising the potential of biological control against the weed Carthamus lanatus. Entomophaga 1997, 42, 559–568. [Google Scholar]
- Vitou, J.; Briese, D.; Sheppard, A.; Thomann, T. Comparative biology of two rosette crown-feeding flies of the genus Botanophila (Dipt., Anthomyiidae) with potential for biological control of their thistle hosts. J. Appl. Entomol. 2001, 125, 89–95. [Google Scholar] [CrossRef]
- Sheppard, A.W.; Vitou, J. The effect of a rosette-crown fly, Botanophila turcica, on growth, biomass allocation and reproduction of the thistle Carthamus lanatus. Acta Oecologica 2000, 21, 337–347. [Google Scholar] [CrossRef]
- Tsialtas, I.T.; Michelsen, V.; Koveos, D.S. First report of Botanophila turcica (Diptera: Anthomyiidae) on safflower Carthamus tinctorius L. in Greece. J. Biol. Res. Thessalon. 2013, 19, 80–82. [Google Scholar]
- GRDC. Safflowers GrownNotes—March 2017; GRDC: Canberra, Australia, 2017. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–346. [Google Scholar]
- Simon, C.; Buckley, T.R.; Frati, F.; Stewart, J.B.; Beckenbach, A.T. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 545–579. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar]
- Meier, R.; Zhang, G.; Ali, F. The use of mean instead of smallest interspecific distances exaggerates the size of the “barcoding gap” and leads to misidentification. Syst. Biol. 2008, 57, 809–813. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [PubMed]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [PubMed]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [PubMed]
- ABRS. Flora of Australia Volume 37, Asteraceae 1; Wilson, A., Ed.; ABRS/CSIRO: Melbourne, Australia, 2015; p. 638. [Google Scholar]
- Fu, Z.X.; Jiao, B.H.; Nie, B.; Zhang, G.J.; Gao, T.G.; Consortium, C.P. A comprehensive generic-level phylogeny of the sunflower family: Implications for the systematics of Chinese Asteraceae. J. Syst. Evol. 2016, 54, 416–437. [Google Scholar]
- Barres, L.; Sanmartín, I.; Anderson, C.L.; Susanna, A.; Buerki, S.; Galbany-Casals, M.; Vilatersana, R. Reconstructing the evolution and biogeographic history of tribe Cardueae (Compositae). Am. J. Bot. 2013, 100, 867–882. [Google Scholar]
- Briese, D. Translating host-specificity test results into the real world: The need to harmonize the yin and yang of current testing procedures. Biol. Control 2005, 35, 208–214. [Google Scholar]
- Wapshere, A. A strategy for evaluating the safety of organisms for biological weed control. Ann. Appl. Biol. 1974, 77, 201–211. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 65, 1–48. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. survminer: Drawing Survival Curves Using ‘ggplot2’. R Package, Version 0.3; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Dajue, L.; Mündel, H.-H. Safflower, Carthamus tinctorius L. Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetic Resources Institute: Rome, Italy, 1996; Volume 7, p. 83. [Google Scholar]
- Parsons, W.T.; Parsons, W.T.; Cuthbertson, E. Noxious Weeds of Australia; CSIRO Publishing: Clayton, Austria, 2001. [Google Scholar]
- Hay, G.; Facelli, J.M.; Panetta, F.D. Invasive potential and competitive ability of the Eurasian herb Centaurea solstitialis L. In Proceedings of the Fifteenth Australian Weeds Conference, Adelaide, Australia, 24–28 September 2006; pp. 719–722. [Google Scholar]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar]
- Komzáková, O.; Rozkošný, R. Identification of central European species of Botanophila Lioy, 1864, based on the female terminalia (Diptera: Anthomyiidae). Acta Zool. Acad. Sci. Hung 2009, 55, 321–337. [Google Scholar]
- Michelsen, V. Report on three unrecognised European species of Anthomyiidae described by O. ringdahl (Insecta: Diptera). Genus 2009, 20, 1–12. [Google Scholar]
- Brown, S.D.; Collins, R.A.; Boyer, S.; Lefort, M.C.; Malumbres-Olarte, J.; Vink, C.J.; Cruickshank, R.H. Spider: An R package for the analysis of species identity and evolution, with particular reference to DNA barcoding. Mol. Ecol. Resour. 2012, 12, 562–565. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
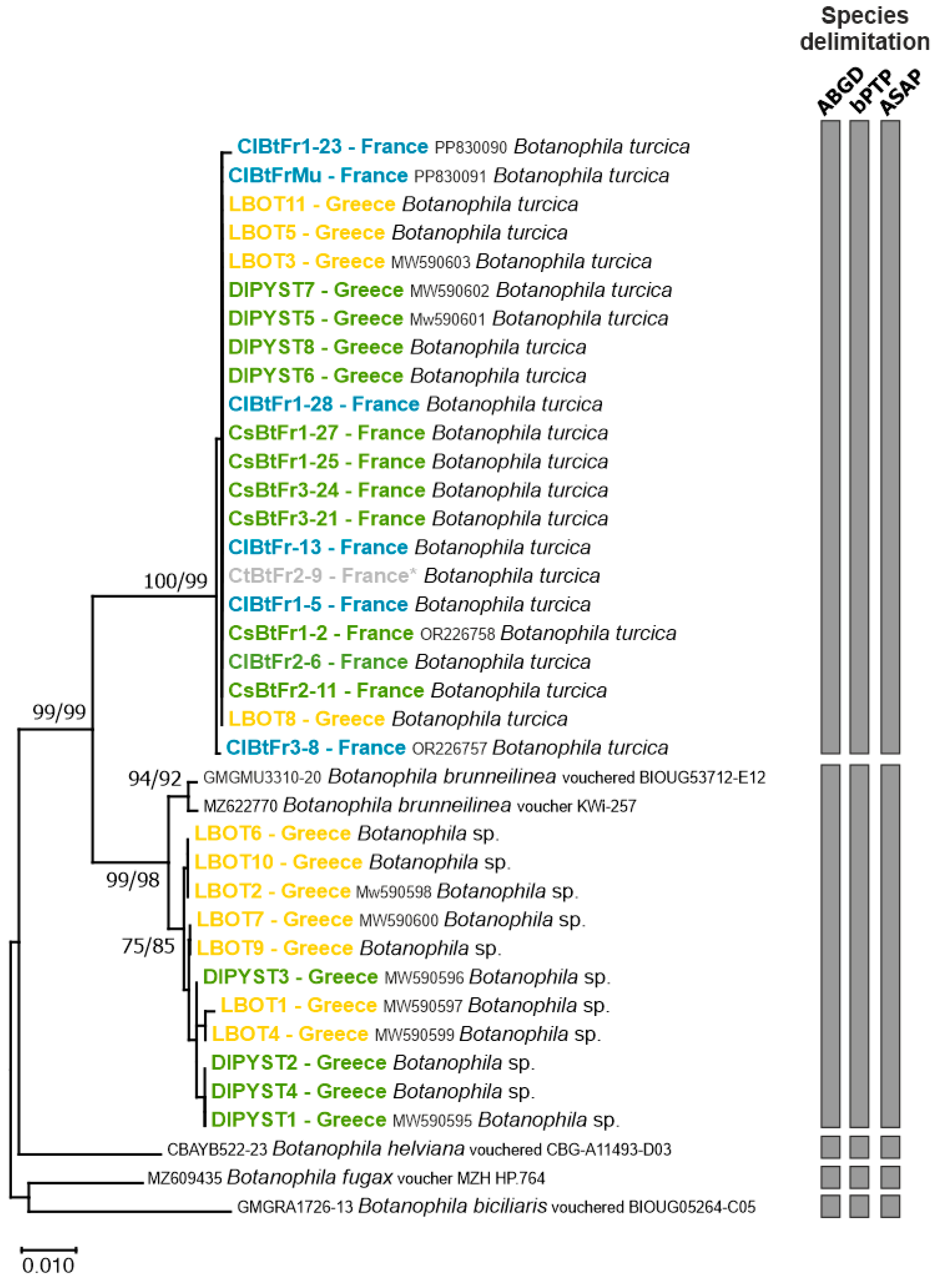

| Sample ID | Host-Plant | Country | Location | GPS Coordinates | Collection Date | Sample Stage | GenBank Accession N° |
|---|---|---|---|---|---|---|---|
| ClBtFrMu | Carthamus lanatus | France | Murles (Herault) | 43°40′57.0″ N 3°44′47.0″ E | 02/05/1995 | Adult | PP830091 |
| CsBtFr1-2 | Centaurea solstitialis | France | Viols-en-Laval (Herault) | 43°45′06.3″ N 3°43′15.4″ E | 26/01/2021 | Larva | OR226758 |
| ClBtFr1-5 | Carthamus lanatus | France | Viols-en-Laval (Herault) | 43°45′06.3″ N 3°43′15.4″ E | 12/01/2021 | Larva | =CsBtFr1-2 |
| ClBtFr1-23 | Carthamus lanatus | France | Viols-en-Laval (Herault) | 43°45′06.3″ N 3°43′15.4″ E | 15/12/2021 | Larva | PP830090 |
| ClBtFr1-28 | Carthamus lanatus | France | Viols-en-Laval (Herault) | 43°45′06.3″ N 3°43′15.4″ E | 01/12/2021 | Larva | =CsBtFr1-2 |
| ClBtFr2-6 | Carthamus lanatus | France | Saint-Clément-de-Rivière (Herault) | 43°41′26.6″ N 3°50′47.1″ E | 19/01/2021 | Larva | =CsBtFr1-2 |
| ClBtFr-13 | Carthamus lanatus | France | Saint-Clément-de-Rivière (Herault) | 43°41′26.6″ N 3°50′47.1″ E | 16/12/2020 | ♂ Adult | =CsBtFr1-2 |
| ClBtFr3-8 | Carthamus lanatus | France | Le Triadou (Herault) | 43°44′49.8″ N 3°51′30.3″ E | 17/11/2021 | Larva | OR226757 |
| CtBtFr2-9 | Carthamus tinctorius * | France | Saint-Clément-de-Rivière (Herault) | 43°41′26.6″ N 3°50′47.1″ E | 16/12/2020 | Adult | =CsBtFr1-2 |
| CsBtFr2-11 | Centaurea solstitialis | France | Saint-Clément-de-Rivière (Herault) | 43°41′26.6″ N 3°50′47.1″ E | 16/12/2020 | ♂ Adult | =CsBtFr1-2 |
| CsBtFr3-21 | Centaurea solstitialis | France | Le Triadou (Herault) | 43°44′49.8″ N 3°51′30.3″ E | 01/12/2021 | Larva | =CsBtFr1-2 |
| CsBtFr3-24 | Centaurea solstitialis | France | Le Triadou (Herault) | 43°44′49.8″ N 3°51′30.3″ E | 15/12/2021 | Larva | =CsBtFr1-2 |
| CsBtFr1-25 | Centaurea solstitialis | France | Viols-en-Laval (Herault) | 43°45′06.3″ N 3°43′15.4″ E | 01/12/2021 | Larva | =CsBtFr1-2 |
| CsBtFr1-27 | Centaurea solstitialis | France | Viols-en-Laval (Herault) | 43°45′06.3″ N 3°43′15.4″ E | 15/12/2021 | Larva | =CsBtFr1-2 |
| DIPYST1 | Centaurea solstitialis | Greece | Galani, Kozani (Macedonia) | 40°22′4.60″ N 21°52′20.84″ E | 05/12/2018 | Larva | MW590595 |
| DIPYST2 | Centaurea solstitialis | Greece | Galani, Kozani (Macedonia) | 40°22′4.60″ N 21°52′20.84″ E | 05/12/2018 | Larva | =DIPYST1 |
| DIPYST3 | Centaurea solstitialis | Greece | Galani, Kozani (Macedonia) | 40°22′4.60″ N 21°52′20.84″ E | 05/12/2018 | Larva | MW590596 |
| DIPYST4 | Centaurea solstitialis | Greece | Galani, Kozani (Macedonia) | 40°22′4.60″ N 21°52′20.84″ E | 05/12/2018 | Larva | =DIPYST1 |
| DIPYST5 | Centaurea solstitialis | Greece | Galani, Kozani (Macedonia) | 40°22′4.60″ N 21°52′20.84″ E | 05/12/2018 | Larva | MW590601 |
| DIPYST6 | Centaurea solstitialis | Greece | Galani, Kozani (Macedonia) | 40°22′4.60″ N 21°52′20.84″ E | 05/12/2018 | Larva | =DIPYST5 |
| DIPYST7 | Centaurea solstitialis | Greece | Galani, Kozani (Macedonia) | 40°22′4.60″ N 21°52′20.84″ E | 05/12/2018 | Egg batch | MW590602 |
| DIPYST8 | Centaurea solstitialis | Greece | Galani, Kozani (Macedonia) | 40°22′4.60″ N 21°52′20.84″ E | 05/12/2018 | Egg batch | =DIPYST7 |
| LBOT1 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 19/06/2002 | Larva | MW590597 |
| LBOT2 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 19/06/2002 | Larva | MW590598 |
| LBOT3 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 19/06/2002 | Larva | MW590603 |
| LBOT4 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 19/06/2002 | Larva | MW590599 |
| LBOT5 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 20/06/2002 | Larva | =LBOT3 |
| LBOT6 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 20/06/2002 | Larva | =LBOT2 |
| LBOT7 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 20/06/2002 | Larva | MW590600 |
| LBOT8 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 20/06/2002 | Larva | =LBOT3 |
| LBOT9 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 20/06/2002 | Larva | =LBOT7 |
| LBOT10 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 20/06/2002 | Larva | =LBOT2 |
| LBOT11 | Centaurea diffusa | Greece | Kilkis near Thessaloniki (Macedonia) | 40°56′20.23″ N 22°50′22.47″ E | 20/06/2002 | Larva | =LBOT3 |
| Sites | Plant Species | Year | n | Infestation Rate (%) |
|---|---|---|---|---|
| Viols-en-Laval | Carthamus lanatus | 2020–2021 | 270 | 7.41 |
| 2021–2022 | 120 | 5.56 | ||
| Centaurea solstitialis | 2020–2021 | 270 | 2.59 | |
| 2021–2022 | 120 | 5.56 | ||
| Saint-Clément-de-Rivière | Carthamus lanatus | 2020–2021 | 270 | 27.3 |
| 2021–2022 | 120 | 12.5 | ||
| Le Triadou | Centaurea solstitialis | 2021–2022 | 120 | 16.7 |
| Sub-Tribe | Species—Treatment | Damaged Plants (%) | Type of Damage | Successful Adult Emergence |
|---|---|---|---|---|
| Centaureinae | Carthamus lanatus | 70 a | Damaged root crown | Yes |
| Carthamus tinctorius—seedlings | 30 ab | Plant death | No | |
| Carthamus tinctorius—leaves | 0 b | None | / | |
| Carthamus tinctorius—flower buds | 85 a | Destroyed seeds | Yes | |
| Centaurea solstitialis | 60 a | Damaged root crown | Yes | |
| Rhaponticumaustrale | 42 ab | Damaged stem | No | |
| Carduinae | Onopordum illyricum | 0 b | None | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesieur, V.; Thomann, T.; Jourdan, M.; Kashefi, J.; Bon, M.-C. Fly in the Ointment: Host-Specificity Challenges for Botanophila turcica, a Candidate Agent for the Biological Control of Saffron Thistle in Australia. Insects 2025, 16, 357. https://doi.org/10.3390/insects16040357
Lesieur V, Thomann T, Jourdan M, Kashefi J, Bon M-C. Fly in the Ointment: Host-Specificity Challenges for Botanophila turcica, a Candidate Agent for the Biological Control of Saffron Thistle in Australia. Insects. 2025; 16(4):357. https://doi.org/10.3390/insects16040357
Chicago/Turabian StyleLesieur, Vincent, Thierry Thomann, Mireille Jourdan, Javid Kashefi, and Marie-Claude Bon. 2025. "Fly in the Ointment: Host-Specificity Challenges for Botanophila turcica, a Candidate Agent for the Biological Control of Saffron Thistle in Australia" Insects 16, no. 4: 357. https://doi.org/10.3390/insects16040357
APA StyleLesieur, V., Thomann, T., Jourdan, M., Kashefi, J., & Bon, M.-C. (2025). Fly in the Ointment: Host-Specificity Challenges for Botanophila turcica, a Candidate Agent for the Biological Control of Saffron Thistle in Australia. Insects, 16(4), 357. https://doi.org/10.3390/insects16040357






