Immune-Related Genes in the Honey Bee Mite Varroa destructor (Acarina, Parasitidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Overview of the Immune Gene Survey
3.2. Recognition Genes
| Gene Name | Role | D. melanogaster | V. destructor 1 | E-Value | Identity | Coverage | Transcription |
|---|---|---|---|---|---|---|---|
| PGRP-LC, peptidoglycan recognition protein | bacterial recognition | AAF50302.3 | XP_022660134.1 | 2 × 10−30 | 36.71% | 31% | YES |
| PGRP-LE, peptidoglycan recognition protein | activation of PPO cascade and autophagy | NP_573078.1 | XP_022660134.1 | 5 × 10−25 | 31.01% | 45% | YES |
| PGRP-SA, peptidoglycan recognition protein | bacterial recognition | AAF48056.1 | XP_022660135.1 | 9 × 10−34 | 32.93% | 82% | YES |
| PGRP-SD, peptidoglycan recognition protein | bacterial recognition | CAD89193.1 | XP_022660134.1 | 5 × 10−27 | 33.54% | 86% | YES |
| PGRP-LB, peptidoglycan recognition protein | bacterial recognition | NP_650079.1 | XP_022660134.1 | 2 × 10−31 | 35.09% | 79% | YES |
| PGRP-SC1a, peptidoglycan recognition protein | bacterial recognition | CAD89161.1 | XP_022660134.1 | 3 × 10−32 | 30.62% | 86% | YES |
| PGRP-SC2, peptidoglycan recognition protein | bacterial recognition | CAD89187.1 | XP_022660134.1 | 3 × 10−35 | 31.71% | 89% | YES |
| PGRP-SB1, peptidoglycan recognition protein | PGN degradation and antibacterial activity | CAD89136.1 | XP_022660135.1 | 6 × 10−35 | 35.22% | 83% | YES |
| PGRP-LF, peptidoglycan recognition protein | blocking of IMD pathway | NP_648299.3 | XP_022660134.1 | 3 × 10−38 | 37.35% | 79% | YES |
| PGRP-LA, peptidoglycan recognition protein | activation of IMD pathway | AAF50304.2 | Not found | Not found | Not found | Not found | - |
| GNBP1, Gram-negative binding protein 1 | bacterial and fungal pattern recognition | Q9NHB0.2 | Not found | Not found | Not found | Not found | - |
| GNBP2, Gram-negative binding protein 2 | bacterial and fungal pattern recognition | ACU30172.1 | Not found | Not found | Not found | Not found | - |
| GNBP3, Gram-negative binding protein 3 | bacterial and fungal pattern recognition | CAJ18910.1 | Not found | Not found | Not found | Not found | - |
| DL1, c-type lectin 1 | bacterial recognition, induction of PPO cascade | AAF53793.1 | Not found | Not found | Not found | Not found | - |
| DL2, c-type lectin 2 | bacterial recognition, induction of PPO cascade | NP_001014489.1 | Not found | Not found | Not found | Not found | - |
| DL3, c-type lectin 3 or solute carrier | bacterial recognition, induction of PPO cascade | NP_001014490.1 | XP_022646715.1 | 4 × 10−7 | 23.58% | 79% | YES |
| galectin 4 | several roles have been hypothesized | ADZ99399.1 | XP_022654763.1 | 1 × 10−9 | 34.75% | 29% | YES |
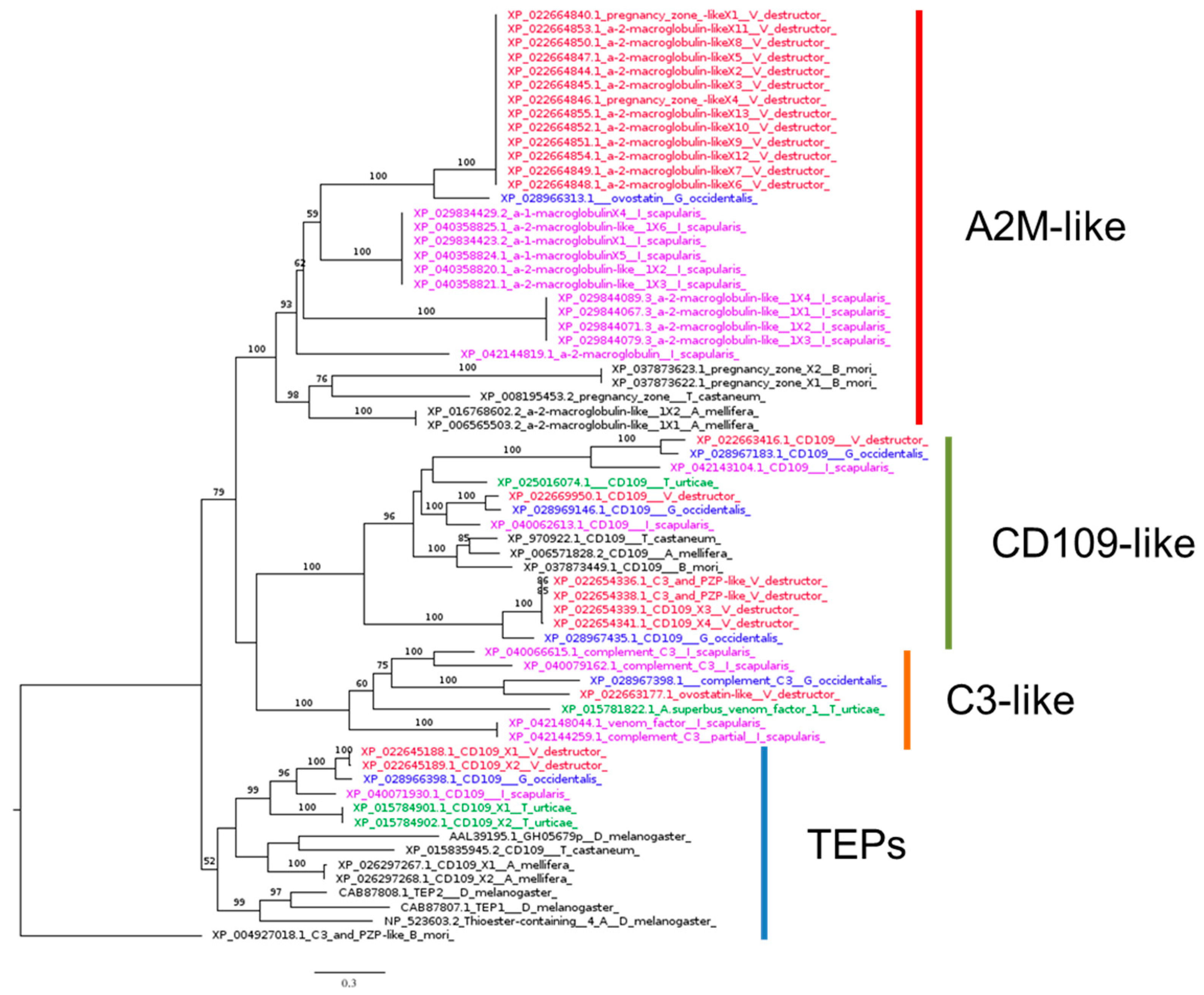
| TEP1, CD109 antigen-like | mark pathogens for phagocytosis | CAB87807.1 | XP_022645189.1 | 0.0 | 31.32% | 98% | YES |
| TEP2, CD109 antigen-like | mark pathogens | CAB87808.1 | XP_022645189.1 | 0.0 | 33.79% | 98% | YES |
| TEP3, CD109 antigen-like | mark pathogens | AAL39195.1 | XP_022645188.1 | 0.0 | 32.35% | 97% | YES |
| TEP4, CD109 antigen-like | mark pathogens | NP_523603.2 | XP_022645188.1 | 0.0 | 30.90% | 98% | YES |
| Pes, scavenger receptor class B member 1-like | bacterial and fungal recognition | AHN54246.1 | XP_022673026.1 | 4 × 10−61 | 29.15% | 78% | YES |
| Crq, croquemort, lysosome membrane protein 2-like | bacterial and fungal recognition | AAF51494.1 | XP_022672747.1 | 5 × 10−70 | 28.81% | 96% | YES |
| Drpr, protein draper-like | bacterial and fungal recognition | NP_477450.1 | XP_022656525.1 | 5 × 10−69 | 39.01% | 72% | YES |
| sr-CI, scavenger receptor class C, type i | bind to lipoproteins and bacteria | AAW79470.1 | XP_022658153.1 | 3 × 10−24 | 28.62% | 45% | YES |
| sr-CII, scavenger receptor class C, type ii | bind to lipoproteins and bacteria | AAF58551.1 | XP_022658154.1 | 4 × 10−23 | 27.16% | 49% | YES |
| sr-CIII, scavenger receptor class C, type iii | bind to lipoproteins and bacteria | AAF37564.1 | XP_022658154.1 | 2 × 10−9 | 21.00% | 90% | YES |
| sr-CIV, scavenger receptor class C, type iv | bind to lipoproteins and bacteria | AAF51092.1 | XP_022658154.1 | 5 × 10−19 | 26.59% | 73% | YES |
| eater | receptor in phagocytosis and microbial binding | AAF56664.5 | XP_022668925.1 | 2 × 10−17 | 33.72% | 77% | NO |
| Drp, protein draper-like | receptor in phagocytosis and microbial binding | AAF53364.2 | XP_022656529.1 | 6 × 10−12 | 27.66% | 62% | YES |
3.2.1. Peptidoglycan Receptor Proteins
3.2.2. Gram-Negative Binding Proteins
3.2.3. Lectins
3.2.4. Thioester-Containing Proteins
3.3. Signaling Pathways
| Gene Name | Role | D. melanogaster | V. destructor 1 | E-Value | Identity | Coverage | Transcript |
|---|---|---|---|---|---|---|---|
| Spz1-1, spätzle 1B | Toll pathway | NP_733188.1 | XP_022644257.1 | 6 × 10−7 | 25.32% | 62% | NO |
| Spz1-2, spätzle 1Bii | Toll pathway | NP_001138116.1 | XP_022644257.1 | 2 × 10−7 | 25.32% | 52% | NO |
| Spz2, spätzle 2, neurotrophin 1 | Toll pathway | NP_001261417.1 | XP_022654810.1 | 3 × 10−15 | 27.85% | 14% | YES |
| Spz3, spätzle 3 | Toll pathway | NP_609160.2 | XP_022643975.1 | 3 × 10−61 | 40.00% | 43% | NO |
| Spz4, spätzle 4 | Toll pathway | NP_609504.2 | XP_022644415.1 | 5 × 10−51 | 63.55% | 17% | YES |
| Spz5, spätzle 5 | Toll pathway | NP_647753.1 | XP_022663991.1 | 1 × 10−14 | 38.98% | 29% | YES |
| Spz6, spätzle 6 | Toll pathway | NP_611961.1 | XP_022645393.1 | 2 × 10−30 | 63.75% | 18% | YES |
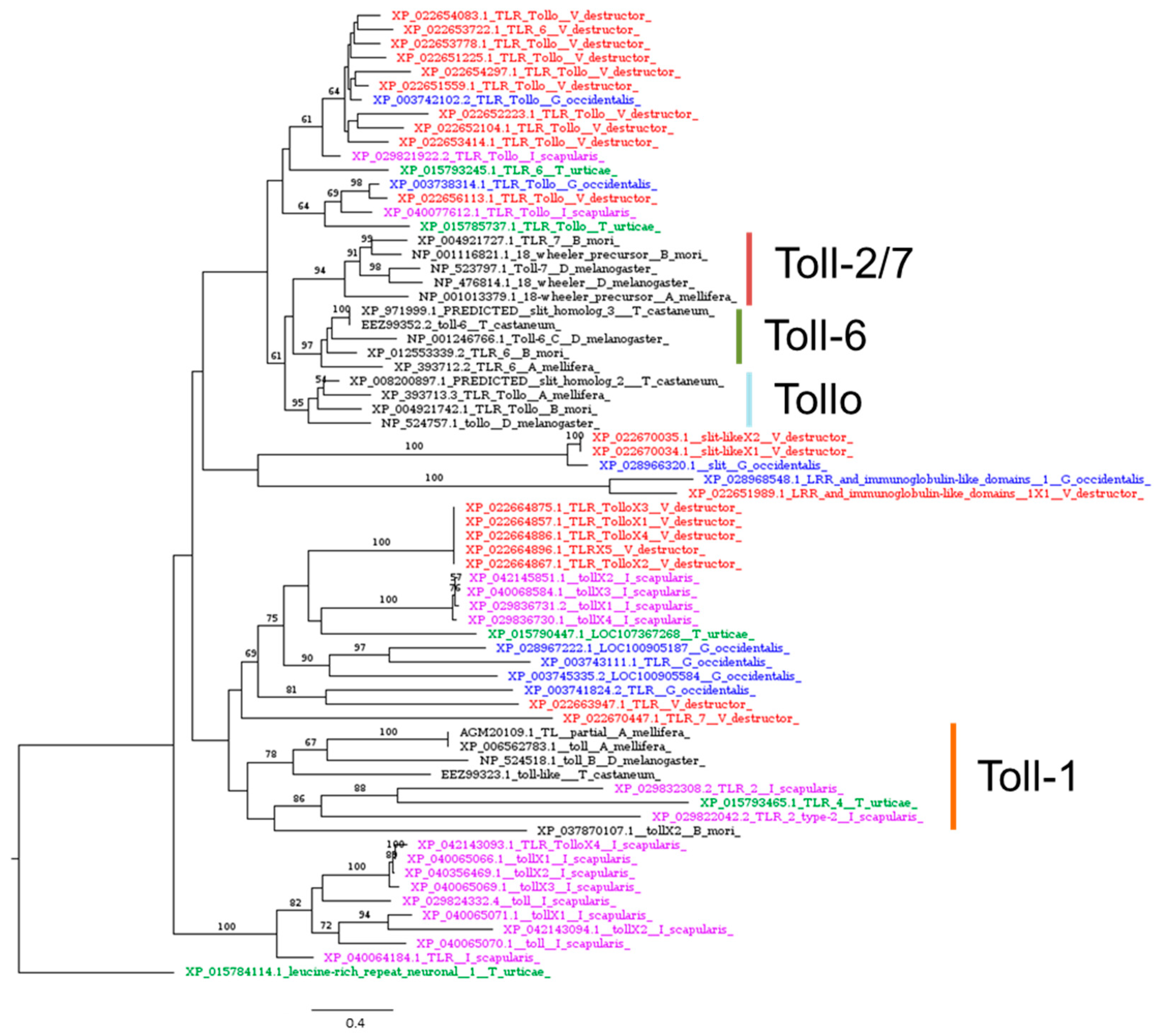
| Toll-1, protein Toll | Toll pathway | NP_524518.1 | XP_022664896.1 | 3 × 10−93 | 29.56% | 74% | YES |
| 18 wheeler, Toll-2 | Toll pathway | NP_476814.1 | XP_022651559.1 | 0.0 | 38.74% | 83% | YES |
| Toll-6 | Toll pathway | NP_001246766.1 | XP_022653722.1 | 0.0 | 40.50% | 77% | NO |
| Toll-7 | Toll pathway | NP_523797.1 | XP_022656113.1 | 0.0 | 41.21% | 82% | YES |
| Tollo, Toll-8 | Toll pathway | NP_524757.1 | XP_022651559.1 | 0.0 | 40.47% | 89% | YES |
| Tube 2, interleukin-1 receptor-associated kinase 4 | Toll pathway | NP_001189164.1 | Not found. A putative homolog was found by using the query from the T. urticae genome 2. | Not found | Not found | Not found | - |
| Myd88, myeloid differentiation primary response gene 2 | Toll pathway | AAF58953.1 | Not found. A putative homolog was found by using the query from the T. urticae genome 2. | Not found | Not found | Not found | - |
| Pll, pelle | Toll pathway | AAF56686.1 | XP_022652064.1 | 1 × 10−64 | 42.48% | 60% | YES |
| Cact, cactus | Toll pathway | AAN10936.1 | XP_022667531.1 | 8 × 10−17 | 30.21% | 48% | YES |
| Cactin | Toll pathway | NP_523422.4 | XP_022668977.1 | 0.0 | 52.09% | 82% | YES |
| Pli, pellino | Toll pathway | NP_524466.1 | XP_022668132.1 | 8 × 10−68 | 42.61% | 59% | YES |
| Traf1, TNF-receptor-associated factor 1 | Toll pathway | AAD34346.1 | XP_022668810.1 | 8 × 10−101 | 46.20% | 66% | YES |
| Traf2,TNF-receptor-associated factor 2 | Toll pathway | AAF46338.1 | XP_022668812.1 | 7 × 10−9 | 24.84% | 30% | YES |
| Traf3, TNF-receptor-associated factor 3 | Toll pathway | NP_727976.1 | XP_022687269.1 | 2 × 10−15 | 25.00% | 38% | YES |
| Dl, dorsal | Toll pathway | AAF53611.1 | XP_022665229.1 | 8 × 10−87 | 50.37% | 39% | YES |
| Dome, domeless 1, interleukine JAK/STAT receptor | JAK/STAT pathway | CAD12503.1 | XP_022659768.1 | 2 × 10−21 | 21.79% | 39% | YES |
| Hops, hopscotch, Janus kinase | JAK/STAT pathway | NP_511119.2 | XP_022650171.1 | 2 × 10−52 | 42.59% | 61% | YES |
| STAT92E, signal transducer and activator of transcription, marelle | JAK/STAT pathway | AAX33462.1 | XP_022661521.1 | 1 × 10−129 | 36.85% | 94% | YES |
| unpaired 1 | JAK/STAT pathway | NP_525095.2 | Not found | Not found | Not found | Not found | - |
| unpaired 2 | JAK/STAT pathway | NP_001356882.1 | Not found | Not found | Not found | Not found | - |
| unpaired 3 | JAK/STAT pathway | NP_001097014.1 | Not found | Not found | Not found | Not found | - |
| IMD, immune deficiency | IMD pathway | NP_573394.1 | Not found | Not found | Not found | Not found | - |
| dFADD | IMD pathway | NP_651006.1 | Not found | Not found | Not found | Not found | - |
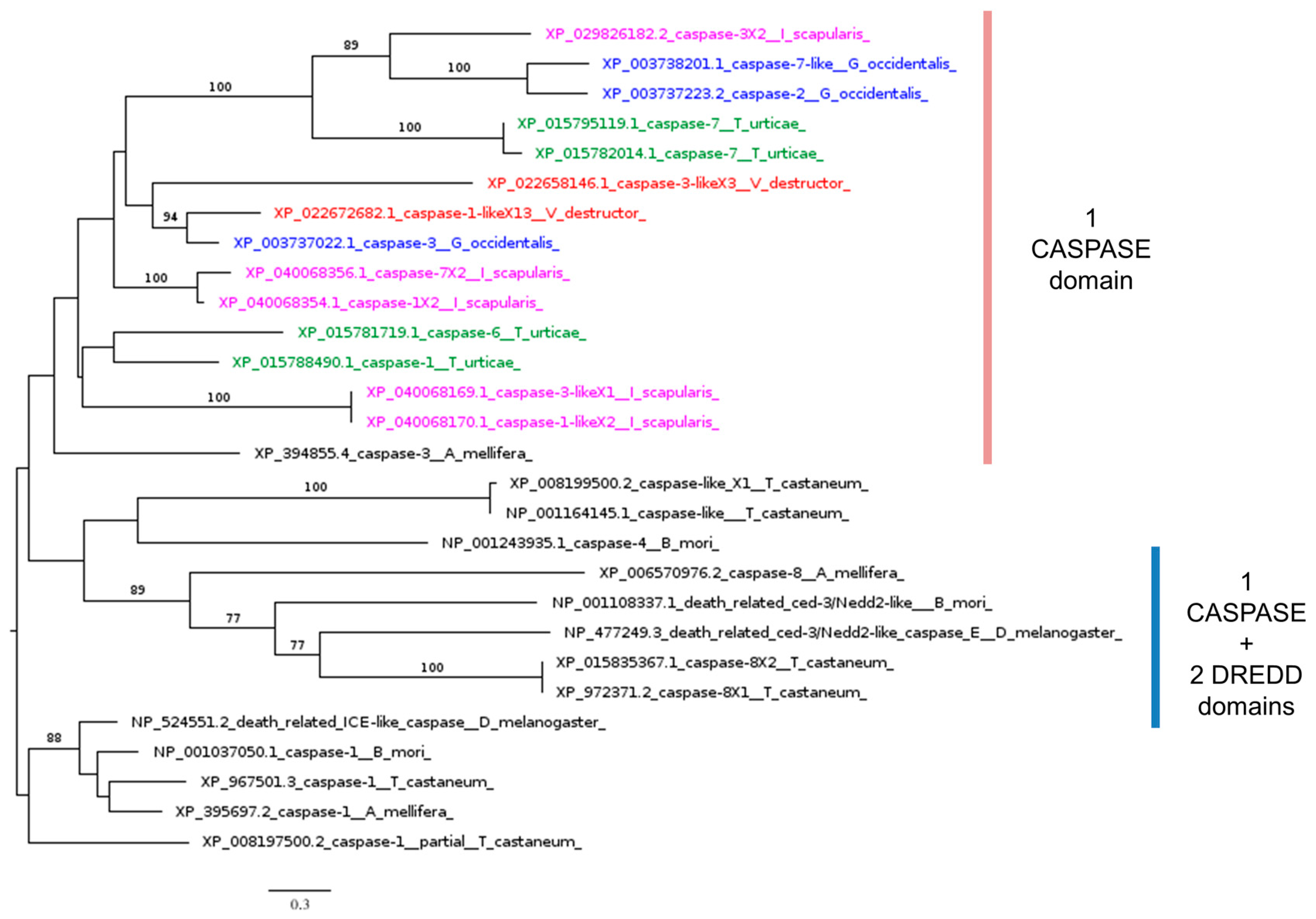
| Dredd, death-related ced-3, caspase-1 | IMD pathway | NP_477249.3 | XP_022672682.1 | 2 × 10−17 | 28.98% | 46% | YES |
| Rel, Relish 3 | IMD pathway | NP_477094.1 | XP_022656314.1 | 3 × 10−11 | 33.81% | 14% | YES |
| TAB2, TAK1-associated binding protein 2 | IMD pathway | NP_611408.2 | Not found | Not found | Not found | Not found | - |
| TAK1, TGF-β-activated kinase 1 | IMD pathway | AAF50895.1 | XP_022652311.1 | 8 × 10−81 | 41.39% | 64% | YES |
| Key, Kenny | IMD pathway | NP_523856.2 | Not found | Not found | Not found | Not found | - |
| DIAP2/XIAP, death-associated inhibitor of apoptosis 2 | IMD pathway | NP_477127.1 | XP_022671835.1 | 2 × 10−59 | 31.12% | 86% | YES |
| IRD5, immune response deficiency 5, IK-β, IKKB, I-kappa-B kinase beta | IMD pathway | NP_524751.3 | XP_022693386.1 | 9 × 10−37 | 38.05% | 31% | YES |
| Hep, hemipterous | JNK pathway | NP_727661.1 | XP_022657489.1 | 4 × 10−110 | 56.25% | 25% | YES |
| Bsk, basket | JNK pathway | P92208.1 | XP_022646497.1 | 0.0 | 84.72% | 96% | YES |
| Jra, Jun-related antigen | JNK pathway | AAF58845.1 | XP_022649508.1 | 2 × 10−32 | 34.36% | 78% | YES |
| Kay, kayak | JNK pathway | NP_001027579.1 | XP_022661416.1 | 2 × 10−10 | 41.44% | 23% | YES |
| Egr, Eiger | JNK pathway | AAF58848.2 | Not found | Not found | Not found | Not found | - |
3.3.1. The Toll Signaling Pathway
3.3.2. The JAK/STAT Signaling Pathway
3.3.3. IMD and JNK Signaling Pathways
3.4. Response Genes
| Gene Name | Role | D. melanogaster | V. destructor 1 | E-Value | Identity | Coverage | Transcript |
|---|---|---|---|---|---|---|---|
| Att, Attacin | antimicrobial peptide | NP_523745.1 | Not found | Not found | Not found | Not found | - |
| Cec, Cecropin | antimicrobial peptide | C0HKQ7.1 | Not found | Not found | Not found | Not found | - |
| Def, Defensin | antimicrobial peptide | ANY27112.1 | Not found | Not found | Not found | Not found | - |
| Dro, Dosocin | antimicrobial peptide | XP_016946682.1 | Not found | Not found | Not found | Not found | - |
| Mtk, Metchnikowin | antimicrobial peptide | AAO72489.1 | Not found | Not found | Not found | Not found | - |
| Andropin | antimicrobial peptide | P21663.1 | Not found | Not found | Not found | Not found | - |
| Diptericin | antimicrobial peptide | QER92349.1 | Not found | Not found | Not found | Not found | - |
| Drs, Drosomycin | antimicrobial peptide | ANY27466.1 | Not found | Not found | Not found | Not found | - |
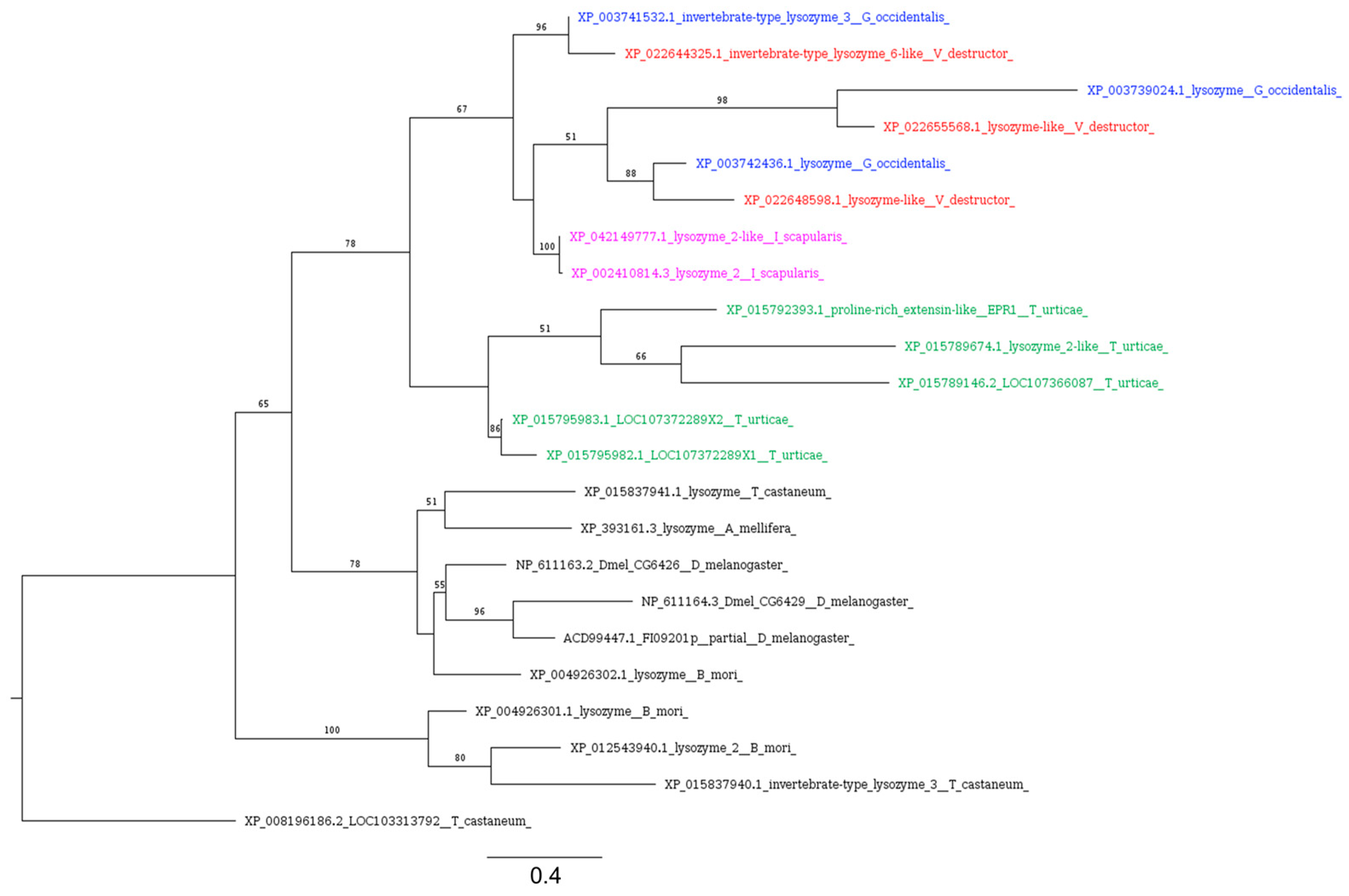
| LysX, Lysozyme X, i-type | microbial degradation | CAL85493.1 | XP_022671742.1 | 2 × 10−30 | 38.30% | 96% | YES |
| LysB, Lysozyme B, i-type | microbial degradation | NP_001261245.1 | XP_022671739.1 | 2 × 10−33 | 41.01% | 97% | YES |
| LysP, Lysozyme, i-type | microbial degradation | NP_476828.1 | XP_022643422.1 | 4 × 10−37 | 43.28% | 95% | NO |
| LysE, Lysozyme E | microbial degradation | CAA80228 | XP_022669180.1 | 3 × 10−32 | 43.09% | 87% | YES |
| LysD, Lysozyme D | microbial degradation | NP_476823.1 | XP_022671742.1 | 7 × 10−33 | 43.90% | 87% | YES |
| LysE, Lysozyme E | microbial degradation | NP_476827.2 | XP_022671742.1 | 6 × 10−32 | 39.42% | 97% | YES |
| LysS, Lysozyme S | microbial degradation | NP_476829.1 | XP_022671742.1 | 1 × 10−34 | 44.36% | 95% | YES |
| Lysozyme E, i-type | microbial degradation | ACD99447.1 | XP_022648598.1 | 5 × 10−23 | 34.55% | 89% | YES |
| Lysozyme, i-type | microbial degradation | NP_611164.3 | XP_022655568.1 | 6 × 10−19 | 35.17% | 88% | YES |
| Lysozyme, i-type | microbial degradation | NP_611163.2 | XP_022644325.1 | 4 × 10−19 | 37.30% | 73% | YES |
| Cht2, Chitinase-like protein 4, flocculation protein | fungal degradation | NP_001261282.1 | XP_022662471.1 | 2 × 10−85 | 40.37% | 73% | YES |
| Cht4, Chitinase-like protein 2, mucin | fungal degradation | NP_524962.2 | XP_022662425.1 | 9 × 10−112 | 38.54% | 94% | YES |
| Cht5, Chitinase-like protein 5, endochitinase | fungal degradation | NP_650314.1 | XP_022664603.1 | 7 × 10−165 | 43.43% | 95% | YES |
| Cht7, Chitinase-like protein 7, chitinase 10 | fungal degradation | NP_647768.3 | XP_022669697.1 | 0.0 | 52.05% | 99% | NO |
| Cht6, Chitinase 6 | fungal degradation | NP_001245599.1 | XP_022662476.1 | 0.0 | 49.73% | 47% | YES |
| idgf6 | fungal degradation | NP_001286499.1 | XP_022662425.1 | 1 × 10−45 | 27.81% | 98% | YES |
| PPO1, Prophenoloxidase 1 | prophenoloxidase response | NP_476812.1 | Not found | Not found | Not found | Not found | - |
| PPO2, Prophenoloxidase 2 | prophenoloxidase response | NP_610443.1 | Not found | Not found | Not found | Not found | - |
| PAF2, Phenoloxidase-activating factor 2 | phenoloxidase activation | AAO24923.1 | XP_022659018.1 | 2 × 10−82 | 41.99% | 77% | YES |
| SP, Serine protease-like precursor | phenoloxidase activation | NP_001097766.1 | XP_022662993.1 | 2 × 10−43 | 37.84% | 56% | YES |
| Hmct, Hemolectin, hemocytin | cell aggregation | NP_001261809.1 | XP_022654008.1 | 8 × 10−158 | 32.23% | 66% | YES |
| Nos, Nitric oxide synthase | production of nitric oxide | NP_001027243.2 | XP_022665384.1 | 6 × 10−68 | 29.27% | 60% | YES |
| Tg, Transglutaminase | clotting | NP_609174.1 | XP_022666443.1 | 9 × 10−121 | 33.06% | 90% | YES |
3.4.1. Antimicrobial Peptides
3.4.2. Lysozyme
3.4.3. Melanization and Coagulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stokstad, E. The Case of the Empty Hives. Science 2007, 316, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Carreck, N.L. Honey Bee Colony Losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.-P.; vanEngelsdorp, D. Drivers of Colony Losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef]
- Kielmanowicz, M.G.; Inberg, A.; Lerner, I.M.; Golani, Y.; Brown, N.; Turner, C.L.; Hayes, G.J.R.; Ballam, J.M. Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses. PLoS Pathog. 2015, 11, e1004816. [Google Scholar] [CrossRef][Green Version]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa destructor. J. Invertebr. Pathol. 2010, 103 (Suppl. S1), S96–S119. [Google Scholar] [CrossRef]
- Noël, A.; Le Conte, Y.; Mondet, F. Varroa Destructor: How Does It Harm Apis mellifera Honey Bees and What Can Be Done about It? Emerg. Top. Life Sci. 2020, 4, 45–57. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor Feeds Primarily on Honey Bee Fat Body Tissue and Not Hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Han, B.; Wu, J.; Wei, Q.; Liu, F.; Cui, L.; Rueppell, O.; Xu, S. Life-History Stage Determines the Diet of Ectoparasitic Mites on Their Honey Bee Hosts. Nat. Commun. 2024, 15, 725. [Google Scholar] [CrossRef]
- De Jong, D.; De Jong, P.H. Longevity of Africanized Honey Bees (Hymenoptera: Apidae) Infested by Varroa jacobsoni (Parasitiformes: Varroidae). J. Econ. Entomol. 1983, 76, 766–768. [Google Scholar] [CrossRef]
- Bowen-Walker, P.L.; Gunn, A. The Effect of the Ectoparasitic Mite, Varroa destructor on Adult Worker Honeybee (Apis Mellifera) Emergence Weights, Water, Protein, Carbohydrate, and Lipid Levels. Entomol. Exp. Appl. 2001, 101, 207–217. [Google Scholar] [CrossRef]
- Traynor, K.S.; Mondet, F.; de Miranda, J.R.; Techer, M.; Kowallik, V.; Oddie, M.A.Y.; Chantawannakul, P.; McAfee, A. Varroa Destructor: A Complex Parasite, Crippling Honey Bees Worldwide. Trends Parasitol. 2020, 36, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Kralj, J.; Fuchs, S. Parasitic Varroa destructor Mites Influence Flight Duration and Homing Ability of Infested Apis mellifera Foragers. Apidologie 2006, 37, 577–587. [Google Scholar] [CrossRef]
- Yang, X.; Cox-Foster, D.L. Impact of an Ectoparasite on the Immunity and Pathology of an Invertebrate: Evidence for Host Immunosuppression and Viral Amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef] [PubMed]
- Wilfert, L.; Long, G.; Leggett, H.C.; Schmid-Hempel, P.; Butlin, R.; Martin, S.J.M.; Boots, M. Deformed Wing Virus Is a Recent Global Epidemic in Honeybees Driven by Varroa Mites. Science 2016, 351, 594–597. [Google Scholar] [CrossRef]
- Boecking, O.; Genersch, E. Varroosis—The Ongoing Crisis in Bee Keeping. J. Für Verbraucherschutz Und Leb. 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Haber, A.I.; Steinhauer, N.A.; vanEngelsdorp, D. Use of Chemical and Nonchemical Methods for the Control of Varroa destructor (Acari: Varroidae) and Associated Winter Colony Losses in U.S. Beekeeping Operations. J. Econ. Entomol. 2019, 112, 1509–1525. [Google Scholar] [CrossRef]
- Lester, P.J. Integrated Resistance Management for Acaricide Use on Varroa destructor. Front. Bee Sci. 2023, 1, 1297326. [Google Scholar] [CrossRef]
- Way, M.J.; van Emden, H.F. Integrated Pest Management in Practice—Pathways towards Successful Application. Crop Prot. 2000, 19, 81–103. [Google Scholar] [CrossRef]
- Vilarem, C.; Piou, V.; Vogelweith, F.; Vétillard, A. Varroa destructor from the Laboratory to the Field: Control, Biocontrol and IPM Perspectives—A Review. Insects 2021, 12, 800. [Google Scholar] [CrossRef]
- Agrawal, N.; Dasaradhi, P.V.N.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA Interference: Biology, Mechanism, and Applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef]
- Caccia, S.; Astarita, F.; Barra, E.; Di Lelio, I.; Varricchio, P.; Pennacchio, F. Enhancement of Bacillus thuringiensis Toxicity by Feeding Spodoptera littoralis Larvae with Bacteria Expressing Immune Suppressive dsRNA. J. Pest Sci. 2020, 93, 303–314. [Google Scholar] [CrossRef]
- Khila, A.; Grbić, M. Gene Silencing in the Spider Mite Tetranychus urticae: dsRNA and siRNA Parental Silencing of the Distal-Less Gene. Dev. Genes Evol. 2007, 217, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Shen, G.; Christiaens, O.; Smagghe, G.; He, L.; Wang, J. Beyond Insects: Current Status and Achievements of RNA Interference in Mite Pests and Future Perspectives. Pest Manag. Sci. 2018, 74, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Garbian, Y.; Maori, E.; Kalev, H.; Shafir, S.; Sela, I. Bidirectional Transfer of RNAi between Honey Bee and Varroa Destructor: Varroa Gene Silencing Reduces Varroa Population. PLoS Pathog 2012, 8, e1003035. [Google Scholar] [CrossRef]
- McGruddy, R.A.; Smeele, Z.E.; Manley, B.; Masucci, J.D.; Haywood, J.; Lester, P.J. RNA Interference as a Next-Generation Control Method for Suppressing Varroa destructor Reproduction in Honey Bee (Apis mellifera) Hives. Pest Manag. Sci. 2024, 80, 4770–4778. [Google Scholar] [CrossRef]
- Leonard, S.P.; Powell, J.E.; Perutka, J.; Geng, P.; Heckmann, L.C.; Horak, R.D.; Davies, B.W.; Ellington, A.D.; Barrick, J.E.; Moran, N.A. Engineered Symbionts Activate Honey Bee Immunity and Limit Pathogens. Science 2020, 367, 573–576. [Google Scholar] [CrossRef]
- Becchimanzi, A.; Tatè, R.; Campbell, E.M.; Gigliotti, S.; Bowman, A.S.; Pennacchio, F. A Salivary Chitinase of Varroa destructor Influences Host Immunity and Mite’s Survival. PLoS Pathog. 2020, 16, e1009075. [Google Scholar] [CrossRef]
- Becchimanzi, A.; Cacace, A.; Parziale, M.; De Leva, G.; Iacopino, S.; Jesu, G.; Di Lelio, I.; Stillittano, V.; Caprio, E.; Pennacchio, F. The Salivary Gland Transcriptome of Varroa destructor Reveals Suitable Targets for RNAi-Based Mite Control. Insect Mol. Biol. 2024. [Google Scholar] [CrossRef]
- Pathak, J.P.N. Insect immunity; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-94-011-1618-3. [Google Scholar]
- Palmer, W.J.; Jiggins, F.M. Comparative Genomics Reveals the Origins and Diversity of Arthropod Immune Systems. Mol. Biol. Evol. 2015, 32, 2111–2129. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, R.; Zhang, J. The Diversity of Pattern Recognition Receptors (PRRs) Involved with Insect Defense against Pathogens. Curr. Opin. Insect Sci. 2019, 33, 105–110. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, J.; Feng, D.; Wang, X.; Zhang, R. Immune Functions of Pattern Recognition Receptors in Lepidoptera. Front. Immunol. 2023, 14, 1203061. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Strand, M.R. The Insect Cellular Immune Response. Insect Sci. 2008, 15, 1–14. [Google Scholar]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-Mediated Immunity in Insects: Cells, Processes and Associated Components in the Fight against Pathogens and Parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway Jr, C.A. Innate Immunity: Impact on the Adaptive Immune Response. Curr. Opin. Immunol. 1997, 9, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tettamanti, G.; Bassal, T.; Heryanto, C.; Eleftherianos, I.; Mohamed, A. Regulators and Signalling in Insect Antimicrobial Innate Immunity: Functional Molecules and Cellular Pathways. Cell. Signal. 2021, 83, 110003. [Google Scholar]
- O’Neal, A.J.; Singh, N.; Rolandelli, A.; Laukaitis, H.J.; Wang, X.; Shaw, D.K.; Young, B.D.; Narasimhan, S.; Dutta, S.; Snyder, G.A.; et al. Croquemort Elicits Activation of the Immune Deficiency Pathway in Ticks. Proc. Natl. Acad. Sci. USA 2023, 120, e2208673120. [Google Scholar] [CrossRef]
- Smith, A.A.; Pal, U. Immunity-Related Genes in Ixodes scapularis—Perspectives from Genome Information. Front. Cell. Infect. Microbiol. 2014, 4, 116. [Google Scholar] [CrossRef]
- Bechsgaard, J.; Vanthournout, B.; Funch, P.; Vestbo, S.; Gibbs, R.A.; Richards, S.; Sanggaard, K.W.; Enghild, J.J.; Bilde, T. Comparative Genomic Study of Arachnid Immune Systems Indicates Loss of Beta-1,3-Glucanase-Related Proteins and the Immune Deficiency Pathway. J. Evol. Biol. 2016, 29, 277–291. [Google Scholar] [CrossRef]
- Rosa, R.D.; Capelli-Peixoto, J.; Mesquita, R.D.; Kalil, S.P.; Pohl, P.C.; Braz, G.R.; Fogaça, A.C.; Daffre, S. Exploring the Immune Signalling Pathway-Related Genes of the Cattle Tick Rhipicephalus microplus: From Molecular Characterization to Transcriptional Profile upon Microbial Challenge. Dev. Comp. Immunol. 2016, 59, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.K.; Wang, X.; Brown, L.J.; Chávez, A.S.O.; Reif, K.E.; Smith, A.A.; Scott, A.J.; McClure, E.E.; Boradia, V.M.; Hammond, H.L.; et al. Infection-Derived Lipids Elicit an Immune Deficiency Circuit in Arthropods. Nat. Commun. 2017, 8, 14401. [Google Scholar] [CrossRef]
- Gerardo, N.M.; Altincicek, B.; Anselme, C.; Atamian, H.; Barribeau, S.M.; de Vos, M.; Duncan, E.J.; Evans, J.D.; Gabaldón, T.; Ghanim, M.; et al. Immunity and Other Defenses in Pea Aphids, Acyrthosiphon pisum. Genome Biol. 2010, 11, R21. [Google Scholar] [CrossRef]
- Becchimanzi, A.; Nicoletti, R.; Di Lelio, I.; Russo, E. Immune Gene Repertoire of Soft Scale Insects (Hemiptera: Coccidae). Int. J. Mol. Sci. 2024, 25, 4922. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinforma. Oxf. Engl. 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De Novo Transcript Sequence Reconstruction from RNA-Seq Using the Trinity Platform for Reference Generation and Analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum Likelihood-Based Phylogenetic Analyses with Thousands of Taxa and Mixed Models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- de Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE Signature Matches and ProRule-Associated Functional and Structural Residues in Proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM Predicts Alpha and Beta Transmembrane Proteins Using Deep Neural Networks. bioRxiv 2022. [Google Scholar]
- Kurata, S. Peptidoglycan Recognition Proteins in Drosophila Immunity. Dev. Comp. Immunol. 2014, 42, 36–41. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. Immune Properties of Invertebrate Phenoloxidases. Dev. Comp. Immunol. 2021, 122, 104098. [Google Scholar] [CrossRef] [PubMed]
- Franc, N.C.; Dimarcq, J.-L.; Lagueux, M.; Hoffmann, J.; Ezekowitz, R.A.B. Croquemort, A Novel Drosophila Hemocyte/Macrophage Receptor That Recognizes Apoptotic Cells. Immunity 1996, 4, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.-J.; Zhan, M.-Y.; Pan, Y.-M.; Liu, S.; Yang, P.-J.; Yang, L.-L.; Yu, X.-Q. Immune Functions of Insect βGRPs and Their Potential Application. Dev. Comp. Immunol. 2018, 83, 80–88. [Google Scholar] [CrossRef]
- Takahashi, D.; Garcia, B.L.; Kanost, M.R. Initiating Protease with Modular Domains Interacts with β-Glucan Recognition Protein to Trigger Innate Immune Response in Insects. Proc. Natl. Acad. Sci. USA 2015, 112, 13856–13861. [Google Scholar] [CrossRef]
- Warr, E.; Das, S.; Dong, Y.; Dimopoulos, G. The Gram-Negative Bacteria-Binding Protein Gene Family: Its Role in the Innate Immune System of Anopheles gambiae and in Anti-Plasmodium Defence. Insect Mol. Biol. 2008, 17, 39–51. [Google Scholar] [CrossRef]
- Pili-Floury, S.; Leulier, F.; Takahashi, K.; Saigo, K.; Samain, E.; Ueda, R.; Lemaitre, B. In Vivo RNA Interference Analysis Reveals an Unexpected Role for GNBP1 in the Defense against Gram-Positive Bacterial Infection in Drosophila Adults. J. Biol. Chem. 2004, 279, 12848–12853. [Google Scholar] [CrossRef]
- Gottar, M.; Gobert, V.; Matskevich, A.A.; Reichhart, J.-M.; Wang, C.; Butt, T.M.; Belvin, M.; Hoffmann, J.A.; Ferrandon, D. Dual Detection of Fungal Infections in Drosophila via Recognition of Glucans and Sensing of Virulence Factors. Cell 2006, 127, 1425–1437. [Google Scholar] [CrossRef]
- Xia, X.; You, M.; Rao, X.-J.; Yu, X.-Q. Insect C-Type Lectins in Innate Immunity. Dev. Comp. Immunol. 2018, 83, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, G.; Zhuo, X.; Liu, Y.; Tang, L.; Liu, X.; Wang, J. C-Type Lectin-Mediated Microbial Homeostasis Is Critical for Helicoverpa armigera Larval Growth and Development. PLoS Pathog. 2020, 16, e1008901. [Google Scholar] [CrossRef]
- Ao, J.; Ling, E.; Yu, X.-Q. Drosophila C-Type Lectins Enhance Cellular Encapsulation. Mol. Immunol. 2007, 44, 2541–2548. [Google Scholar] [CrossRef]
- Santos-Matos, G.; Wybouw, N.; Martins, N.E.; Zélé, F.; Riga, M.; Leitão, A.B.; Vontas, J.; Grbić, M.; Van Leeuwen, T.; Magalhães, S.; et al. Tetranychus urticae Mites Do Not Mount an Induced Immune Response against Bacteria. Proc. Biol. Sci. 2017, 284, 20170401. [Google Scholar] [CrossRef]
- Pace, K.E.; Baum, L.G. Insect Galectins: Roles in Immunity and Development. Glycoconj. J. 2002, 19, 607–614. [Google Scholar] [CrossRef]
- Drickamer, K. Two Distinct Classes of Carbohydrate-Recognition Domains in Animal Lectins. J. Biol. Chem. 1988, 263, 9557–9560. [Google Scholar]
- Dimopoulos, G.; Richman, A.; della Torre, A.; Kafatos, F.C.; Louis, C. Identification and Characterization of Differentially Expressed cDNAs of the Vector Mosquito, Anopheles Gambiae. Proc. Natl. Acad. Sci. USA 1996, 93, 13066–13071. [Google Scholar] [CrossRef] [PubMed]
- Kamhawi, S.; Ramalho-Ortigao, M.; Pham, V.M.; Kumar, S.; Lawyer, P.G.; Turco, S.J.; Barillas-Mury, C.; Sacks, D.L.; Valenzuela, J.G. A Role for Insect Galectins in Parasite Survival. Cell 2004, 119, 329–341. [Google Scholar] [CrossRef]
- Rao, X.-J.; Wu, P.; Shahzad, T.; Liu, S.; Chen, L.; Yang, Y.-F.; Shi, Q.; Yu, X.-Q. Characterization of a Dual-CRD Galectin in the Silkworm Bombyx Mori. Dev. Comp. Immunol. 2016, 60, 149–159. [Google Scholar] [CrossRef]
- Waterhouse, R.M.; Kriventseva, E.V.; Meister, S.; Xi, Z.; Alvarez, K.S.; Bartholomay, L.C.; Barillas-Mury, C.; Bian, G.; Blandin, S.; Christensen, B.M.; et al. Evolutionary Dynamics of Immune-Related Genes and Pathways in Disease-Vector Mosquitoes. Science 2007, 316, 1738–1743. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, J.-L.; Cheng, Y.; Wang, J.-X.; Zou, Z. Pattern Recognition Receptors from Lepidopteran Insects and Their Biological Functions. Dev. Comp. Immunol. 2020, 108, 103688. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Thangamani, S.; Ho, B.; Ding, J.L. The Ancient Origin of the Complement System. EMBO J. 2005, 24, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, R.; Fujito, N.T.; Nonaka, M. Evolution of the Thioester-Containing Proteins (TEPs) of the Arthropoda, Revealed by Molecular Cloning of TEP Genes from a Spider, Hasarius Adansoni. Dev. Comp. Immunol. 2012, 36, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Sottrup-Jensen, L.; Folkersen, J.; Kristensen, T.; Tack, B.F. Partial Primary Structure of Human Pregnancy Zone Protein: Extensive Sequence Homology with Human Alpha 2-Macroglobulin. Proc. Natl. Acad. Sci. USA 1984, 81, 7353–7357. [Google Scholar] [CrossRef]
- Lin, M.; Sutherland, D.R.; Horsfall, W.; Totty, N.; Yeo, E.; Nayar, R.; Wu, X.-F.; Schuh, A.C. Cell Surface Antigen CD109 Is a Novel Member of the A2 Macroglobulin/C3, C4, C5 Family of Thioester-Containing Proteins. Blood 2002, 99, 1683–1691. [Google Scholar] [CrossRef]
- Li, Z.-F.; Wu, X.; Engvall, E. Identification and Characterization of CPAMD8, a Novel Member of the Complement 3/A2-Macroglobulin Family with a C-Terminal Kazal Domain. Genomics 2004, 83, 1083–1093. [Google Scholar] [CrossRef]
- Lagueux, M.; Perrodou, E.; Levashina, E.A.; Capovilla, M.; Hoffmann, J.A. Constitutive Expression of a Complement-like Protein in Toll and JAK Gain-of-Function Mutants of Drosophila. Proc. Natl. Acad. Sci. USA 2000, 97, 11427–11432. [Google Scholar] [CrossRef]
- Levashina, E.A.; Moita, L.F.; Blandin, S.; Vriend, G.; Lagueux, M.; Kafatos, F.C. Conserved Role of a Complement-like Protein in Phagocytosis Revealed by dsRNA Knockout in Cultured Cells of the Mosquito, Anopheles Gambiae. Cell 2001, 104, 709–718. [Google Scholar] [CrossRef]
- Theopold, U.; Schmid, M. Thioester-Containing Proteins: At the Crossroads of Immune Effector Mechanisms. Virulence 2017, 8, 1468–1470. [Google Scholar] [CrossRef][Green Version]
- Boutros, M.; Agaisse, H.; Perrimon, N. Sequential Activation of Signaling Pathways during Innate Immune Responses in Drosophila. Dev. Cell 2002, 3, 711–722. [Google Scholar] [CrossRef]
- Towb, P.; Sun, H.; Wasserman, S.A. Tube Is an IRAK-4 Homolog in a Toll Pathway Adapted for Development and Immunity. J. Innate Immun. 2009, 1, 309–321. [Google Scholar] [CrossRef]
- Gay, N.J.; Gangloff, M.; Weber, A.N.R. Toll-like Receptors as Molecular Switches. Nat. Rev. Immunol. 2006, 6, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.J.; Gilmore, T.D. Evolutionary Origins of Toll-like Receptor Signaling. Mol. Biol. Evol. 2018, 35, 1576–1587. [Google Scholar] [CrossRef]
- Kanzok, S.M.; Hoa, N.T.; Bonizzoni, M.; Luna, C.; Huang, Y.; Malacrida, A.R.; Zheng, L. Origin of Toll-Like Receptor-Mediated Innate Immunity. J. Mol. Evol. 2004, 58, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Leulier, F.; Lemaitre, B. Toll-like Receptors—Taking an Evolutionary Approach. Nat. Rev. Genet. 2008, 9, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Agaisse, H.; Perrimon, N. The Roles of JAK/STAT Signaling in Drosophila Immune Responses. Immunol. Rev. 2004, 198, 72–82. [Google Scholar] [CrossRef]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.-L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune Pathways and Defence Mechanisms in Honey Bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef]
- Dionne, M.S.; Schneider, D.S. Models of Infectious Diseases in the Fruit Fly Drosophila melanogaster. Dis. Model. Mech. 2008, 1, 43–49. [Google Scholar] [CrossRef]
- Naitza, S.; Rossé, C.; Kappler, C.; Georgel, P.; Belvin, M.; Gubb, D.; Camonis, J.; Hoffmann, J.A.; Reichhart, J.M. The Drosophila Immune Defense against Gram-Negative Infection Requires the Death Protein dFADD. Immunity 2002, 17, 575–581. [Google Scholar] [CrossRef]
- Kaneko, T.; Yano, T.; Aggarwal, K.; Lim, J.-H.; Ueda, K.; Oshima, Y.; Peach, C.; Erturk-Hasdemir, D.; Goldman, W.E.; Oh, B.-H.; et al. PGRP-LC and PGRP-LE Have Essential yet Distinct Functions in the Drosophila Immune Response to Monomeric DAP-Type Peptidoglycan. Nat. Immunol. 2006, 7, 715–723. [Google Scholar] [CrossRef]
- Meinander, A.; Runchel, C.; Tenev, T.; Chen, L.; Kim, C.-H.; Ribeiro, P.S.; Broemer, M.; Leulier, F.; Zvelebil, M.; Silverman, N.; et al. Ubiquitylation of the Initiator Caspase DREDD Is Required for Innate Immune Signalling. EMBO J. 2012, 31, 2770–2783. [Google Scholar] [CrossRef] [PubMed]
- Paquette, N.; Broemer, M.; Aggarwal, K.; Chen, L.; Husson, M.; Ertürk-Hasdemir, D.; Reichhart, J.-M.; Meier, P.; Silverman, N. Caspase Mediated Cleavage, IAP Binding and Ubiquitination: Linking Three Mechanisms Crucial for Drosophila NF-κB Signaling. Mol. Cell 2010, 37, 172. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila Melanogaster—from Microbial Recognition to Whole-Organism Physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef]
- Kleino, A.; Silverman, N. The Drosophila IMD Pathway in the Activation of the Humoral Immune Response. Dev. Comp. Immunol. 2014, 42, 25–35. [Google Scholar] [CrossRef]
- Kleino, A.; Myllymäki, H.; Kallio, J.; Vanha-aho, L.-M.; Oksanen, K.; Ulvila, J.; Hultmark, D.; Valanne, S.; Rämet, M. Pirk Is a Negative Regulator of the Drosophila Imd Pathway. J. Immunol. Baltim. Md 1950 2008, 180, 5413–5422. [Google Scholar] [CrossRef] [PubMed]
- Stöven, S.; Ando, I.; Kadalayil, L.; Engström, Y.; Hultmark, D. Activation of the Drosophila NF-κB Factor Relish by Rapid Endoproteolytic Cleavage. EMBO Rep. 2000, 1, 347–352. [Google Scholar] [CrossRef]
- Stöven, S.; Silverman, N.; Junell, A.; Hedengren-Olcott, M.; Erturk, D.; Engström, Y.; Maniatis, T.; Hultmark, D. Caspase-Mediated Processing of the Drosophila NF-κB Factor Relish. Proc. Natl. Acad. Sci. USA 2003, 100, 5991–5996. [Google Scholar] [CrossRef]
- Ertürk-Hasdemir, D.; Broemer, M.; Leulier, F.; Lane, W.S.; Paquette, N.; Hwang, D.; Kim, C.-H.; Stöven, S.; Meier, P.; Silverman, N. Two Roles for the Drosophila IKK Complex in the Activation of Relish and the Induction of Antimicrobial Peptide Genes. Proc. Natl. Acad. Sci. USA 2009, 106, 9779–9784. [Google Scholar] [CrossRef]
- Fogaça, A.C.; Sousa, G.; Pavanelo, D.B.; Esteves, E.; Martins, L.A.; Urbanová, V.; Kopáček, P.; Daffre, S. Tick Immune System: What Is Known, the Interconnections, the Gaps, and the Challenges. Front. Immunol. 2021, 12, 628054. [Google Scholar] [CrossRef]
- Müller, C.W.; Rey, F.A.; Sodeoka, M.; Verdine, G.L.; Harrison, S.C. Structure of the NF-κB P50 Homodimer Bound to DNA. Nature 1995, 373, 311–317. [Google Scholar] [CrossRef]
- Dushay, M.S.; Asling, B.; Hultmark, D. Origins of Immunity: Relish, a Compound Rel-like Gene in the Antibacterial Defense of Drosophila. Proc. Natl. Acad. Sci. USA 1996, 93, 10343–10347. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, V.; Ayllón, N.; de la Lastra, J.M.P.; Galindo, R.C.; Kocan, K.M.; Blouin, E.F.; Mitra, R.; Alberdi, P.; Villar, M.; Fuente, J. de la Reciprocal Regulation of NF-kB (Relish) and Subolesin in the Tick Vector, Ixodes scapularis. PLoS ONE 2013, 8, e65915. [Google Scholar] [CrossRef]
- Capelli-Peixoto, J.; Carvalho, D.D.; Johnson, W.C.; Scoles, G.A.; Fogaça, A.C.; Daffre, S.; Ueti, M.W. The Transcription Factor Relish Controls Anaplasma marginale Infection in the Bovine Tick Rhipicephalus microplus. Dev. Comp. Immunol. 2017, 74, 32–39. [Google Scholar] [CrossRef]
- Fongsaran, C.; Verhoeve, V.I.; Jirakanwisal, K.; Harris, E.K.; Macaluso, K.R. Identification and Characterization of a Relish-Type NF-κB, DvRelish, in Dermacentor variabilis in Response to Rickettsia Rickettsii Infection. Front. Cell. Infect. Microbiol. 2024, 14, 1494450. [Google Scholar] [CrossRef]
- Jalovecka, M.; Malandrin, L.; Urbanova, V.; Mahmood, S.; Snebergerova, P.; Peklanska, M.; Pavlasova, V.; Sima, R.; Kopacek, P.; Perner, J.; et al. Activation of the Tick Toll Pathway to Control Infection of Ixodes ricinus by the Apicomplexan Parasite Babesia Microti. PLoS Pathog. 2024, 20, e1012743. [Google Scholar] [CrossRef]
- Oliva Chávez, A.S.; Shaw, D.K.; Munderloh, U.G.; Pedra, J.H.F. Tick Humoral Responses: Marching to the Beat of a Different Drummer. Front. Microbiol. 2017, 8, 223. [Google Scholar] [CrossRef]
- Severo, M.S.; Choy, A.; Stephens, K.D.; Sakhon, O.S.; Chen, G.; Chung, D.-W.D.; Le Roch, K.G.; Blaha, G.; Pedra, J.H.F. The E3 Ubiquitin Ligase XIAP Restricts Anaplasma phagocytophilum Colonization of Ixodes scapularis Ticks. J. Infect. Dis. 2013, 208, 1830–1840. [Google Scholar] [CrossRef]
- McClure Carroll, E.E.; Wang, X.; Shaw, D.K.; O’Neal, A.J.; Oliva Chávez, A.S.; Brown, L.J.; Boradia, V.M.; Hammond, H.L.; Pedra, J.H.F. P47 Licenses Activation of the Immune Deficiency Pathway in the Tick Ixodes scapularis. Proc. Natl. Acad. Sci. USA 2019, 116, 205–210. [Google Scholar] [CrossRef]
- Myllymäki, H.; Valanne, S.; Rämet, M. The Drosophila Imd Signaling Pathway. J. Immunol. Baltim. Md 1950 2014, 192, 3455–3462. [Google Scholar] [CrossRef]
- De Gregorio, E.; Spellman, P.T.; Tzou, P.; Rubin, G.M.; Lemaitre, B. The Toll and Imd Pathways Are the Major Regulators of the Immune Response in Drosophila. EMBO J. 2002, 21, 2568–2579. [Google Scholar] [CrossRef]
- Stączek, S.; Cytryńska, M.; Zdybicka-Barabas, A. Unraveling the Role of Antimicrobial Peptides in Insects. Int. J. Mol. Sci. 2023, 24, 5753. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, X.; Chen, Q.; Chen, Z.; Zhang, J.; Yang, L.; Sun, Y.; Wang, G.; Dai, J.; Feng, T. Defensins as a Promising Class of Tick Antimicrobial Peptides: A Scoping Review. Infect. Dis. Poverty 2022, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Kämpfer, U.; Schürch, S.; Schaller, J.; Largiadèr, C.; Nentwig, W.; Kuhn-Nentwig, L. Ctenidins: Antimicrobial Glycine-Rich Peptides from the Hemocytes of the Spider Cupiennius salei. Cell. Mol. Life Sci. 2010, 67, 2787–2798. [Google Scholar] [CrossRef]
- Bachali, S.; Jager, M.; Hassanin, A.; Schoentgen, F.; Jollès, P.; Fiala-Medioni, A.; Deutsch, J.S. Phylogenetic Analysis of Invertebrate Lysozymes and the Evolution of Lysozyme Function. J. Mol. Evol. 2002, 54, 652–664. [Google Scholar] [CrossRef]
- Van Herreweghe, J.M.; Michiels, C.W. Invertebrate Lysozymes: Diversity and Distribution, Molecular Mechanism and in Vivo Function. J. Biosci. 2012, 37, 327–348. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawano, S.; Nakao, S.; Umemiya-Shirafuji, R.; Rahman, M.M.; Boldbaatar, D.; Battur, B.; Liao, M.; Fujisaki, K. The Identification and Characterization of Lysozyme from the Hard Tick Haemaphysalis longicornis. Ticks Tick-Borne Dis. 2010, 1, 178–185. [Google Scholar] [CrossRef]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-System: Pros and Cons for Its Role in Invertebrate Immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [PubMed]
- Burmester, T. Origin and Evolution of Arthropod Hemocyanins and Related Proteins. J. Comp. Physiol. B 2002, 172, 95–107. [Google Scholar] [CrossRef]
- Nagai, T.; Osaki, T.; Kawabata, S. Functional Conversion of Hemocyanin to Phenoloxidase by Horseshoe Crab Antimicrobial Peptides. J. Biol. Chem. 2001, 276, 27166–27170. [Google Scholar] [CrossRef]
- Voit, R.; Feldmaier-Fuchs, G.; Schweikardt, T.; Decker, H.; Burmester, T. Complete Sequence of the 24-Mer Hemocyanin of the Tarantula Eurypelma Californicum. Structure and Intramolecular Evolution of the Subunits. J. Biol. Chem. 2000, 275, 39339–39344. [Google Scholar] [CrossRef]
- Zhioua, E.; Browning, M.; Johnson, P.W.; Ginsberg, H.S.; LeBrun, R.A. Pathogenicity of the Entomopathogenic Fungus Metarhizium Anisopliae (Deuteromycetes) to Ixodes scapularis (Acari: Ixodidae). J. Parasitol. 1997, 83, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Simser, J.A.; Macaluso, K.R.; Mulenga, A.; Azad, A.F. Immune-Responsive Lysozymes from Hemocytes of the American Dog Tick, Dermacentor variabilis and an Embryonic Cell Line of the Rocky Mountain Wood Tick, D. Andersoni. Insect Biochem. Mol. Biol. 2004, 34, 1235–1246. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacace, A.; De Leva, G.; Di Lelio, I.; Becchimanzi, A. Immune-Related Genes in the Honey Bee Mite Varroa destructor (Acarina, Parasitidae). Insects 2025, 16, 356. https://doi.org/10.3390/insects16040356
Cacace A, De Leva G, Di Lelio I, Becchimanzi A. Immune-Related Genes in the Honey Bee Mite Varroa destructor (Acarina, Parasitidae). Insects. 2025; 16(4):356. https://doi.org/10.3390/insects16040356
Chicago/Turabian StyleCacace, Alfonso, Giovanna De Leva, Ilaria Di Lelio, and Andrea Becchimanzi. 2025. "Immune-Related Genes in the Honey Bee Mite Varroa destructor (Acarina, Parasitidae)" Insects 16, no. 4: 356. https://doi.org/10.3390/insects16040356
APA StyleCacace, A., De Leva, G., Di Lelio, I., & Becchimanzi, A. (2025). Immune-Related Genes in the Honey Bee Mite Varroa destructor (Acarina, Parasitidae). Insects, 16(4), 356. https://doi.org/10.3390/insects16040356







