Environmental Sources of Possible Associated Pathogens and Contaminants of Stingless Bees in the Neotropics
Simple Summary
Abstract
1. Introduction
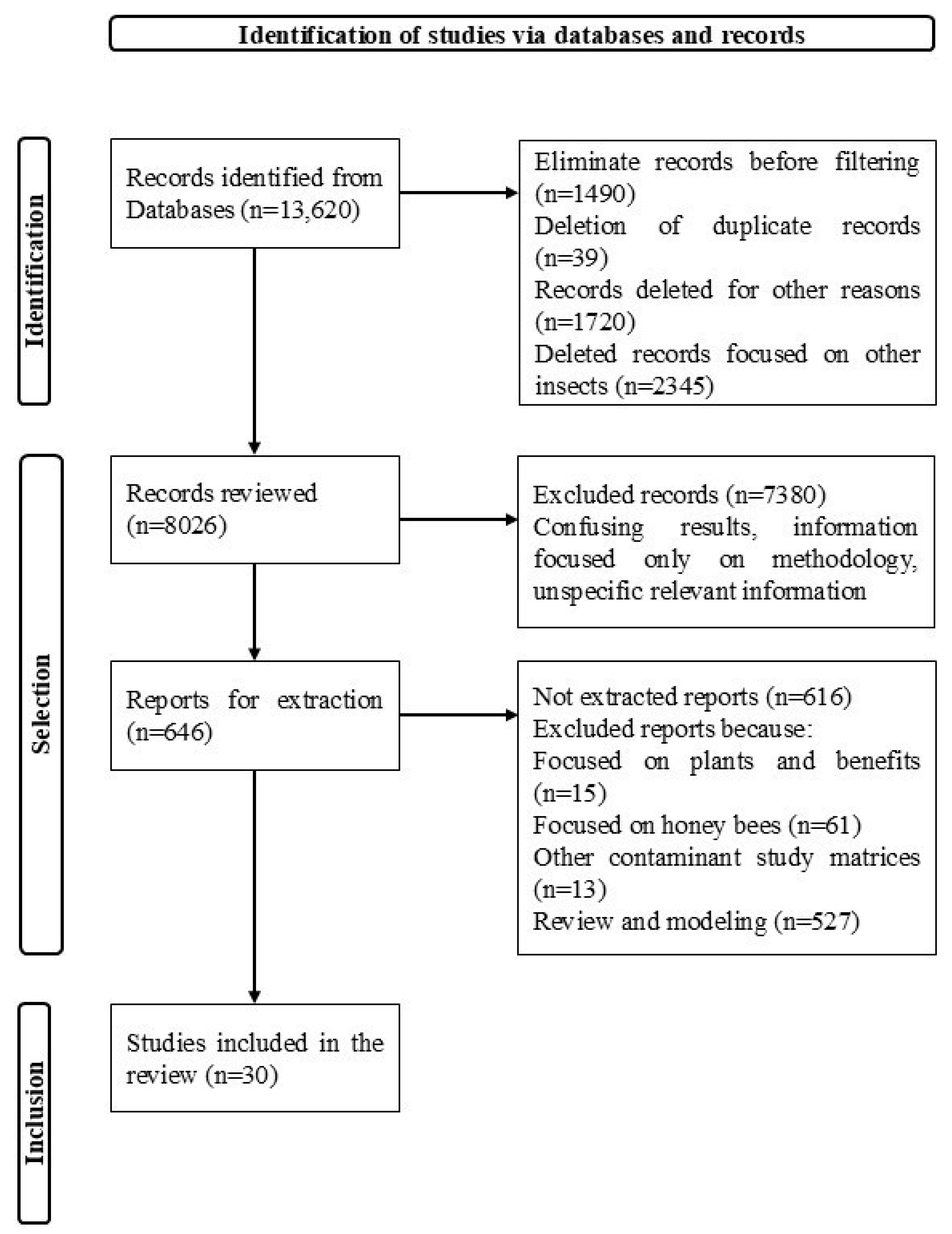
2. Materials and Methods
3. Results
3.1. Study Matrices for Pathogens and Pollutants in Stingless Bees
3.2. Bacterial, Fungi, and Viral Pathogens of Stingless Bees
3.3. Anthropogenic Contaminants in Stingless-Bee By-Products
4. Discussion
4.1. Occurrence and Reporting of Pathogens in Stingless Bees
4.2. Bees and Nest By-Products as Bioindicators of Environmental Health
4.3. Good Management Practices (GMPs) in Meliponiculture
4.4. One Health Approach
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roubik, D.W. Stingless Bee (Apidae: Apinae: Meliponini) Ecology. Annu. Rev. Entomol. 2023, 68, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Dymond, K.; Celis-Diez, J.L.; Potts, S.G.; Howlett, B.G.; Willcox, B.K.; Garratt, M.P.D. The role of insect pollinators in avocado production: A global review. J. Appl. Entomol. 2021, 145, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trusheva, B.; Ilieva, N.; Thanh, L.N.; Lien, N.T.P.; Bankova, V. Mangifera indica as propolis source: What exactly do bees collect? BMC Res. Notes 2021, 14, 448. [Google Scholar] [CrossRef]
- Layek, U.; Kundu, A.; Bisui, S.; Karmakar, P. Impact of managed stingless bee and western honey bee colonies on native pollinators and yield of watermelon: A comparative study. Ann. Agric. Sci. 2021, 66, 38–45. [Google Scholar] [CrossRef]
- Esa, N.E.F.; Ansari, M.N.M.; Razak, S.I.A.; Ismail, N.I.; Jusoh, N.; Zawawi, N.A.; Jamaludin, M.I.; Sagadevan, S.; Nayan, N.H.M. A Review on Recent Progress of Stingless Bee Honey and Its Hydrogel-Based Compound for Wound Care Management. Molecules 2022, 27, 3080. [Google Scholar] [CrossRef]
- Ávila, S.; Beux, M.R.; Hoffmann Ribani, R.; Zambiazi, R.C. Stingless bee honey: Quality parameters, bioactive compounds, health-promotion properties and modification detection strategies. Trends Food Sci. Technol. 2018, 81, 37–50. [Google Scholar] [CrossRef]
- Cabezas-Mera, F.S.; Atiencia-Carrera, M.B.; Villacrés-Granda, I.; Proaño, A.A.; Debut, A.; Vizuete, K.; Herrero-Bayo, L.; Gonzalez-Paramás, A.M.; Giampieri, F.; Abreu-Naranjo, R.; et al. Evaluation of the polyphenolic profile of native Ecuadorian stingless bee honeys (Tribe: Meliponini) and their antibiofilm activity on susceptible and multidrug-resistant pathogens: An exploratory analysis. Curr. Res. Food Sci. 2023, 7, 100543. [Google Scholar] [CrossRef]
- Villacrés-Granda, I.; Coello, D.; Proaño, A.; Ballesteros, I.; Roubik, D.W.; Jijón, G.; Granda-Albuja, G.; Granda-Albuja, S.; Abreu-Naranjo, R.; Maza, F.; et al. Honey quality parameters, chemical composition and antimicrobial activity in twelve Ecuadorian stingless bees (Apidae: Apinae: Meliponini) tested against multiresistant human pathogens. LWT 2021, 140, 110737. [Google Scholar] [CrossRef]
- Hrncir, M.; Jarau, S.; Barth, F.G. Stingless bees (Meliponini): Senses and behavior. J. Comp. Physiol. A 2016, 202, 597–601. [Google Scholar] [CrossRef]
- Martins, A.C.; Proença, C.E.B.; Vasconcelos, T.N.C.; Aguiar, A.J.C.; Farinasso, H.C.; de Lima, A.T.F.; Faria, J.E.Q.; Norrana, K.; Costa, M.B.R.; Carvalho, M.M.; et al. Contrasting patterns of foraging behavior in neotropical stingless bees using pollen and honey metabarcoding. Sci. Rep. 2023, 13, 14474. [Google Scholar] [CrossRef]
- Grüter, C.; Balbuena, M.S.; Valadares, L. Mechanisms and adaptations that shape division of labour in stingless bees. Curr. Opin. Insect Sci. 2023, 58, 101057. [Google Scholar] [CrossRef]
- Jaffé, R.; Pope, N.; Carvalho, A.T.; Maia, U.M.; Blochtein, B.; de Carvalho, C.A.L.; Carvalho-Zilse, G.A.; Freitas, B.M.; Menezes, C.; Ribeiro, M.d.F.; et al. Bees for Development: Brazilian Survey Reveals How to Optimize Stingless Beekeeping. PLoS ONE 2015, 10, e0121157. [Google Scholar] [CrossRef]
- Mustafa, M.Z.; Yaacob, N.S.; Sulaiman, S.A. Reinventing the Honey Industry: Opportunities of the Stingless Bee. Malays. J. Med. Sci. MJMS 2018, 25, 1–5. [Google Scholar] [CrossRef]
- Priyambodo, P.; Rustiati, E.L.; Permatasari, N.; Sidik, M.; Lestari, I.A.; Yani, A.A.; Sa’uddah, L.D. Optimizing honey production in stingless bee farming. J. Community Serv. Empower. 2023, 4, 360–367. [Google Scholar] [CrossRef]
- Supeno, E. The production of honey and pot-pollen from stingless bee Tetragonula clypearis and their contribution to increase the farmers income in West Lombok, Indonesia. Livest. Res. Rural Dev. 2022, 34, 20220221133. [Google Scholar]
- Mayes, D.M.; Bhatta, C.P.; Shi, D.; Brown, J.C.; Smith, D.R. Body Size Influences Stingless Bee (Hymenoptera: Apidae) Communities Across a Range of Deforestation Levels in Rondônia, Brazil. J. Insect Sci. 2019, 19, 23. [Google Scholar] [CrossRef]
- Requier, F.; Leyton, M.S.; Morales, C.L.; Garibaldi, L.A.; Giacobino, A.; Porrini, M.P.; Rosso-Londoño, J.M.; Velarde, R.A.; Aignasse, A.; Aldea-Sánchez, P.; et al. First large-scale study reveals important losses of managed honey bee and stingless bee colonies in Latin America. Sci. Rep. 2024, 14, 10079. [Google Scholar] [CrossRef]
- Lichtenberg, E.M.; Mendenhall, C.D.; Brosi, B. Foraging traits modulate stingless bee community disassembly under forest loss. J. Anim. Ecol. 2017, 86, 1404–1416. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Cobos, M.E.; Jaramillo, J.; Ospina, R. Climate change will reduce the potential distribution ranges of Colombia’s most valuable pollinators. Perspect. Ecol. Conserv. 2021, 19, 195–206. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Oyen, K.; Vitale, N.; Ospina, R. Neotropical stingless bees display a strong response in cold tolerance with changes in elevation. Conserv. Physiol. 2022, 10, coac073. [Google Scholar] [CrossRef]
- Ostwald, M.M.; da Silva, C.R.B.; Seltmann, K.C. How does climate change impact social bees and bee sociality? J. Anim. Ecol. 2024, 93, 1610–1621. [Google Scholar] [CrossRef]
- Becker, T.; Pequeno, P.A.C.L.; Carvalho-Zilse, G.A. Impact of environmental temperatures on mortality, sex and caste ratios in Melipona interrupta Latreille (Hymenoptera, Apidae). Naturwissenschaften 2018, 105, 55. [Google Scholar] [CrossRef]
- Dos Santos, C.F.; Acosta, A.L.; Nunes-Silva, P.; Saraiva, A.M.; Blochtein, B. Climate Warming May Threaten Reproductive Diapause of a Highly Eusocial Bee. Environ. Entomol. 2015, 44, 1172–1181. [Google Scholar] [CrossRef]
- Lima, V.P.; Marchioro, C.A. Brazilian stingless bees are threatened by habitat conversion and climate change. Reg. Environ. Chang. 2021, 21, 14. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Slaa, E.J. Information flow and organization of stingless bee foraging. Apidologie 2004, 35, 143–157. [Google Scholar] [CrossRef]
- Koethe, S.; Banysch, S.; Alves-dos-Santos, I.; Lunau, K. Spectral purity, intensity and dominant wavelength: Disparate colour preferences of two Brazilian stingless bee species. PLoS ONE 2018, 13, e0204663. [Google Scholar] [CrossRef]
- Harrap, M.J.; Rands, S.A.; Hempel de Ibarra, N.; Whitney, H.M. The diversity of floral temperature patterns, and their use by pollinators. eLife 2017, 6, e31262. [Google Scholar] [CrossRef]
- Koethe, S.; Fischbach, V.; Banysch, S.; Reinartz, L.; Hrncir, M.; Lunau, K. A Comparative Study of Food Source Selection in Stingless Bees and Honeybees: Scent Marks, Location, or Color. Front. Plant Sci. 2020, 11, 516. [Google Scholar] [CrossRef]
- Hrncir, M.; Barth, F.G. Vibratory Communication in Stingless Bees (Meliponini): The Challenge of Interpreting the Signals. In Studying Vibrational Communication; Cocroft, R.B., Gogala, M., Hill, P.S.M., Wessel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 349–374. ISBN 978-3-662-43607-3. [Google Scholar]
- Villagómez, G.N.; Keller, A.; Rasmussen, C.; Lozano, P.; Donoso, D.A.; Blüthgen, N.; Leonhardt, S.D. Nutrients or resin?—The relationship between resin and food foraging in stingless bees. Ecol. Evol. 2024, 14, e10879. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.D.; Blüthgen, N. A Sticky Affair: Resin Collection by Bornean Stingless Bees. Biotropica 2009, 41, 730–736. [Google Scholar] [CrossRef]
- Nicholls, E.; Rands, S.A.; Botías, C.; Hempel de Ibarra, N. Flower sharing and pollinator health: A behavioural perspective. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210157. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef]
- de Souza, F.S.; Kevill, J.L.; Correia-Oliveira, M.E.; de Carvalho, C.A.L.; Martin, S.J. Occurrence of deformed wing virus variants in the stingless bee Melipona subnitida and honey bee Apis mellifera populations in Brazil. J. Gen. Virol. 2019, 100, 289–294. [Google Scholar] [CrossRef]
- Dalmon, A.; Desbiez, C.; Coulon, M.; Thomasson, M.; Le Conte, Y.; Alaux, C.; Vallon, J.; Moury, B. Evidence for positive selection and recombination hotspots in Deformed wing virus (DWV). Sci. Rep. 2017, 7, 41045. [Google Scholar] [CrossRef]
- Morfin, N.; Gashout, H.A.; Macías-Macías, J.O.; De la Mora, A.; Tapia-Rivera, J.C.; Tapia-González, J.M.; Contreras-Escareño, F.; Guzman-Novoa, E. Detection, replication and quantification of deformed wing virus-A, deformed wing virus-B, and black queen cell virus in the endemic stingless bee, Melipona colimana, from Jalisco, Mexico. Int. J. Trop. Insect Sci. 2021, 41, 1285–1292. [Google Scholar] [CrossRef]
- Shanks, J.L.; Haigh, A.M.; Riegler, M.; Spooner-Hart, R.N. First confirmed report of a bacterial brood disease in stingless bees. J. Invertebr. Pathol. 2017, 144, 7–10. [Google Scholar] [CrossRef]
- Teixeira, É.W.; Ferreira, E.A.; da Luz, C.F.P.; Martins, M.F.; Ramos, T.A.; Lourenço, A.P. European Foulbrood in stingless bees (Apidae: Meliponini) in Brazil: Old disease, renewed threat. J. Invertebr. Pathol. 2020, 172, 107357. [Google Scholar] [CrossRef]
- Porrini, M.P.; Porrini, L.P.; Garrido, P.M.; de Melo e Silva Neto, C.; Porrini, D.P.; Muller, F.; Nuñez, L.A.; Alvarez, L.; Iriarte, P.F.; Eguaras, M.J. Nosema ceranae in South American Native Stingless Bees and Social Wasp. Microb. Ecol. 2017, 74, 761–764. [Google Scholar] [CrossRef]
- Al Toufailia, H.; Alves, D.A.; Bento, J.M.S.; Marchini, L.C.; Ratnieks, F.L.W. Hygienic behaviour in Brazilian stingless bees. Biol. Open 2016, 5, 1712–1718. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.; Saegerman, C.; Cox-Foster, D.L. Colony Collapse Disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE 2017, 12, e0179535. [Google Scholar] [CrossRef]
- Oliveira, G.d.L.T. Political ecology of soybeans in South America. In Political Ecology of Industrial Crops; Routledge: London, UK, 2021; pp. 201–220. ISBN 978042935110. [Google Scholar]
- Gemmill-Herren, B.; Garibaldi, L.A.; Kremen, C.; Ngo, H.T. Building effective policies to conserve pollinators: Translating knowledge into policy. Curr. Opin. Insect Sci. 2021, 46, 64–71. [Google Scholar] [CrossRef]
- Lourencetti, A.P.S.; Azevedo, P.; Miotelo, L.; Malaspina, O.; Nocelli, R.C.F. Surrogate species in pesticide risk assessments: Toxicological data of three stingless bees species. Environ. Pollut. 2023, 318, 120842. [Google Scholar] [CrossRef]
- de Morais, C.R.; Travençolo, B.A.N.; Carvalho, S.M.; Beletti, M.E.; Vieira Santos, V.S.; Campos, C.F.; de Campos Júnior, E.O.; Pereira, B.B.; Carvalho Naves, M.P.; de Rezende, A.A.A.; et al. Ecotoxicological effects of the insecticide fipronil in Brazilian native stingless bees Melipona scutellaris (Apidae: Meliponini). Chemosphere 2018, 206, 632–642. [Google Scholar] [CrossRef]
- Bogo, G.; Caringi, V.; Albertazzi, S.; Capano, V.; Colombo, R.; Dettori, A.; Guerra, I.; Lora, G.; Bortolotti, L.; Medrzycki, P. Residues of agrochemicals in beebread as an indicator of landscape management. Sci. Total Environ. 2024, 945, 174075. [Google Scholar] [CrossRef]
- Conceição de Assis, J.; Tadei, R.; Menezes-Oliveira, V.B.; Silva-Zacarin, E.C.M. Are native bees in Brazil at risk from the exposure to the neonicotinoid imidacloprid? Environ. Res. 2022, 212, 113127. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Estrella-Maldonado, H.; Paxton, R.J.; Solís, T.; Quezada-Euán, J.J.G. The Insecticide Imidacloprid Decreases Nannotrigona Stingless Bee Survival and Food Consumption and Modulates the Expression of Detoxification and Immune-Related Genes. Insects 2022, 13, 972. [Google Scholar] [CrossRef]
- Bogo, G.; Porrini, M.P.; Aguilar-Monge, I.; Aldea-Sánchez, P.; de Groot, G.S.; Velarde, R.A.; Xolalpa-Aroche, A.; Vázquez, D.E. Current status of toxicological research on stingless bees (Apidae, Meliponini): Important pollinators neglected by pesticides’ regulations. Sci. Total Environ. 2025, 959, 178229. [Google Scholar] [CrossRef]
- Díaz, S.; de Souza Urbano, S.; Caesar, L.; Blochtein, B.; Sattler, A.; Zuge, V.; Haag, K.L. Report on the microbiota of Melipona quadrifasciata affected by a recurrent disease. J. Invertebr. Pathol. 2017, 143, 35–39. [Google Scholar] [CrossRef]
- Caesar, L.; Haag, K.L. Tailed bacteriophages (Caudoviricetes) dominate the microbiome of a diseased stingless bee. Genet. Mol. Biol. 2024, 46, e20230120. [Google Scholar] [CrossRef]
- Sousa, L.P. de Bacterial communities of indoor surface of stingless bee nests. PLoS ONE 2021, 16, e0252933. [Google Scholar] [CrossRef]
- Guimarães-Cestaro, L.; Martins, M.F.; Martínez, L.C.; Alves, M.L.T.M.F.; Guidugli-Lazzarini, K.R.; Nocelli, R.C.F.; Malaspina, O.; Serrão, J.E.; Teixeira, É.W. Occurrence of virus, microsporidia, and pesticide residues in three species of stingless bees (Apidae: Meliponini) in the field. Sci. Nat. 2020, 107, 16. [Google Scholar] [CrossRef]
- de Oliveira, F.A.; de Abreu, A.T.; de Oliveira Nascimento, N.; Froes-Silva, R.E.S.; Antonini, Y.; Nalini, H.A.; de Lena, J.C. Evaluation of matrix effect on the determination of rare earth elements and As, Bi, Cd, Pb, Se and In in honey and pollen of native Brazilian bees (Tetragonisca angustula—Jataí) by Q-ICP-MS. Talanta 2017, 162, 488–494. [Google Scholar] [CrossRef]
- Bonsucesso, J.S.; Gloaguen, T.V.; do Nascimento, A.S.; de Carvalho, C.A.L.; Dias, F.d.S. Metals in geopropolis from beehive of Melipona scutellaris in urban environments. Sci. Total Environ. 2018, 634, 687–694. [Google Scholar] [CrossRef]
- Viana, T.A.; Botina, L.L.; Bernardes, R.C.; Barbosa, W.F.; Xavier, T.K.D.; Lima, M.A.P.; Araújo, R.D.S.; Martins, G.F. Ingesting microplastics or nanometals during development harms the tropical pollinator Partamona helleri (Apinae: Meliponini). Sci. Total Environ. 2023, 893, 164790. [Google Scholar] [CrossRef]
- Rani-Borges, B.; Nicolosi Arena, M.V.; Naiara Gomes, I.; de Carvalho Lins, L.H.F.; Camargo Cestaro, L.d.S.; Pompêo, M.; Augusto Ando, R.; Alves-dos-Santos, I.; Hartung Toppa, R.; Roberto Martines, M.; et al. More than just sweet: Current insights into microplastics in honey products and a case study of Melipona quadrifasciata honey. Environ. Sci. Processes Impacts 2024, 26, 2132–2144. [Google Scholar] [CrossRef]
- Zhou, X.; Taylor, M.P.; Davies, P.J. Tracing natural and industrial contamination and lead isotopic compositions in an Australian native bee species. Environ. Pollut. 2018, 242, 54–62. [Google Scholar] [CrossRef]
- Pucholobek, G.; de Andrade, C.K.; Rigobello, E.S.; Wielewski, P.; de Toledo, V.d.A.A.; Quináia, S.P. Determination of the Ca, Mn, Mg and Fe in honey from multiple species of stingless bee produced in Brazil. Food Chem. 2022, 367, 130652. [Google Scholar] [CrossRef]
- Marcolin, L.C.; de Oliveira Arias, J.L.; Kupski, L.; Barbosa, S.C.; Primel, E.G. Polycyclic Aromatic Hydrocarbons (PAHs) in honey from stingless bees (Meliponinae) in southern Brazil. Food Chem. 2023, 405, 134944. [Google Scholar] [CrossRef]
- da Cruz Ferreira, R.; de Souza Dias, F.; de Aragão Tannus, C.; Santana, F.B.; Dos Santos, D.C.M.B.; de Souza Dias, F.; de Castro, M.S.; Brandão, H.N.; de Freitas Santos Júnior, A.; Cerqueira E Silva, L.C.R.; et al. Essential and Potentially Toxic Elements from Brazilian Geopropolis Produced by the Stingless Bee Melipona quadrifasciata anthidioides Using ICP OES. Biol. Trace Elem. Res. 2021, 199, 3527–3539. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Vandame, R.; Castro-Chan, R.A.; Penilla-Navarro, R.P.; Gómez, J.; Sánchez, D. Organochlorine Pesticides in Honey and Pollen Samples from Managed Colonies of the Honey Bee Apis mellifera Linnaeus and the Stingless Bee Scaptotrigona mexicana Guérin from Southern, Mexico. Insects 2018, 9, 54. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef]
- Sulborska, A.; Horecka, B.; Cebrat, M.; Kowalczyk, M.; Skrzypek, T.H.; Kazimierczak, W.; Trytek, M.; Borsuk, G. Microsporidia Nosema spp.—Obligate bee parasites are transmitted by air. Sci. Rep. 2019, 9, 14376. [Google Scholar] [CrossRef]
- Mutinelli, F. The spread of pathogens through trade in honey bees and their products (including queen bees and semen): Overview and recent developments. Rev. Sci. Tech. Int. Off. Epizoot. 2011, 30, 257–271. [Google Scholar] [CrossRef]
- Graystock, P.; Jones, J.C.; Pamminger, T.; Parkinson, J.F.; Norman, V.; Blane, E.J.; Rothstein, L.; Wäckers, F.; Goulson, D.; Hughes, W.O.H. Hygienic food to reduce pathogen risk to bumblebees. J. Invertebr. Pathol. 2016, 136, 68–73. [Google Scholar] [CrossRef]
- Grüter, C.; von Zuben, L.G.; Segers, F.H.I.D.; Cunningham, J.P. Warfare in stingless bees. Insectes Sociaux 2016, 63, 223–236. [Google Scholar] [CrossRef]
- Valera, F.; Gómez-Moracho, T.; Yuan, H.-W.; Muñoz, I.; De la Rúa, P.; Martín-Hernández, R.; Chen, Y.-L.; Higes, M. Any role for the dissemination of Nosema spores by the blue-tailed bee-eater Merops philippinus? J. Apic. Res. 2017, 56, 262–269. [Google Scholar] [CrossRef]
- Fleites-Ayil, F.A.; Medina-Medina, L.A.; Quezada Euán, J.J.G.; Stolle, E.; Theodorou, P.; Tragust, S.; Paxton, R.J. Trouble in the tropics: Pathogen spillover is a threat for native stingless bees. Biol. Conserv. 2023, 284, 110150. [Google Scholar] [CrossRef]
- Tôrres, W.d.L.; Vilvert, J.C.; Carvalho, A.T.; Leite, R.H.d.L.; dos Santos, F.K.G.; Aroucha, E.M.M. Quality of Apis mellifera honey after being used in the feeding of jandaira stingless bees (Melipona subnitida). Acta Sci. Anim. Sci. 2021, 43, e50383. [Google Scholar] [CrossRef]
- Kathe, E.; Seidelmann, K.; Lewkowski, O.; Le Conte, Y.; Erler, S. Changes in chemical cues of Melissococcus plutonius infected honey bee larvae. Chemoecology 2021, 31, 189–200. [Google Scholar] [CrossRef]
- de Paula, G.T.; Menezes, C.; Pupo, M.T.; Rosa, C.A. Stingless bees and microbial interactions. Curr. Opin. Insect Sci. 2021, 44, 41–47. [Google Scholar] [CrossRef]
- Granberg, F.; Vicente-Rubiano, M.; Rubio-Guerri, C.; Karlsson, O.E.; Kukielka, D.; Belák, S.; Sánchez-Vizcaíno, J.M. Metagenomic detection of viral pathogens in Spanish honeybees: Co-infection by Aphid Lethal Paralysis, Israel Acute Paralysis and Lake Sinai Viruses. PLoS ONE 2013, 8, e57459. [Google Scholar] [CrossRef]
- Li, J.L.; Cornman, R.S.; Evans, J.D.; Pettis, J.S.; Zhao, Y.; Murphy, C.; Peng, W.J.; Wu, J.; Hamilton, M.; Boncristiani, H.F.; et al. Systemic spread and propagation of a plant-pathogenic virus in European honeybees, Apis mellifera. mBio 2014, 5, e00898-13. [Google Scholar] [CrossRef]
- Galbraith, D.A.; Fuller, Z.L.; Ray, A.M.; Brockmann, A.; Frazier, M.; Gikungu, M.W.; Martinez, J.F.I.; Kapheim, K.M.; Kerby, J.T.; Kocher, S.D.; et al. Investigating the viral ecology of global bee communities with high-throughput metagenomics. Sci. Rep. 2018, 8, 8879. [Google Scholar] [CrossRef]
- Zhang, X.; He, S.Y.; Evans, J.D.; Pettis, J.S.; Yin, G.F.; Chen, Y.P. New evidence that deformed wing virus and black queen cell virus are multi-host pathogens. J. Invertebr. Pathol. 2012, 109, 156–159. [Google Scholar] [CrossRef]
- Singh, R.; Levitt, A.L.; Rajotte, E.G.; Holmes, E.C.; Ostiguy, N.; vanEngelsdorp, D.; Lipkin, W.I.; dePamphilis, C.W.; Toth, A.L.; Cox-Foster, D.L. RNA Viruses in Hymenopteran Pollinators: Evidence of Inter-Taxa Virus Transmission via Pollen and Potential Impact on Non-Apis Hymenopteran Species. PLoS ONE 2010, 5, e14357. [Google Scholar] [CrossRef]
- Farias, R.A.; Nunes, C.N.; Quináia, S.P. Bees reflect better on their ecosystem health than their products. Environ. Sci. Pollut. Res. 2023, 30, 79617–79626. [Google Scholar] [CrossRef]
- Smith, J.P.; Heard, T.A.; Beekman, M.; Gloag, R. Flight range of the Australian stingless bee Tetragonula carbonaria (Hymenoptera: Apidae). Austral Entomol. 2017, 56, 50–53. [Google Scholar] [CrossRef]
- Bartha, S.; Taut, I.; Goji, G.; Vlad, I.A.; Dinulică, F. Heavy Metal Content in PolyfloralHoney and Potential Health Risk. A Case Study of Copșa Mică, Romania. Int. J. Environ. Res. Public Health 2020, 17, 1507. [Google Scholar] [CrossRef]
- Cozmuta, A.M.; Bretan, L.; Cozmuta, L.M.; Nicula, C.; Peter, A. Lead traceability along soil-melliferous flora-bee family-apiary products chain. J. Environ. Monit. 2012, 14, 1622–1630. [Google Scholar] [CrossRef]
- Formicki, G.; Greń, A.; Stawarz, R.; Zyśk, B.; Gał, A. Metal Content in Honey, Propolis, Wax, and Bee Pollen and Implications for Metal Pollution Monitoring. Pol. J. Environ. Stud. 2013, 22, 99–106. [Google Scholar]
- Huber, M.; Welker, A.; Helmreich, B. Critical review of heavy metal pollution of traffic area runoff: Occurrence, influencing factors, and partitioning. Sci. Total Environ. 2016, 541, 895–919. [Google Scholar] [CrossRef]
- Ye, J.; Li, J.; Wang, P.; Ning, Y.; Liu, J.; Yu, Q.; Bi, X. Inputs and sources of Pb and other metals in urban area in the post leaded gasoline era. Environ. Pollut. 2022, 306, 119389. [Google Scholar] [CrossRef]
- Dong, C.; Taylor, M.P. Applying geochemical signatures of atmospheric dust to distinguish current mine emissions from legacy sources. Atmos. Environ. 2017, 161, 82–89. [Google Scholar] [CrossRef]
- Sharma, R.; Agrawal, P.R.; Chankit; Chanchal; Ittishree; Kashyap, V.; Sharma, A.K.; Alagesan, V. Industrial Waste-Derived Materials for Adsorption of Heavy Metals from Polluted Water. In Remediation of Heavy Metals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2024; pp. 169–197. ISBN 978-1-119-85358-9. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119853589.ch9 (accessed on 27 January 2025).
- Kristensen, L.J.; Taylor, M.P.; Odigie, K.O.; Hibdon, S.A.; Flegal, A.R. Lead isotopic compositions of ash sourced from Australian bushfires. Environ. Pollut. 2014, 190, 159–165. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ye, J.; Zhang, Y.; Ok, Y.S.; Song, Y.; Coulon, F.; Peng, T.; Tian, L. Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications. Environ. Int. 2018, 121, 85–101. [Google Scholar] [CrossRef]
- Yakhshieva, Z.Z.; Usmanova, K.U.; Zhuraev, K.B.; Akhmadjonova, Y.T.; Umarov, F.A.; Karabaeva, G.B. Development of Methods for the Determination of Aluminum in Water. J. Surv. Fish. Sci. 2023, 10, 3322–3337. [Google Scholar]
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.T.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary supplement use in the United States, 2003–2006. J. Nutr. 2011, 141, 261–266. [Google Scholar] [CrossRef]
- JECFA. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2011. Aluminium—Containing Food Additives. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/6179 (accessed on 12 February 2025).
- Scott, S.B.; Lanno, R.; Gardiner, M.M. Acute toxicity and bioaccumulation of common urban metals in Bombus impatiens life stages. Sci. Total Environ. 2024, 915, 169997. [Google Scholar] [CrossRef]
- Feldhaar, H.; Otti, O. Pollutants and Their Interaction with Diseases of Social Hymenoptera. Insects 2020, 11, 153. [Google Scholar] [CrossRef]
- Bashir, S.; Ghosh, P.; Lal, P. Dancing with danger-how honeybees are getting affected in the web of microplastics—A review. NanoImpact 2024, 35, 100522. [Google Scholar] [CrossRef]
- Li, Z.; Guo, D.; Wang, C.; Chi, X.; Liu, Z.; Wang, Y.; Wang, H.; Guo, X.; Wang, N.; Xu, B.; et al. Toxic effects of the heavy metal Cd on Apis cerana cerana (Hymenoptera: Apidae): Oxidative stress, immune disorders and disturbance of gut microbiota. Sci. Total Environ. 2024, 912, 169318. [Google Scholar] [CrossRef]
- Pinheiro, A.I.; Milhome, M.A.L.; Ferreira, F.E.F.R.; da Costa, R.S.; dos Santos, J.L.G.; de Oliveira, L.K.B.; Amorim, A.V. Potencial de contaminação em águas superficiais pelo uso de agrotóxicos em Iguatu, Ceará. Rev. Craibeiras Agroecol. 2017, 1, 1–5. [Google Scholar]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Serafini Poeta Silva, A.P.; Khan, K.; Corbellini, L.G.; Medeiros, A.A.; Silva, G.S. Compliance of biosecurity practices for compartmentalization to foot-mouth disease and classical swine fever viruses in commercial swine companies from southern Brazil. Front. Vet. Sci. 2023, 10, 1125856. [Google Scholar] [CrossRef]
- Ocaña-Cabrera, J.S.; Martin-Solano, S.; Saegerman, C. Development of Tools to Understand the Relationship between Good Management Practices and Nest Losses in Meliponiculture: A Pilot Study in Latin American Countries. Insects 2024, 15, 715. [Google Scholar] [CrossRef]
- Neiva de Jesus, J.; Chambó, E.D.; da Silva Sodré, G.; de Oliveira, N.T.E.; de Carvalho, C.A.L. Hygienic behavior in Melipona quadrifasciata anthidioides (Apidae, Meliponini). Apidologie 2017, 48, 504–512. [Google Scholar]
- Vit, P.; Chuttong, B.; Ramírez-Arriaga, E.; Enríquez, E.; Wang, Z.; Cervancia, C.; Vossler, F.; Kimoloi, S.; Engel, M.S.; Contreras, R.R.; et al. Stingless bee honey: Nutraceutical properties and urgent call for proposed global standards. Trends Food Sci. Technol. 2024, 157, 104844. [Google Scholar]
- Gutiérrez-Chacón, C.; Mueses-Cisneros, J.; Carvalho, A.; González, V. Marco Regulatorio Para la Meliponicultura en Latinoamérica: Aspectos Vlave y Extractos Relevantes; Wildlife Conservation Society: Cali, Colombia, 2025; 38p. [Google Scholar] [CrossRef]
- Prata, J.C.; Martins da Costa, P. Honeybees and the One Health Approach. Environments 2024, 11, 161. [Google Scholar] [CrossRef]
- Salkova, D.; Panayotova-Pencheva, M. Honey bees and their products as indicators of environmental pollution: A review. Agric. Sci. Technol. 2016, 8, 175–182. [Google Scholar] [CrossRef]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
| Stingless-Bee Species | Study Matrix | Disease/Pathogen | Study Prevalence | Detection Method | Habitat/Season | Country | Publication |
|---|---|---|---|---|---|---|---|
| Melipona quadrifasciata | Unhealthy and healthy adult individuals | Unknown annual colony collapse syndrome Disorder Firmicutes Group U (23%), Firmicutes Group Z (23%), and Acetobacteraceae (16%) | 52 positives/76 samples = 0.68 | PCR and Illumina MiSeq sequencing to analyze the variable region V1-V3 of the 16S rDNA gene | Managed nest Summer | Brazil | [50] |
| Melipona marginata Melipona quadrifasciata Melipona mandacaia Melipona compressipes Melipona rufiventris Melipona mondury | Brood, pollen, and honey | European Foulbrood Melissococcus plutonius Brood (66%), pollen (6%), honey (33%) | 18 positives/30 mixed samples = 0.6 | PCR and Sanger sequencing and fragment analysis applications, to analyze 16S rDNA gene | Managed nest distributed in an open and roofed area, in an orchard Spring | Brazil | [38] |
| Tetragonula carbonaria Austroplebeia australis | Workers and queen larvae, brood cell provisions, and honey pots | Bacterial brood disease Lysinibacillus sphaericus (Firmicutes, Bacillaceae) strains | Not specified | Characterization and pathogenicity by microbiology. PCR of the 16s rDNA gene, and cloning. Multilocus sequence typing (MLST) analysis | Managed colonies Summer | Australia | [37] |
| Melipona subnitida | Workers | Deformed wing virus variants DWV-A and DWV-C The average total viral loads per bee was 8.8 × 107 | 21 stingless-bee positive/100 (10 pools of 10) = 0.21 | RT-PCR of total RNA | Managed colonies Spring | Brazil | [34] |
| Tetragonisca fiebrigi Scaptotrigona jujuyensis Tetragonisca angustula Melipona fasciculata Melipona quadrifasciata anthidioides Melipona marginata Melipona rufiventris Melipona mandacaia | Adult individuals | Nosemosis Nosema ceranae | 7 positives/8 species = 0.87 | Duplex PCR of the 16S rRNA locus | Managed and wild colonies. Sampling over 5 years in Argentina, and one year in Brazil | Argentina and Brazil | [39] |
| Melipona quadrifasciata | Healthy and diseased forager bees | Tailed viruses (Caudoviricetes) | Not specified | DNA and RNA metagenomic | Not specified | Brazil | [51] |
| Frieseomelitta varia Tetragonisca angustula Trigona spinipes Melipona quadrifasciata | Adult individuals | Unknown annual syndrome Pseudomonas sp. Sphingomonas sp. Escherichia coli Alcaligenes faecalis | Not specified | PCR of the 16S rRNA gene (V3/V4 regions) and the MiSeq sequencing system | Managed colonies Spring–Summer | Brazil | [52] |
| Nannotrigona testaceicornis Tetragonisca angustula Tetragona elongata | Adult individuals | Nosema ceranae Acute bee paralysis virus (APBV) (10.8%) Deformed wing virus (DWV) (5.1%) Black queen cell virus (BQCV) (5.1%) | Histology detected spores in 100% stingless-bee bodies. Not detected in the midgut by PCR Viruses were found in 23.4% of stingless-bee samples. | Duplex PCR of 16S ribosomal gene RT-qPCR of mRNA from stingless bees | Managed nests Autumn–winter | Brazil | [53] |
| Stingless-Bee Species | Study Matrix | Contaminant [Min–Max] | Habitat/Season | Country | Publication |
|---|---|---|---|---|---|
| Tetragonisca angustula | Honey and pollen | As [1.70 ± 0.01–361.30 ± 18.88] μg kg−1 Cd [0.11 ± 0.01–1.64 ± 0.01] μg kg−1 In [0.08 ± 0.01–0.53 ± 0.29] μg kg−1 Pb [1.20 ± 0.01–463.31 ± 35.16] μg kg−1 | Not specified | Brazil | [54] |
| Melipona scutellaris | Geopropolis | Cr [6.5–39.0] mg kg−1 Cu [1.9–8.4] mg kg−1 Mo [0.6–2.5] mg kg−1 Ni [0.8–6.8] mg kg−1 Pb [1.6–8.9] mg kg−1 Zn [1.2–21] mg kg−1 Cd [0.2–1.2] mg kg−1 | Managed nests Urban environment Sampling over one year | Brazil | [55] |
| Partamona helleri | Larvae midguts | 500 ng/bee of plastic microparticles of polystyrene (PS), and polyethylene terephthalate (PET) 10 μg/bee of nanoparticles of a metal oxide (titanium dioxide—TiO2) | Bioassay (laboratory conditions)* | Brazil | [56] |
| Melipona quadrifasciata | Honey | 0.1 to 2.6 particles per honey mL of microplastics (primarily composed of polypropylene) | Managed nests Built-up and vegetated areas | Brazil | [57] |
| Tetragonula carbonaria | Bees, honey, and wax | As [12–140] μg kg−1 Pb [11–2050] μg kg−1 Mn [410–46,400] μg kg−1 Zn [490–73,000] μg kg−1 | Managed nests Summer | Australia | [58] |
| Scaptotrigona bipunctata Tetragonisca angustula Melipona quadrifasciata Tetragonisca weyrauchi Tetragona clavipes Scaptotrigona postica Melipona marginata | Honey | Ca [0.70 ± 0.06–123.92 ± 1.49] μg g−1 Mn [0.66 ± 0.06–41.92 ± 4.67] μg g−1 Mg [1.60 ± 0.25–351.48 ± 9.58] μg g−1 Fe [13.04 ± 0.39–363.77 ± 6.41] μg g−1 | Managed nests Atlantic Forest, and Amazon River Sampling over 4 years | Brazil | [59] |
| Tetragonisca angustula Scaptotrigona depilis Scaptotrigona postica Melipona quadrifasciata Scaptotrigona bipunctata Melipona marginata Melipona bicolor | Honey | 1.4 to 23.3 μg kg−1 of polycyclic Aromatic Hydrocarbons (PAHs) | Managed nests Native forests and industrial areas Summer | Brazil | [60] |
| Melipona quadrifasciata anthidioides | Geopropolis | Al [20,414.40–36,911.1] mg kg−1 As [4.37] mg kg−1 Cr [17.41–38.07] mg kg−1 Ni [2.28–21.74] mg kg−1 Pb [3.45–8.55] mg kg−1 Sb [1.34–1.64] mg kg−1 Sn [4.92–16.14] mg kg−1 | Managed nests Summer | Brazil | [61] |
| Scaptotrigona mexicana | Honey and pollen | Organochlorine compounds: Heptaclor [96.4–645.08] μg kg−1 γ-HCH [8.8–207.15] μg kg−1 α-HCH [3.8–4.79] μg kg−1 β-HCH [26.1–68.41] μg kg−1 p,p’-DDE [25.1–34.1] μg kg−1 Heptachlor epoxide [18.1–21.68] μg kg−1 α-Endosulfan [51–59.12] μg kg−1 p,p’-DDT [99–440.78] μg kg−1 | Managed nests Sampling over one year | Mexico | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocaña-Cabrera, J.S.; Martin-Solano, S.; Saegerman, C. Environmental Sources of Possible Associated Pathogens and Contaminants of Stingless Bees in the Neotropics. Insects 2025, 16, 350. https://doi.org/10.3390/insects16040350
Ocaña-Cabrera JS, Martin-Solano S, Saegerman C. Environmental Sources of Possible Associated Pathogens and Contaminants of Stingless Bees in the Neotropics. Insects. 2025; 16(4):350. https://doi.org/10.3390/insects16040350
Chicago/Turabian StyleOcaña-Cabrera, Joseline Sofía, Sarah Martin-Solano, and Claude Saegerman. 2025. "Environmental Sources of Possible Associated Pathogens and Contaminants of Stingless Bees in the Neotropics" Insects 16, no. 4: 350. https://doi.org/10.3390/insects16040350
APA StyleOcaña-Cabrera, J. S., Martin-Solano, S., & Saegerman, C. (2025). Environmental Sources of Possible Associated Pathogens and Contaminants of Stingless Bees in the Neotropics. Insects, 16(4), 350. https://doi.org/10.3390/insects16040350








