Patterns of Detoxification Enzyme Activities During the Selection of Phortica okadai Resistant to β-Cypermethrin Under Laboratory Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Reagents
2.3. Determination of Sublethal Concentrations and Tolerance Bioassay
2.4. Resistance Selection
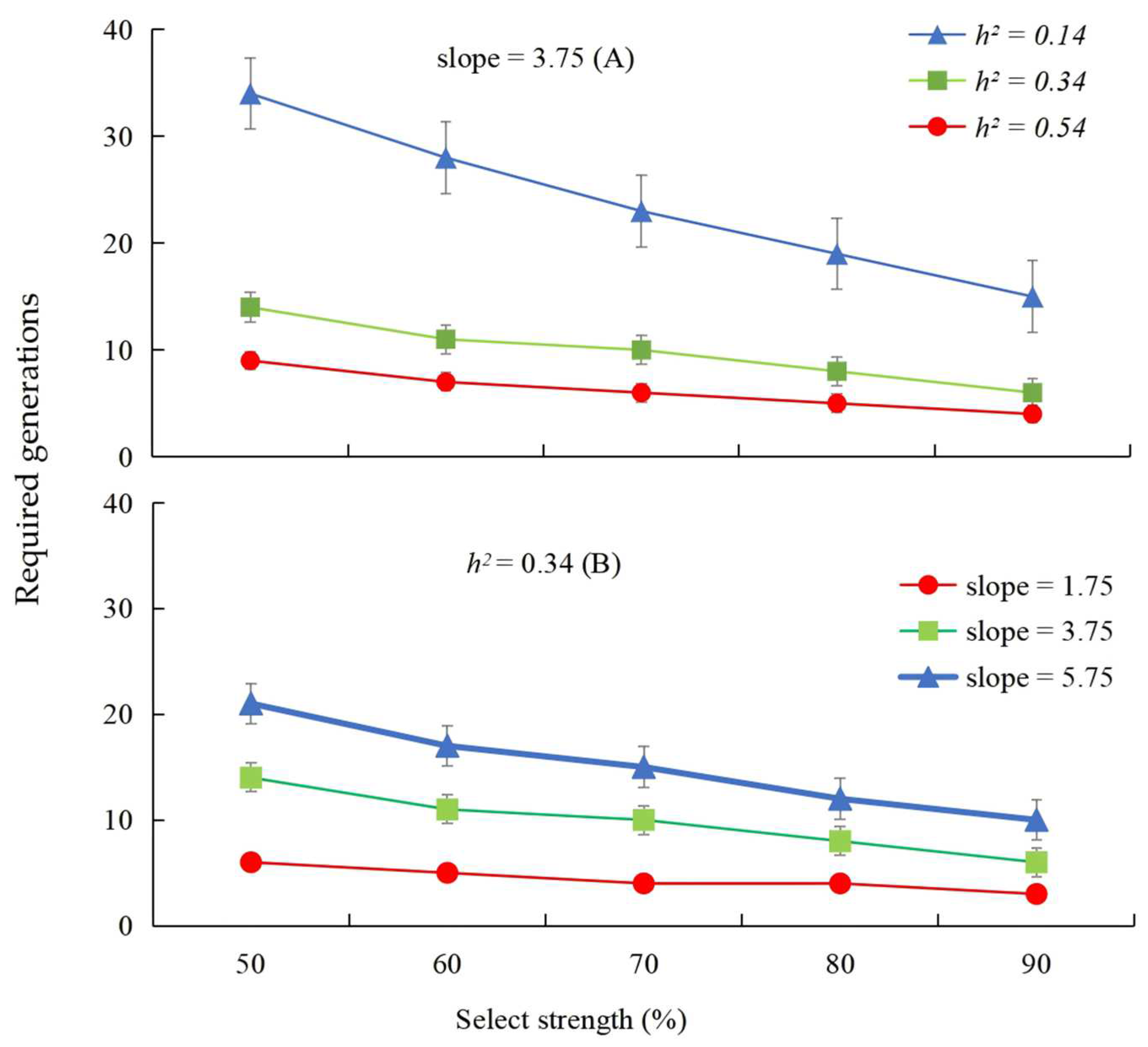
2.5. Estimation of Realized Heritability (h2)
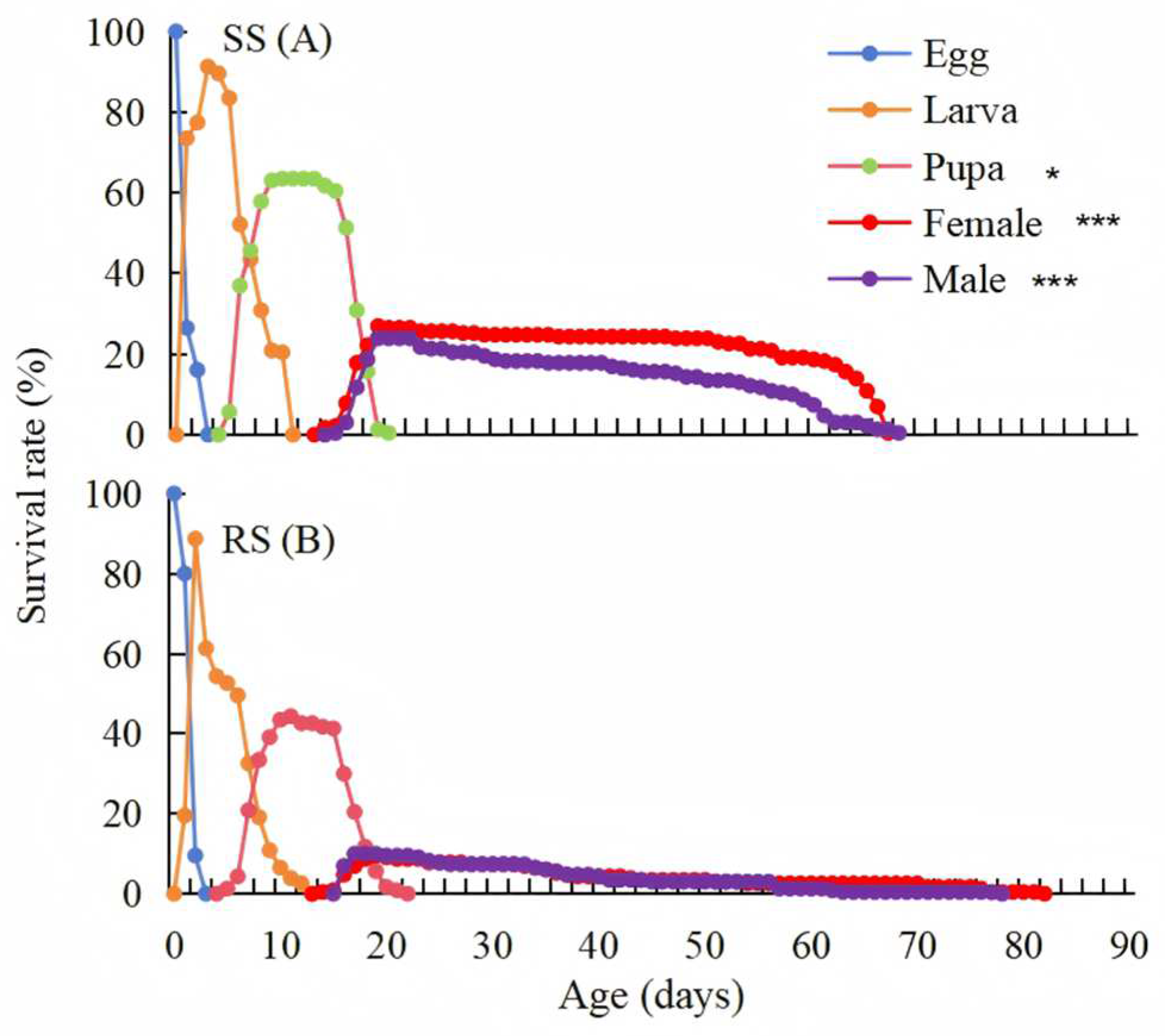
2.6. Comparison of the Development Duration and Life Table Between the RS and the SS
2.7. Statistical Analysis
2.8. Determination of Detoxification Enzyme Activities
3. Results
3.1. Estimation of Realized Heritability and Resistance Rate
3.2. Comparative Development Duration and Life Table Between the RS and SS
3.3. Effect on Detoxification Enzyme Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.J.; Li, D.; Yin, C.; Tang, H.; Luo, B.; Yan, R.; Shen, Y.; Liu, H. Laboratory Culture and Life Cycle of Thelazia callipaeda in Intermediate and Definitive Hosts. Pathogens 2022, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Mendoza-Roldan, J.A.; Dantas-Torres, F. Thelazia callipaeda. Trends Parasitol. 2021, 37, 263–264. [Google Scholar] [PubMed]
- Bezerra-Santos, M.A.; Moroni, B.; Mendoza-Roldan, J.A.; Perrucci, S.; Cavicchio, P.; Cordon, R.; Cianfanelli, C.; Lia, R.P.; Rossi, L.; Otranto, D. Wild carnivores and Thelazia callipaeda zoonotic eyeworms: A focus on wolves. Int. J. Parasitol. Parasites Wildl. 2022, 17, 239–243. [Google Scholar] [PubMed]
- Liu, S.N.; Xu, F.F.; Chen, W.Q.; Jiang, P.; Cui, J.; Wang, Z.Q.; Zhang, X. A Case of Human Thelaziasis and Review of Chinese Cases. Acta Parasitol. 2020, 65, 783–786. [Google Scholar]
- Khan, H.A.A.; Akram, W.; Shad, S.A. Resistance to conventional insecticides in Pakistani populations of Musca domestica L. (Diptera: Muscidae): A potential ectoparasite of dairy animals. Ecotoxicology 2013, 22, 522–527. [Google Scholar]
- Kumar Singh, A.; Nath Tiwari, M.; Prakash, O.; Pratap Singh, M. A Current Review of Cypermethrin-Induced Neurotoxicity and Nigrostriatal Dopaminergic Neurodegeneration. Curr. Neuropharmacol. 2012, 10, 64–71. [Google Scholar]
- Robert, W.M.; Samuel, D.; Haina, S.; Gregory, L.; Jeffrey, G. Selection for, and characterization of, malathion and zeta-cypermethrin resistance in vineyard collected Drosophila melanogaster. Pest. Manag. Sci. 2022, 79, 1623–1627. [Google Scholar]
- Ismail, S.M. Synergistic effects of suppress resistance of cypermethrin and chlorpyrifos in the cotton leafworm, Spodoptera littoralis (Boisduval) populations in Egypt. Int. J. Trop. Insect Sci. 2023, 43, 1669–1674. [Google Scholar]
- Ahmad, M.; McCaffery, A.R. Penetration and metabolism of trans-cypermethrin in a susceptible and a pyrethroid-resistant strain of Helicoverpa armigera. Pestic. Biochem. Physiol. 1999, 65, 6–14. [Google Scholar]
- Ishaaya, I. Insect detoxifying enzymes: Their importance in pesticide synergism and resistance. Arch. Insect Biochem. Physiol. 1993, 22, 263–276. [Google Scholar]
- Hemingway, J.; Ranson, H. Insecticide Resistance in Insect Vectors of Human Disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Ingham, V.A.; Balabanidou, V.; Ranson, H. The Anopheles gambiae ATP-binding cassette transporter family: Phylogenetic analysis and tissue localization provide clues on function and role in insecticide resistance. Insect Mol. Biol. 2018, 27, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, M.; Gong, Y.; Liu, N. Cytochrome P450s-Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Physiol. 2015, 120, 77–81. [Google Scholar] [CrossRef]
- Hatfield, M.J.; Umans, R.A.; Hyatt, J.L.; Edwards, C.C.; Wierdl, M.; Tsurkan, L.; Taylor, M.R.; Potter, P.M. Carboxylesterases: General detoxifying enzymes. Chem.-Biol. Interact. 2016, 259, 327–331. [Google Scholar] [CrossRef]
- Chatterjee, A.; Gupta, S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett. 2018, 433, 33–42. [Google Scholar] [CrossRef]
- Li, R.; Zhu, B.; Shan, J.; Li, L.; Liang, P.; Gao, X. Functional analysis of a carboxylesterase gene involved in beta-cypermethrin and phoxim resistance in Plutella xylostella (L.). Pest. Manag. Sci. 2021, 77, 2097–2105. [Google Scholar] [CrossRef]
- Gao, Y.; Kim, J.H.; Jeong, I.H.; Clark, J.M.; Lee, S.H. Transcriptomic identification and characterization of genes commonly responding to sublethal concentrations of six different insecticides in the common fruit fly, Drosophila melanogaster. Pestic. Biochem. Physiol. 2021, 175, 104852. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. Available online: https://www.antpedia.com/standard/6160888-1.html (accessed on 28 March 2024).
- Abbas, N.; Hafez, A.M. Alpha-Cypermethrin Resistance in Musca domestica: Resistance Instability, Realized Heritability, Risk Assessment, and Insecticide Cross-Resistance. Insects 2023, 14, 233. [Google Scholar] [CrossRef]
- National Public Service Platform for Standards Information. Available online: https://std.samr.gov.cn/hb/search/stdHBDetailed?id=AF7D4A1CE6581558E05397BE0A0A9D1D (accessed on 28 March 2024).
- Falconer, D.S.; Mackay, T.F. Introduction to Quantitative Genetics, 4th ed.; Longman: Harlow, UK, 1996; p. 280. [Google Scholar]
- Tabashnik, B.E. Determining the mode of inheritance of pesticide resistance with backcross experiments. J. Econ. Entomol. 1991, 84, 703–712. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Mcgaughey, W.H. Resistance risk assessment for single and multiple insecticides: Responses of indianmeal moth (Lepidoptera: Pyralidae) to Bacillus thuringiensis. J. Econ. Entomol. 1994, 87, 834–841. [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Agestage, Two-Sex LIFE Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2015. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Jen, T.S.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest. Manag. Sci. 2014, 70, 805–813. [Google Scholar]
- Cao, Y.; Yang, H.; Gao, Y.L.; Wang, L.J.; Li, J.; Wang, C.; Li, C. Effect of elevated CO2 on the population development of the invasive species Frankliniella occidentalis and native species Thrips hawaiiensis and activities of their detoxifying enzymes. J. Pest. Sci. 2021, 94, 29–42. [Google Scholar]
- Ijaz, M.; Shad, S.A. Realized heritability, cross-resistance and high risk of resistance development to spirotetramat in dusky cotton bug, Oxycarenus hyalinipennis Costa (Hemiptera: Lygaeidae), an emerging threat to BT cotton in Pakistan. Phytoparasitica 2021, 50, 453–463. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, W.F.; Miao, S.J.; Lu, Y.J. Progress in research on the relative fitness of insecticide-resistance insects. Chin. J. Appl. Entomol. 2021, 58, 487–496. [Google Scholar]
- Ayla, K.; Zafer, B. Effect of cypermethrin on some developmental stages of Drosophila melanogaster. Bull. Environ. Contam. Toxicol. 2009, 82, 738–742. [Google Scholar]
- Mukhopadhyay, I.; Nazir, A.; Saxena, D.K.; Chowdhuri, D.K. Toxicity of cypermethrin: hsp70 as a biomarker of response in transgenic Drosophila. Biomarkers 2002, 7, 501–510. [Google Scholar]
- Li, X.; Schuler, L.M.; Berenbaum, M. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [PubMed]
- Small, G.J.; Karunaratne, S.H.P.P.; Hemingway, J. Characterization of amplified esterase Estβ12 associated with organophosphate resistance in a multi-resistant population of the mosquito Culex quinquefasciatus from Cuba. Med. Vet. Entomol. 1998, 12, 187–191. [Google Scholar] [PubMed]
- Li, R.; Liang, L.; Zhao, Y.; Zhang, J.; Hao, Z.; Zhao, H.; Liang, P. Functional Analysis of Two Carboxylesterase Genes Involved in Beta-Cypermethrin and Phoxim Resistance in Plutella xylostella (L.). Agronomy 2024, 14, 2781. [Google Scholar] [CrossRef]
- Liang, L.; Li, J.; Jin, L.; Yan, K.P.; Pan, Y.; Shang, Q.L. Identification of inducible CYP3 and CYP4 genes associated with abamectin tolerance in the fat body and Malpighian tubules of Spodoptera litura. Pestic. Biochem. Physiol. 2024, 198, 105751. [Google Scholar]
- Mukhopadhyay, I.; Chowdhuri, D.K.; Bajpayee, M.; Dhawan, A. Evaluation of in vivo genotoxicity of cypermethrin in Drosophila melanogaster using the alkaline Comet assay. Mutagenesis 2004, 19, 85–90. [Google Scholar]




| Generations | Slope ± SE | LC50 (95% Confidence Interval) (mg/L) | Resistance Ratio (RR) | X2 (df) | p (95% Confidence Interval) |
|---|---|---|---|---|---|
| F0 | 1.496 ± 0.357 | 0.166 (0.085, 0.228) | 1.000 | 1.143 (3) | 0.767 (0.851, 2.988) |
| F2 | 2.667 ± 0.281 | 0.408 (0.383, 0.434) | 2.460 | 0.984 (3) | 0.805 (5.224, 7.839) |
| F4 | 2.776 ± 0.221 | 1.450 (1.281, 1.698) | 8.730 | 5.531 (3) | 0.137 (1.580, 2.248) |
| F8 | 4.659 ± 0.517 | 1.666 (1.609, 1.727) | 10.040 | 4.023 (3) | 0.259 (2.180, 3.414) |
| G | Estimation of Mean Selection Response per Generation | Estimation of Mean Selection Differential per Generation | Estimation of Heritability (h2) | |||||
|---|---|---|---|---|---|---|---|---|
| Initial LC50 (95% Confidence Interval) | Final LC50 (95% Confidence Interval) | Response to Selection (R) | Intensity of Selection (i) | Mean Slope | Phenotypic Variation (σp) | Selection Differential (S) | ||
| F0–F8 | 0.408 (0.383–0.434) | 1.667 (1.609–1.727) | 0.08 | 0.85 | 3.75 | 0.27 | 0.23 | 0.34 |
| Insect Stage | Resistant Strain (Mean ± SEM) (d) | Susceptible Strain (Mean ± SEM) (d) | t (df) | p (95% Confidence Interval) |
|---|---|---|---|---|
| Duration in the egg stage | 1.90 ± 0.04 a | 1.43 ± 0.05 b | 7.766 (457) | <0.001 (0.354, 0.593) |
| Duration in the larval stage | 6.26 ± 0.13 a | 5.62 ± 0.09 b | 4.11 (252) | <0.001 (0.336, 0.954) |
| Duration in the pupal stage | 9.27 ± 0.14 a | 10.84 ± 0.1 b | 8.417 (163) | <0.001 (−1.965, 1.191) |
| APOPs | 11.12 ± 0.78 a | 9.74 ± 0.35 a | 1.74 (72) | 0.086 (−2.969, −0.201) |
| TPOPs | 27.68 ± 0.89 a | 26.69 ± 0.41 a | 0.783 (72) | 0.436 (−2.56, 0.116) |
| Parameter | Resistant Strain (Mean ± SE) | Susceptible Strain (Mean ± SEM) | t (df) | p (95% Confidence Interval) |
|---|---|---|---|---|
| Intrinsic rate of increase, r (day−1) | 0.0448 ± 0.0093 a | 0.090 ± 0.0039 b | 38.85 (199) | <0.001 (−0.49, −0.45) |
| Finite rate of increase, λ (day−1) | 1.0458 ± 0.0097 a | 1.0943 ± 0.0042 b | 37.82 (199) | <0.001 (−0.52, −0.48) |
| Net reproductive rate, R0 (offspring) | 6.3260 ± 2.1712 a | 32.1565 ± 4.1889 b | 14.15 (199) | <0.001 (−27.7, −25.8) |
| Mean generation time, T (days) | 41.205 ± 1.7490 a | 38.5930 ± 0.5900 a | 0.31 (199) | 0.757 (2.396, 3.062) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wu, F.; Luo, Y.; Chen, Z.; Long, D.; Liu, H.; Luo, B.; Yan, R.; Wang, L. Patterns of Detoxification Enzyme Activities During the Selection of Phortica okadai Resistant to β-Cypermethrin Under Laboratory Conditions. Insects 2025, 16, 346. https://doi.org/10.3390/insects16040346
Zhou J, Wu F, Luo Y, Chen Z, Long D, Liu H, Luo B, Yan R, Wang L. Patterns of Detoxification Enzyme Activities During the Selection of Phortica okadai Resistant to β-Cypermethrin Under Laboratory Conditions. Insects. 2025; 16(4):346. https://doi.org/10.3390/insects16040346
Chicago/Turabian StyleZhou, Juan, Fang Wu, Yang Luo, Zhenfu Chen, Donghua Long, Hui Liu, Bo Luo, Rong Yan, and Lingjun Wang. 2025. "Patterns of Detoxification Enzyme Activities During the Selection of Phortica okadai Resistant to β-Cypermethrin Under Laboratory Conditions" Insects 16, no. 4: 346. https://doi.org/10.3390/insects16040346
APA StyleZhou, J., Wu, F., Luo, Y., Chen, Z., Long, D., Liu, H., Luo, B., Yan, R., & Wang, L. (2025). Patterns of Detoxification Enzyme Activities During the Selection of Phortica okadai Resistant to β-Cypermethrin Under Laboratory Conditions. Insects, 16(4), 346. https://doi.org/10.3390/insects16040346





