Simple Summary
In this review, we summarize caste determination systems in eusocial insects, with a focus on temperate Polistes paper wasps. We describe the plasticity of caste determination, a foundational characteristic of eusociality, during the adult stage. Studies associated with caste determination have advanced in honey bees and are progressing in paper wasps. Honey bees have morphological and physiological caste differences at emergence as a result of different nutrition provision during the larval stage. By contrast, castes in temperate Polistes wasps are ultimately determined by external factors during the adult stage, with preimaginal caste biases during the larval stage; therefore, paper wasps show a high degree of plasticity of caste determination during the adult stage. Thus, studies that explore the caste determination system in temperate Polistes wasps will contribute to understanding how eusociality evolves. We also consider the factors that led to the loss or maintenance of plasticity, based on differences in life history across species, and provide insight into the evolution of eusociality in the Hymenoptera.
Abstract
The reproductive division of labor is a fundamental characteristic of eusociality; thus, understanding the caste determination system underlying the reproductive division of labor would shed more light on the evolution of eusociality. In this review, we summarize the factors associated with caste determination in temperate Polistes paper wasps and focus on life histories associated with the loss or maintenance of caste plasticity during the adult stage among eusocial Hymenoptera. In many species of eusocial Hymenoptera, caste trajectories are differentiated by nutrition during the larval stages, indicating that caste plasticity is either absent or has not yet been confirmed. However, in temperate Polistes wasps, nutrition during the larval stage only causes biases in caste trajectory, with castes ultimately determined by environmental factors, such as day length and temperature, and colony conditions during the adult stage, indicating high caste plasticity during this stage. Therefore, morphological dimorphism and physiological differences between castes, such as in dopamine levels, have not been found in temperate Polistes wasps at emergence. This plasticity in temperate paper wasps could reflect the fact that females destined to be workers also have a chance to mate with males (especially early males) after emergence, leaving the possibility that they can produce daughters in the emerging year.
Keywords:
biogenic amine; brain; caste plasticity; dopamine; eusociality; Hymenoptera; photoperiod; Polistes; reproduction; social insect 1. Introduction
The reproductive division of labor is a foundational characteristic of the eusociality of Hymenoptera [1,2]. Adult females differentiate into either queens specialized for reproduction or infertile workers engaged in labor other than egg-laying (sometimes as helpers and soldiers, depending on the type of labor). Among eusocial species, the level of social development varies depending on the degree of morphological and/or physiological differentiation between castes and the degree of behavioral specialization for the task [2,3,4,5]. The most derived level of sociality occurs in insects that are advanced eusocial, characterized by morphological dimorphism between castes, a distinct division of labor regarding reproduction and swarm nest founding [5]. A level of sociality with characteristics similar to advanced eusocial, but where nests are founded by a single female, is highly eusocial. In both advanced and highly eusocial species, females are thought to have evolved (or degenerated) the morphology required for each type of labor, such as body size, reproductive organs (number of ovarioles and developmental status of the spermatheca), exocrine glands, and brain and nervous systems [5,6,7,8,9,10,11]. By contrast, groups that lack morphological and physiological dimorphism between castes are primitively eusocial [5]. Here, differences in body size between queens and workers are continuous and sometimes caste switching can occur.
Eusocial Hymenoptera are represented by three main groups: wasps (Vespidae), bees (Apidae + Halictidae), and ants (Formicidae), and eusociality has evolved independently in each group [12,13]. This means that even social structures defined as eusocial among the Hymenoptera might differ in terms of their developmental mechanisms and the factors driving their evolution, influenced by regional and climatic differences. In addition, these groups have adapted to a range of environments, from tropical to subarctic climates [14,15,16,17]. In their expansion into temperate and subarctic regions, seasonal adaptation is important for survival, that is, they must survive winters that are unsuitable for reproduction and development [18,19]. Overwintering systems in eusocial groups in temperate zones can be divided into two types. First, both queens and workers overwinter but do not diapause (perennial species): the overwintering adults are considered to be less active than during the warm season, but begin to act immediately upon warming. Such overwintering systems are only found in advanced and highly eusocial species, represented by honey bees and ants. Second, only reproductive castes (specifically gynes, which are queen candidates) overwinter (annual species). Such overwintering systems are found in both primitively and highly eusocial species, represented by Polistes paper wasps, bumble bees, hornets, and yellow jackets. The gynes mate with males during the autumn, overwinter, and then initiate the founding of a nest the following spring, whereas workers cannot survive the winter. Therefore, it is possible to determine the caste by whether the female prepares for overwintering. Gynes do not develop mature eggs during the year of emergence and instead accumulate high levels of lipids [20,21,22,23]. Thus, a mechanism for sensing the seasons might be incorporated into the caste determination system in species with an annual colony cycle [24].
Caste is determined before and/or after emergence. In species with morphological and physiological dimorphism between castes, castes begin to differentiate during the larval stage and determine their morphology before emergence. This means that there is a lack of plasticity in caste determination during the adult stage. In advanced and highly eusocial Hymenoptera, caste differentiation is promoted by food quality (e.g., royal jelly) and quantity during the larval stage, and caste is already determined at emergence [2,3,10,25,26]. However, in other Hymenoptera, especially those with an annual colony cycle, such as Polistes wasps and bumble bees, the mechanism of caste differentiation or determination has not yet been fully elucidated.
2. Caste Determination System in Temperate Polistes Wasps
2.1. Preimaginal Caste-Fate Biases
In temperate Polistes wasps, caste-fate bias is considered to occur during immature stages (larval and pupal stages), with caste being determined at the adult stage [27,28,29,30,31,32]. Factors causing preimaginal biases include food quality/quantity, vibratory stimuli, and day length [21,30,32,33,34,35,36,37]. Food quantity during the larval stage causes differences in body sizes [33], whereby individuals with a restricted food supply (protein source) have smaller bodies [38]. Likewise, food quality during the larval stage also induces physiological differences at emergence, with individuals that consume more carbohydrates during the larval stage having a higher lipid content at emergence [35,39]. This nutrition-dependent caste determination (bias) has also been reported in highly eusocial vespid wasps [40,41], which share an origin of eusociality with Polistinae [13]. There are morphological differences between worker and gyne castes in several species of vespid wasps, considered to be affected by the amount of food after the third instar of the larval phase [42,43] (noting that some vespid wasps have a lack of morphological differences between castes, such as Vespa ducalis). Therefore, the nutrition-dependent caste-fate biases observed in primitive eusocial wasps might be a fundamental developmental process across eusocial wasp species and an ancestral trait of caste determination in eusocial Hymenoptera.
Vibratory stimuli are an external factor that characterizes the common ancestor of the Polistinae and Vespidae [34]. Antennal drumming behavior by adults affects the subsequent caste trajectories of larval Polistes fuscatus, with larvae that experience high frequencies of antennal drumming developing into individuals with a worker-type physiology at emergence [44,45]. In addition, recent work showed that the combination of nutrition and vibrational stimuli affects metabolic systems and diapause-related gene expression through different molecular control mechanisms [36]. Finally, photoperiod is also related to caste-fate biases in temperate paper wasps, whereby individuals that experience increasing light periods from the pupal to adult stage contain more developed eggs at 2 weeks after emergence (i.e., reproductive workers) [32,37]. However, it has not yet been possible to separate the effects of photoperiod and body size (perhaps indirectly reflecting larval-stage food availability), meaning that further experimental manipulation, such as combining feed limitation and day length, would clarify the effects of day length during the immature stage.
2.2. Imaginal Caste Determination Factors
Caste is ultimately determined by factors during the adult stage in temperate Polistes paper wasps [31,32,37,46,47,48]. Emerged females are divided into two types: nondiapause (mainly workers) and diapause (gynes) females. In nondiapause females, newly emerged adults can either stay or leave the natal nest. This determination is based mainly on colony status, such as the presence of queens and/or immature individuals and colony size [29,46,47,48]. The early stage of colony activity is also at high risk of disruption by predators such as birds, mammals, arthropods, and mollusks [42,49,50,51], which might also be a factor in leaving the natal nest. Nondiapause females that stay in the natal nest are engaged in internal (e.g., brood care) and/or external roles (e.g., foraging) as workers and some become reproductive workers. Within workers, there is a dominance–subordinate hierarchy in which individuals are ranked according to their aggressive behavior, with dominant individuals being more likely to be reproductive workers (generally, they oviposit unfertilized eggs and produce males) (e.g., [52,53,54,55,56]). However, this worker reproduction is generally suppressed by queen aggression and/or queen-/worker-policing behaviors (e.g., [56,57]). When the queen disappears, the most dominant female mates and becomes the successive queen [58]. In addition, in Polistes jokahamae, which has a dominance hierarchy based on intergroup conflict, abdominal rubbing behavior is also observed by the queen and dominant egg-laying workers [55] (note that only the behavior of reproductive workers in the queenright colony was observed in this study). This abdominal rubbing-like behavior has been reported in the tropical paper wasp Ropalidia marginata, in which it is thought to apply queen pheromone [59]. Therefore, it is possible that the successive queen also controls the reproductive physiology of her nestmates (i.e., fixes them to the worker caste) through pheromones.
Next, females leaving the natal nest can become either mid-season foundresses or drifters [31,33]. Mid-season foundresses found a nest by themselves and produce the next generation. In some Polistes species, males are produced at the same time as the first brood (often called early males). Therefore, workers have an opportunity to mate with early males and can lay both haploid and diploid eggs [54,60,61,62,63,64]. By contrast, drifters enter other colonies [65,66] and produce males [67]. Such individuals might appear primarily under long daylight (day length close to the summer solstice) because females reared individually (i.e., leaving the nest) are more likely to develop ovaries [32,37].
Finally, the decision to become a diapausing or nondiapausing individual is determined mainly by day length [32,37,68]. When adult females experience short days during the adult stage under isolated conditions, they do not develop ovaries and instead accumulate high levels of lipids in the abdomen. Thus, the day length during the adult stage is a cue to determine the time remaining until overwintering and influences caste determination. However, the photoperiod-related caste determination mechanisms might not be consistent among temperate species. The site of origin of paper wasps is considered to be in the Old World tropics [16], suggesting that temperate adaptation has likely occurred multiple times in temperate Polistinae wasps and that their photoperiodic responsiveness and intensity might also differ.
4. Conclusions and Future Directions
The plasticity of caste determination during the adult stage differs significantly between Polistes paper wasps and other eusocial Hymenoptera, such as bumble bees: temperate Polistes paper wasps have a high plasticity of caste determination during the adult stage, whereas bumble bees have caste determination during the larval stage and low plasticity during the adult stage (Figure 1). Why is there such a large difference in the plasticity of caste determination in the adult stage among primitive eusocial groups? Considering the evolution of social structure from solitary to eusocial, plasticity originally existed in all individuals, and then, during the evolution of social structure, some groups or species lost plasticity in the adult stage [97,98]. Here, we consider the factors that led to the loss or maintenance of plasticity, based on the differences in their life histories.
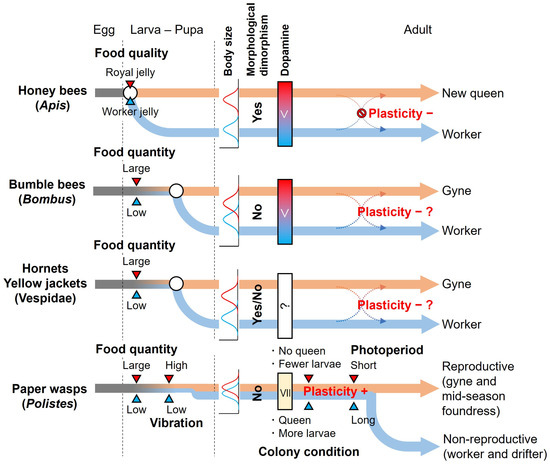
Figure 1.
Timing of caste determination and plasticity of caste determination during adult stage in representative temperate eusocial bees and wasps. White circles indicate the timing of caste determination. In the body size column, blue and red indicate the histogram of the body size of worker (-like) and gyne (-like) individuals in the adult stage, respectively. The body sizes of honey bees and many Vespidae are bimodal distribution, while those of bumble bees and Polistes paper wasps are overlapping or continuous and indistinct. In addition, honey bees and some Vespidae show morphological dimorphism between castes, but bumble bees and Polistes paper wasps do not. In honey bees and bumble bees, there is a caste difference in the amount of dopamine of newly emerged females: the reproductive caste has higher levels of dopamine in the brain than the non-reproductive caste. On the other hand, caste differences in the amounts of dopamine of newly emerged females are much smaller in paper wasps. In adult stage plasticity, “−” and “+” indicate the group without and with plasticity of caste determination during the adult stage, respectively. In bumble bees and Vespidae wasps, no clear answer on plasticity has been obtained. The workers of these groups have not shown evidence of mating with males in the field, but they do not lose spermatheca as known in honey bees, meaning that workers have the possibility of becoming a reproductive caste. Hence, the plasticity of caste determination during the adult stage in bumble bees and Vespidae wasps is “−?”.
First, in many temperate eusocial wasps and bees, larval food quantity and/or quality is an important factor in bias toward caste differentiation or caste decision [26,33,35,36,38,39,42,43]. However, food sources differ significantly between wasps and bees. Wasps feed their larvae on both animal (protein) and plant (nectar) food resources with progressive provisioning, whereas bees feed on only plant food resources (pollen and nectar). In addition, honey bees and some bumble bee species store larval food in the cell (i.e., mass provisioning). Considering the stability of the food supply for larvae, mass provisioning is more stable than progressive provisioning. Hence, the larvae of the species with mass provisioning might be able to determine their caste at immature stages because they can fully develop without being affected by unstable and uncertain environmental conditions, such as a lack of food. However, there are counterarguments to this hypothesis. In Polistes paper wasps, larval cannibalism has been observed when the colony experiences restricted food availability [99,100]; thus, the larvae can serve as an animal resource (i.e., protein storage) in situations of food deprivation. Such protein storage may be a means of temporarily tolerating the unstable environment. Moreover, temperate Vespidae wasps also have distinct morphological differences between castes, even though they display the same progressive provisioning as the Polistes paper wasps. These differences cannot be explained by food quantity. Recently, LeBoeuf et al. (2016) [101] revealed that many growth-related proteins and juvenile hormones are included in the trophallaxis fluid exchanged between adults and larvae. Research on trophallaxis in Vespinae wasps has focused on larvae to adults [102], and future work to determine the components of trophallaxis fluid in adults to larvae may provide clues to understand morphological caste differences.
Second, the nest structure differs greatly between temperate Polistes wasps and other eusocial Hymenoptera: Polistes paper wasps found a nest in an open space without an envelope. However, hornets and yellow jackets typically build a nest covered with an envelope in an open space and/or in closed spaces, such as underground or in tree hollows [41], as do temperate honey bees and bumble bees, although bee hives are not covered with an envelope. Nests built in enclosed spaces and covered with an envelope could protect larvae from harsh weather and maintain a consistent temperature [103,104,105], resulting in a stable developmental period during the immature stage.
Finally, several options to increase direct fitness after emergence may contribute to maintaining the plasticity of caste-fate determination during the adult stage. In particular, whether they have an opportunity to mate and produce both females (fertilized eggs) and males (unfertilized eggs) in the emerging year would be a significant point. Some temperate Polistes paper wasps produce early males, as described in Section 2.2. These early males mate with early-emerged females (usually considered to be workers) and mated female individuals can produce both males and females [54,61,62,63,64]. Early males have also been identified in hornets [106], but it is not known whether they mate with early-emerged females. However, early male production is rare in bumble bees; therefore, early-emerged females are unlikely to produce female offspring. Future comparisons of the percentage of early males in the field or the degree of female production by early females and the degree of plasticity of caste determination during the adult stage among groups of eusocial species and among species within each eusocial group could provide clues to understanding why there is such a large difference in the plasticity of caste determination in the adult stage among primitive eusocial insects.
In conclusion, we introduced preimaginal and imaginal caste determination systems in eusocial Hymenoptera and compared caste determination systems between Polistes species and other temperate eusocial species. We have discussed three possible factors that maintain the plasticity of caste determination during the adult stage in temperate Polistes wasps from the perspective of life history by comparing with other eusocial Hymenoptera groups: (1) the stability of food availability during the larval stage, (2) nest structure, and (3) an opportunity to mate and produce fertilized eggs in the emerging year. However, none of the three factors in this review is sufficient to completely explain plasticity based on current research findings. Therefore, a first step for a future study would be to determine the degree of variation in the strength of the plasticity during the adult stage among temperate Polistes species. In addition, it would be necessary to steadily clarify the pieces of life history and caste determinants (e.g., function of the early male in bumble bees and Vespidae wasps, and physiology of reproductive regulation in Vespidae wasps).
Author Contributions
Conceptualization, H.Y. and K.S.; writing—original draft preparation, H.Y.; writing—reviewing and editing, K.S.; visualization, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We thank our colleagues in the Honeybee Science Research Center in Tamagawa University for their many helpful suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Michener, C.D. Comparative social behavior of bees. Ann. Rev. Entomol. 1969, 14, 299–342. [Google Scholar]
- Wilson, E.O. The Insect Societies; Harvard University Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Michener, C.D. The Social Behavior of the Bees; Harvard University Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Jeanne, R.L. Social complexity in the Hymenoptera, with special attention to the wasps. In Genes, Behaviors and Evolution of Social Insects; Kikuchi, T., Azuma, N., Higashi, S., Eds.; Hokkaido University Press: Sapporo, Japan, 2003; pp. 81–131. [Google Scholar]
- Jandt, J.M.; Toth, A.L. Physiological and genomic mechanisms of social organization in wasps (Family: Vespidae). Adv. Insect Physiol. 2015, 48, 95–130. [Google Scholar] [CrossRef]
- Gobin, B.; Ito, F.; Peeters, C.; Billen, J. Queen-worker differences in spermatheca reservoir of phylogenetically basal ants. Cell Tissue Res. 2006, 326, 169–178. [Google Scholar] [CrossRef]
- Khila, A.; Abouheif, E. Reproductive constraint is a developmental mechanism that maintains social harmony in advanced ant societies. Proc. Natl. Acad. Sci. USA 2008, 105, 17884–17889. [Google Scholar] [CrossRef]
- Kugler, J.; Orion, T.; Ishay, J. The number of ovarioles in the Vespinae (Hymenoptera). Insectes Sociaux 1976, 23, 525–533. [Google Scholar] [CrossRef]
- Ito, F.; Ohkawara, K. Spermatheca size differentiation between queens and workers in primitive ants. Naturwissenschaften 1994, 81, 138–140. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Groh, C.; Rössler, W. Caste-specific postembryonic development of primary and secondary olfactory centers in the female honeybee brain. Arthropod Struct. Dev. 2008, 37, 459–468. [Google Scholar] [CrossRef]
- Hughes, W.O.H.; Oldroyd, B.P.; Beekman, M.; Ratnieks, F.L.W. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 2008, 320, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Piekarski, P.K.; Carpenter, J.M.; Lemmon, A.R.; Lemmon, E.M.; Sharanowski, B.J. Phylogenomic evidence overturns current conceptions of social evolution in wasps (Vespidae). Mol. Biol. Evol. 2018, 35, 2097–2109. [Google Scholar] [CrossRef]
- Hines, H.M. Historical biogeography, divergence times, and diversification patterns of bumble bees (Hymenoptera: Apidae: Bombus). Syst. Biol. 2008, 57, 58–75. [Google Scholar] [CrossRef]
- Dunn, R.R.; Agosti, D.; Andersen, A.N.; Arnan, X.; Bruhl, C.A.; Cerdá, X.; Ellison, A.M.; Fisher, B.L.; Fitzpatrick, M.C.; Gibb, H.; et al. Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol. Lett. 2009, 12, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.F.; Payne, A.; Pickett, K.M.; Carpenter, J.M. Phylogeny and historical biogeography of the paper wasp genus Polistes (Hymenoptera: Vespidae): Implications for the overwintering hypothesis of social evolution. Cladistics 2015, 31, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Economo, E.P.; Narula, N.; Friedman, N.R.; Weiser, M.D.; Guénard, B. Macroecology and macroevolution of the latitudinal diversity gradient in ants. Nat. Commun. 2018, 9, 1778. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Denlinger, D.L. Insect Diapause; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Eickwort, K. Separation of the castes of Polistes exclamans and notes on its biology (Hym.: Vespidae). Insectes Sociaux 1969, 16, 67–72. [Google Scholar] [CrossRef]
- Keeping, M.G. Reproductive and worker castes in the primitively eusocial wasp Belonogaster petiolata (DeGeer) (Hymenoptera: Vespidae): Evidence for pre-imaginal differentiation. J. Insect Physiol. 2002, 48, 867–879. [Google Scholar] [CrossRef]
- Toth, A.L.; Bilof, K.B.J.; Henshaw, M.T.; Hunt, J.H.; Robinson, G.E. Lipid stores, ovary development, and brain gene expression in Polistes metricus females. Insectes Sociaux 2009, 56, 77–84. [Google Scholar] [CrossRef]
- Judd, T.M.; Magnus, R.M.; Fasnacht, M.P. A nutritional profile of the social wasp Polistes metricus: Differences in nutrient levels between castes and changes within castes during the annual life cycle. J. Insect Physiol. 2010, 56, 42–56. [Google Scholar] [CrossRef]
- Hunt, J.H. Evolution of castes in Polistes. Ann. Zool. Fennici. 2006, 43, 407–422. [Google Scholar]
- Wilde, J.D.; Beetsma, J. The physiology of caste development in social insects. Adv. Insect Physiol. 1982, 16, 167–246. [Google Scholar] [CrossRef]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef]
- O’Donnell, S. Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae). Annu. Rev. Entomol. 1998, 43, 323–346. [Google Scholar] [CrossRef] [PubMed]
- Berens, A.J.; Hunt, J.H.; Toth, A.L. Nourishment level affects caste related gene expression in Polistes wasps. BMC Genom. 2015, 16, 235. [Google Scholar] [CrossRef]
- Judd, T.M. Effect of the presence of brood on the behavior and nutrient level of emerging individuals in field colonies of Polistes metricus. Insectes Sociaux 2018, 65, 171–182. [Google Scholar] [CrossRef]
- Hunt, J.H. Origin of an evolutionary novelty: The worker phenotype of eusocial wasps. Insectes Sociaux 2021, 68, 303–318. [Google Scholar] [CrossRef]
- Yoshimura, H.; Sasaki, K. Factor that affect reproductive states in female eusocial Hymenoptera. In Advances in Animal Science and Zoology; Jenkins, O.P., Ed.; Nova Science Publishers: New York, NY, USA, 2020; Volume 15, pp. 133–161. [Google Scholar]
- Yoshimura, H.; Yamada, Y.Y. Preimaginal caste-related bias in the paper wasp Polistes jokahamae is limited to the first brood. Insectes Sociaux 2021, 68, 133–143. [Google Scholar] [CrossRef]
- Hunt, J.H.; Amdam, G.V. Bivoltinism as an antecedent to eusociality in the paper wasp genus Polistes. Science 2005, 308, 264–267. [Google Scholar] [CrossRef]
- Jeanne, R.L.; Suryanarayanan, S. A new model for caste development in social wasps. Commun. Integr. Biol. 2011, 4, 373–377. [Google Scholar] [CrossRef]
- Judd, T.M.; Teal, P.E.A.; Hernandez, E.J.; Choudhury, T.; Hunt, J.H. Quantitative differences in nourishment affect caste-related physiology and development in the paper wasp Polistes metricus. PLoS ONE 2015, 10, e0116199. [Google Scholar] [CrossRef]
- Jandt, J.M.; Suryanarayanan, S.; Hermanson, J.C.; Jeanne, R.L.; Toth, A.L. Maternal and nourishment factors interact to influence offspring developmental trajectories in social wasps. Proc. R. Soc. B 2017, 284, 20170651. [Google Scholar] [CrossRef]
- Yoshimura, H.; Yamada, Y.Y. Caste-fate determination primarily occurs after adult emergence in a primitively eusocial paper wasp: Significance of the photoperiod during the adult stage. Sci. Nat. 2018, 105, 15. [Google Scholar] [CrossRef]
- Karsai, I.; Hunt, J.H. Food quantity affect traits of offspring in the paper wasp Polistes metricus (Hymenoptera: Vespidae). Environ. Entomol. 2002, 31, 99–106. [Google Scholar] [CrossRef]
- Rossi, A.M.; Hunt, J.H. Honey supplementation and its developmental consequences: Evidence for food limitation in a paper wasp, Polistes metricus. Ecol. Entomol. 1988, 13, 437–442. [Google Scholar] [CrossRef]
- Wheeler, D.E. Developmental and physiological determinants of caste in social Hymenoptera: Evolutionary implications. Am. Nat. 1986, 128, 13–34. [Google Scholar] [CrossRef]
- Matsuura, M.; Yamane, S. Biology of the Vespine Wasps; Springer: Berlin, Germany, 1990. [Google Scholar]
- Brian, M.V.; Brian, A.D. The wasp, Vespula sylvestris Scopoli: Feeding, foraging and colony development. Trans. R. Entomol. Soc. Lond. 1952, 103, 1–26. [Google Scholar] [CrossRef]
- Ishay, J. Caste determination by social wasps: Cell size and building behaviour. Anim. Behav. 1975, 23, 425–431. [Google Scholar] [CrossRef]
- Suryanarayanan, S.; Hantschel, A.E.; Torres, C.G.; Jeanne, R.L. Changes in the temporal pattern of antennal drumming behavior across the Polistes fuscatus colony cycle (Hymenoptera, Vespidae). Insectes Sociaux 2011, 58, 97–106. [Google Scholar] [CrossRef]
- Suryanarayanan, S.; Hermanson, J.C.; Jeanne, R.L. A mechanical signal biases caste development in a social wasp. Curr. Biol. 2011, 21, 231–235. [Google Scholar] [CrossRef]
- Solís, C.R.; Strassmann, J.E. Presence of brood affects caste differentiation in the social wasp, Polistes exclamans Viereck (Hymenoptera: Vespidae). Funct. Ecol. 1990, 4, 531–541. [Google Scholar] [CrossRef]
- Reeve, H.K.; Peters, J.M.; Nonacs, P.; Starks, P.T. Dispersal of first “workers” in social wasps: Causes and implications of an alternative reproductive strategy. Proc. Natl. Acad. Sci. USA 1998, 95, 13737–13742. [Google Scholar] [CrossRef]
- Tibbetts, E.A. Dispersal decisions and predispersal behavior in Polistes paper wasp ‘workers’. Behav. Ecol. Sociobiol. 2007, 61, 1877–1883. [Google Scholar] [CrossRef]
- Strassmann, J.E. Parasitoids, predators, and group size in the paper wasp, Polistes exclamans. Ecology 1981, 62, 1225–1233. [Google Scholar] [CrossRef]
- Furuichi, S. Field observation of predation on paper wasp nests by introduced terrestrial slugs. Insectes Sociaux 2014, 61, 95–96. [Google Scholar] [CrossRef]
- Kozyra, K.B.; Baraniak, E. Causes of mortality of Polistes nimpha colonies. Insectes Sociaux 2016, 63, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Pardi, L. Dominance order in Polistes wasp. Physiol. Zool. 1948, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Strassmann, J.E.; Meyer, D.C. Gerontocracy in the social wasp, Polistes exclamans. Anim. Behav. 1983, 31, 431–438. [Google Scholar] [CrossRef]
- Reeve, H.K. Polistes. In The Social Biology of Wasps; Ross, K.G., Matthews, R.W., Eds.; Comstock Publicating Associates, A Division of Cornell University Press: London, UK, 1991; pp. 99–148. [Google Scholar]
- Yoshimura, H.; Yamada, J.; Yamada, Y.Y. The queen of the paper wasp Polistes jokahamae (Hymenoptera: Polistinae) is not aggressive but maintains her reproductive priority. Sociobiology 2019, 66, 166–178. [Google Scholar] [CrossRef]
- Jandt, J.M.; Tibbetts, E.A.; Toth, A.L. Polistes paper wasps: A model genus for the study of social dominance hierarchies. Insectes Sociaux 2014, 61, 11–27. [Google Scholar] [CrossRef]
- Gamboa, G.J.; Wacker, T.L.; Scope, J.A.; Cornell, T.J.; Shellman-Reeve, J. The mechanism of queen regulation of foraging by workers in paper wasps (Polistes fuscatus, Hymenoptera: Vespidae). Ethology 1990, 85, 335–343. [Google Scholar] [CrossRef]
- Miyano, S. Worker reproduction and related behavior in orphan colonies of a Japanese paper wasp, Polistes jadwigae (Hymenoptera, Vespidae). J. Ethol. 1991, 9, 135–146. [Google Scholar] [CrossRef]
- Mitra, A. Queen pheromone and monopoly of reproduction by the queen in the social wasp Ropalidia marginata. Proc. Natl. Acad. Sci. USA 2014, 80, 1025–1044. [Google Scholar] [CrossRef]
- Strassmann, J.E. Evolutionary implications of early male and satellite nest production in Polistes exclamans colony cycles. Behav. Ecol. Sociobiol. 1981, 8, 55–64. [Google Scholar] [CrossRef]
- Kasuya, E. Social behavior of early emerging males of a Japanese paper wasp, Polistes chinensis antennalis (Hymenoptera: Vespidae). Res. Popul. Ecol. 1983, 25, 143–149. [Google Scholar] [CrossRef]
- Suzuki, T. Mating and laying of female-producing eggs by orphaned workers of a paper wasp, Polistes snelleni (Hymenoptera: Vespidae). Ann. Entomol. Soc. Am. 1985, 78, 736–739. [Google Scholar] [CrossRef]
- Suzuki, T. Paradox of worker reproduction and worker mating in temperate paper wasps, Polistes chinensis and P. snelleni (Hymenoptera: Vespidae). Ethol. Ecol. Evol. 1998, 10, 347–359. [Google Scholar] [CrossRef]
- Yamasaki, K.; Takahashi, J.; Ono, M.; Tsuchida, K. Reproductivity of early males of the temperate paper wasp Polistes rothneyi iwatai. Entomol. Sci. 2011, 14, 383–386. [Google Scholar] [CrossRef]
- Kasuya, E. Internidal drifting of workers in the Japanese paper wasp Polistes chinensis antennalis (Vespidae; Hymenoptera). Insectes Sociaux 1981, 28, 343–346. [Google Scholar] [CrossRef]
- Sumner, S.; Lucas, E.; Barker, J.; Isaac, N. Radio-tagging technology reveals extreme nest-drifting behavior in a eusocial insect. Curr. Biol. 2007, 17, 140–145. [Google Scholar] [CrossRef]
- Nishimura, M.; Ono, M. Evidence of alternative reproduction by drifting workers in the Japanese paper wasp, Polistes rothneyi Cameron, 1900 (Hymenoptera: Vespidae). Entomol. Sci. 2021, 24, 111–115. [Google Scholar] [CrossRef]
- Bohm, M.K. Effects of environment and juvenile hormone on ovaries of the wasp, Polistes metricus. J. Insect Physiol. 1972, 18, 1875–1883. [Google Scholar] [CrossRef]
- Goulson, D. Bumblebees: Behaviour, Ecology, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Zhuang, M.; Colgan, T.J.; Guo, Y.; Zhang, Z.; Liu, F.; Xia, Z.; Dai, X.; Zhang, Z.; Li, Y.; Wang, L.; et al. Unexpected worker mating and colony-founding in a superorganism. Nat. Commun. 2023, 14, 5499. [Google Scholar] [CrossRef]
- Röseler, P.-F.; Röseler, I. Caste specific differences in fat body glycogen metabolism of the bumblebee, Bombus terrestris. Insect Biochem. 1986, 16, 501–508. [Google Scholar] [CrossRef]
- Hunt, J.H.; Wolschin, F.; Henshaw, M.T.; Newman, T.C.; Toth, A.L.; Amdam, G.V. Differential gene expression and protein abundance evince ontogenetic bias toward castes in a primitively eusocial wasp. PLoS ONE 2010, 5, e10674. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.H.; Kensinger, B.J.; Kossuth, J.A.; Henshaw, M.T.; Norberg, K.; Wolschin, F.; Amdam, G.V. A diapause pathway underlies the gyne phenotype in Polistes wasps, revealing an evolutionary route to caste-containing insect societies. Proc. Natl. Acad. Sci. USA 2007, 104, 14020–14025. [Google Scholar] [CrossRef]
- Wheeler, D.E.; Buck, N.; Evans, J.D. Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Mol. Biol. 2006, 15, 597–602. [Google Scholar] [CrossRef]
- Wolschin, F.; Mutti, N.S.; Amdam, G.V. Insulin receptor substrate influences female caste development in honeybees. Biol. Lett. 2011, 7, 112–115. [Google Scholar] [CrossRef]
- Mutti, N.S.; Dolezal, A.G.; Wolschin, F.; Mutti, J.S.; Gill, K.S.; Amdam, G.V. IRS and TOR nutrient-signaling pathways act via juvenile hormone to influence honey bee caste fate. J. Exp. Biol. 2011, 214, 3977–3984. [Google Scholar] [CrossRef]
- Wheeler, D.E.; Buck, N.A.; Evans, J.D. Expression of insulin/insulin-like signalling and TOR pathway genes in honey bee caste determination. Insect Mol. Biol. 2014, 23, 113–121. [Google Scholar] [CrossRef]
- Patel, A.; Fondrk, M.K.; Kaftanoglu, O.; Emore, C.; Hunt, G.; Frederick, K.; Amdam, G.V. The making of a queen: TOR pathway is a key player in diphenic caste development. PLoS ONE 2007, 2, e509. [Google Scholar] [CrossRef] [PubMed]
- Hartfelder, K.; Guidugli-Lazzarini, K.R.; Cervoni, M.S.; Santos, D.E.; Humann, F.C. Old threads make new tapestry—Rewiring of signalling pathways underlies caste phenotypic plasticity in the honey bee, Apis mellifera L. Adv. Insect Physiol. 2015, 48, 1–36. [Google Scholar] [CrossRef]
- Corona, M.; Libbrecht, R.; Wheeler, D.E. Molecular mechanisms of phenotypic plasticity in social insects. Curr. Opin. Insect Sci. 2016, 13, 55–60. [Google Scholar] [CrossRef]
- Buttstedt, A.; Ihling, C.H.; Pietzsch, M.; Moritz, R.F.A. Royalactin is not a royal making of a queen. Nature 2016, 537, E10–E12. [Google Scholar] [CrossRef] [PubMed]
- Maleszka, R. Beyond royalactin and a master inducer explanation of phenotypic plasticity in honey bees. Commun. Biol. 2018, 1, 8. [Google Scholar] [CrossRef]
- Hartfelder, K.; Engels, W. Social insect polymorphism: Hormonal regulation of plasticity in development and reproduction in the honeybee. Curr. Top. Dev. Biol. 1998, 40, 45–77. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Yoshimura, H.; Yokoi, K. Brain physiology during photoperiod-related caste determination in the primitively eusocial wasp Polistes jokahamae. Sci. Rep. 2024, 14, 30399. [Google Scholar] [CrossRef]
- Formesyn, E.M.; Cardoen, D.; Ernst, U.R.; Danneels, E.L.; van Vaerenbergh, M.; de Koker, D.; Verleyen, P.; Wenseleers, T.; Schoofs, L.; de Graaf, D.C. Reproduction of honeybee workers is regulated by epidermal growth factor receptor signaling. Gen. Comp. Endocrinol. 2014, 197, 1–4. [Google Scholar] [CrossRef]
- Sasaki, K.; Ugajin, A.; Harano, K. Caste-specific development of the dopaminergic system during metamorphosis in female honey bees. PLoS ONE 2018, 13, e0206624. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Yokoi, K.; Toga, K. Bumble bee queens activate dopamine production and gene expression in nutritional signaling pathways in the brain. Sci. Rep. 2021, 11, 5526. [Google Scholar] [CrossRef]
- Harano, K.; Sasaki, M.; Nagao, T.; Sasaki, K. Dopamine influences locomotor activity in honeybee queens: Implications for a behavioural change after mating. Physiol. Entomol. 2008, 33, 395–399. [Google Scholar] [CrossRef]
- Sasaki, K.; Harada, M. Dopamine production in the brain is associated with caste-specific morphology and behavior in an artificial intermediate honey bee caste. PLoS ONE 2020, 15, e0244140. [Google Scholar] [CrossRef]
- Morigami, A.; Sasaki, K. Physiological specialization of the brain in bumble bee castes: Roles of dopamine in mating-related behaviors in female bumble bees. PLoS ONE 2024, 19, e0298682. [Google Scholar] [CrossRef]
- Sasaki, K.; Yoshimura, H.; Nishimura, M. Caste-specific storage of dopamine-related substances in the brains of four Polistes paper wasp species. PLoS ONE 2023, 18, e0280881. [Google Scholar] [CrossRef]
- Sasaki, K.; Yamasaki, K.; Tsuchida, K.; Nagao, T. Gonadotrophic effects of dopamine in isolated workers of the primitively eusocial wasp, Polistes chinensis. Naturwissenschaften 2009, 96, 625–629. [Google Scholar] [CrossRef]
- Toth, A.L.; Varala, K.; Newman, T.C.; Miguez, F.E.; Hutchison, S.K.; Willoughby, D.A.; Simons, J.F.; Egholm, M.; Hunt, J.H.; Hudson, M.E.; et al. Wasp gene expression supports an evolutionary link between material behavior and eusociality. Science 2007, 318, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Jedlička, P.; Ernst, U.R.; Votavová, A.; Hanus, R.; Valterová, I. Gene expression dynamics in major endocrine regulatory pathways along the transition from solitary to social life in a bumble bee, Bombus terrestris. Front. Physiol. 2016, 7, 574. [Google Scholar] [CrossRef]
- Chandra, V.; Fetter-Pruneda, I.; Oxley, P.R.; Ritger, A.L.; McKenzie, S.K.; Libbrecht, R.; Kronauer, D.J.C. Social regulation of insulin signaling and the evolution of eusociality in ants. Science 2018, 361, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Gruntenko, N.E.; Rauschenbach, I.Y. The role of insulin signalling in the endocrine stress response in Drosophila melanogaster: A mini-review. Gen. Comp. Endocrinol. 2018, 258, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.H. The Evolution of Social Wasps; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Yoshimura, H.; Yamada, Y.Y. The first brood emerges smaller, lighter, and with lower lipid stores in the paper wasp Polistes jokahamae (Hymenoptera: Vespidae). Insectes Sociaux 2018, 65, 473–481. [Google Scholar] [CrossRef]
- Mead, F.; Habersetzer, C.; Gabouriaut, D.; Gervet, J. Dynamics of colony development in the paper wasp Polistes dominulus Christ (Hymenoptera, Vespidae): The influence of prey availability. J. Ethol. 1994, 12, 43–51. [Google Scholar] [CrossRef]
- Kudô, K.; Shirai, A. Effect of food availability on larval cannibalism by foundresses of the paper wasp Polistes chinensis antennalis. Insectes Sociaux 2012, 59, 279–284. [Google Scholar] [CrossRef]
- LeBoeuf, A.C.; Waridel, P.; Brent, C.S.; Gonçalves, A.N.; Menin, L.; Ortiz, D.; Riba-Grognuz, O.; Koto, A.; Soares, Z.G.; Privman, E.; et al. Oral transfer of chemical cues, growth proteins and hormones in social insects. eLife 2016, 5, e20375. [Google Scholar] [CrossRef]
- Jeanne, R.L. Evolution of social behavior in the Vespidae. Ann. Rev. Entomol. 1980, 25, 371–396. [Google Scholar] [CrossRef]
- Jone, J.C.; Oldroyd, B.P. Nest thermoregulation in social insects. Adv. Insect Physiol. 2007, 33, 153–191. [Google Scholar] [CrossRef]
- Kovac, H.; Nagy, J.M.; Käfer, H.; Stabentheiner, A. Relationship between nest and body temperature and microclimate in the paper wasp Polistes dominula. Insects 2023, 14, 886. [Google Scholar] [CrossRef] [PubMed]
- Gradišek, A.; Bizjak, J.; Popovski, A.; Grad, J. Bumble bee nest thermoregulation: A field study. J. Apic. Res. 2023, 62, 634–642. [Google Scholar] [CrossRef]
- Darrouzet, E.; Gévar, J.; Guignard, Q.; Aron, S. Production of early diploid males by European colonies of the invasive hornet Vespa velutina nigrithorax. PLoS ONE 2015, 10, e0136680. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).