Transformation of Internal Thoracic Structures of Callobruchus maculatus (Coleoptera: Bruchidae) from Larva to Adult
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Rearing
2.2. Larval Specimen Collection
2.3. Pupal Specimen Collection
2.4. Micro-CT and 3D Reconstruction
2.5. Terminology and Abbreviations
3. Results
3.1. Larval Development
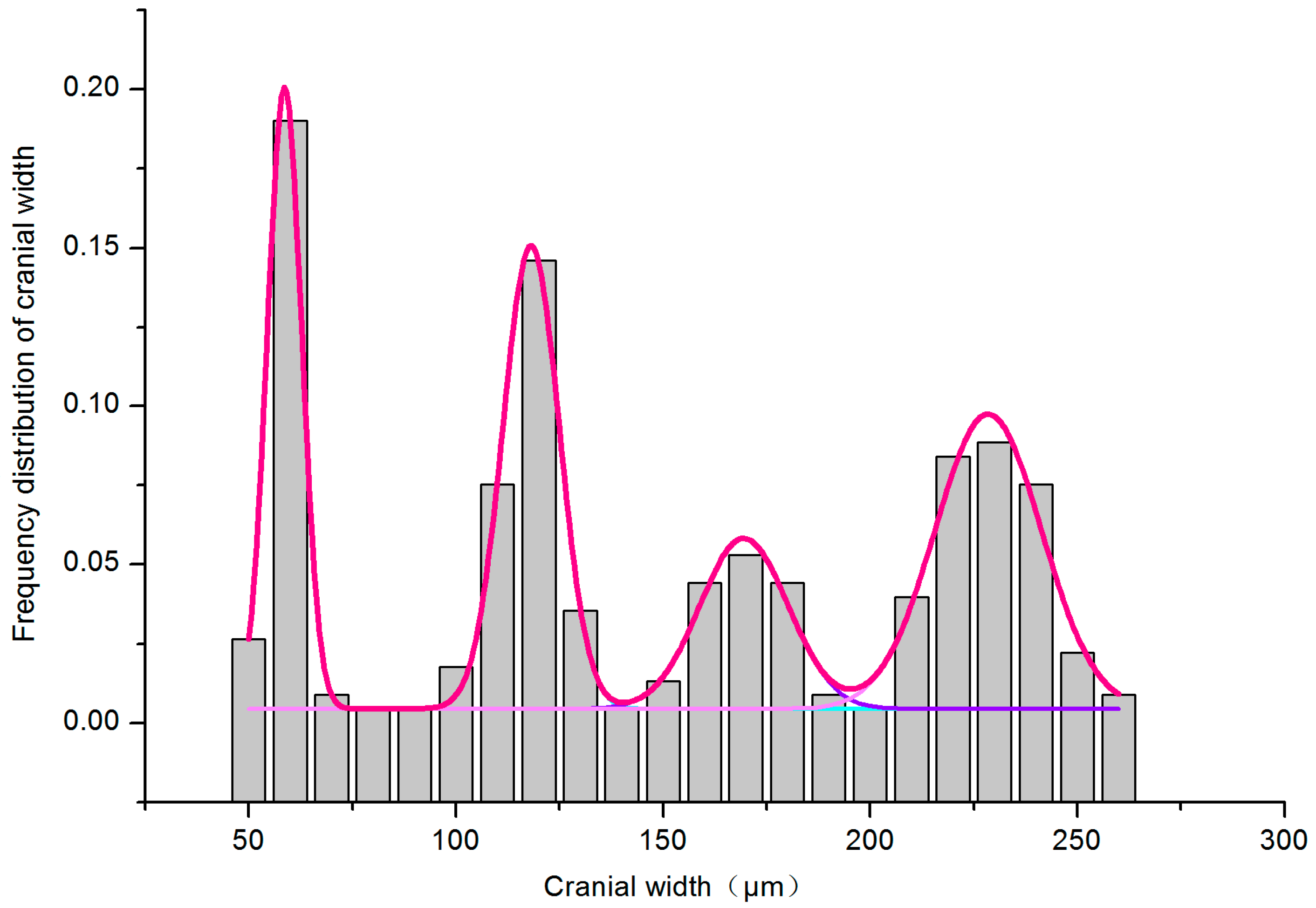
3.1.1. Multiple-Peak Fit
3.1.2. Cuticles
3.1.3. Muscles
3.1.4. Digestive System, Respiratory System and Nervous System
3.2. Pupal Development
3.2.1. Skeletons
3.2.2. Muscles
3.2.3. Digestive System, Respiratory System and Nervous System
4. Discussion
4.1. Skeleto-Muscular Transformations During Larval Development
4.2. Skeleto-Muscular Transformations During Pupal Development
4.3. Transformation of Other Organs During Larval and Pupal Stages
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruan, Q.C.; Lin, X. Study on the biology of Callosobruchus maculatus. Food Storage 1988, 17, 3–9. [Google Scholar]
- Ma, Z.; Jiang, C.Y.; Zhang, R.Z.; Liu, H.; Qin, M.; Feng, X.D.; Zhang, R.Z. Distribution and spread of national quarantine insects of agriculatural plants in China. Chin. J. Appl. Entomol. 2018, 55, 1–11. [Google Scholar]
- Utida, S. “Phase” dimorphism observed in the laboratory population of the cowpea weevil, Callosobruchus quadrimaculatus 2nd report: Differential effects of temperature, humidity and population density upon some ecological characters of the two phases. Res. Popul. Ecol. 1956, 3, 93–104. [Google Scholar] [CrossRef]
- Du, Z.; Liu, X.K.; Liu, S.P.; Jiang, L.; Zong, L.; Li, W.J.; Fan, W.L.; Zhang, L.J.; Wu, F.M.; Ge, S.Q. Divergence in the morphology and energy metabolism of adult polyphenism in the cowpea beetle Callosobruchus maculatus. Insects 2024, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Rolff, J.; Johnston, P.R.; Reynolds, S. Complete metamorphosis of insects. Philos. Trans. R Soc. Lond. B Biol. Sci. 2019, 374, 20190063. [Google Scholar] [CrossRef]
- Richards, C.S.; Simonsen, T.J.; Abel, R.L.; Hall, M.J.R.; Schwyn, D.A.; Wicklein, M. Virtual forensic entomology: Improving estimates of minimum post-mortem interval with 3D micro-computed tomography. Forensic Sci. Int. 2012, 220, 251–264. [Google Scholar] [CrossRef]
- Lowe, T.; Garwood, R.J.; Simonsen, T.J.; Bradley, R.S.; Withers, P.J. Metamorphosis revealed: Time-lapse three-dimensional imaging inside a living chrysalis. J. R. Soc. Interface 2013, 10, 20130304. [Google Scholar] [CrossRef]
- Martín-Vega, D.; Simonsen, T.J.; Hall, M.J.R. Looking into the puparium: Micro-CT visualization of the internal morphological changes during metamorphosis of the blow fly, Calliphora vicina, with the first quantitative analysis of organ development in cyclorrhaphous dipterans. J. Morphol. 2017, 278, 629–651. [Google Scholar] [CrossRef]
- Helm, B.R.; Payne, S.; Rinehart, J.P.; Yocum, G.D.; Bowsher, J.H.; Greenlee, K.J. Micro-computed tomography of pupal metamorphosis in the solitary bee Megachile rotundata. Arthropod. Struct. Dev. 2018, 47, 521–528. [Google Scholar] [CrossRef]
- Zhao, C.J.; Ang, Y.C.; Wang, M.Q.; Gao, C.X.; Zhang, K.Y.; Tang, C.F.; Liu, X.Y.; Li, M.; Yang, D.; Meier, R. Contribution to understanding the evolution of holometaboly: Transformation of internal head structures during the metamorphosis in the green lacewing Chrysopa pallens (Neuroptera: Chrysopidae). BMC Evol. Biol. 2020, 20, 79. [Google Scholar] [CrossRef]
- Zhao, C.J.; Wang, M.Q.; Gao, C.X.; Li, M.; Zhang, K.Y.; Yang, D.; Liu, X.Y. Evolution of holometaboly revealed by developmental transformation of internal thoracic structures in a green lacewing Chrysopa pallens (Neuroptera: Chrysopidae). Insect Sci. 2022, 29, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Yin, H.D.; Li, W.J.; Qin, Z.H.; Yang, Y.; Huang, Z.Z.; Zong, L.; Liu, X.K.; Du, Z.; Fan, W.L.; et al. The morphological transformation of the thorax during the eclosion of Drosophila melanogaster (Diptera: Drosophilidae). Insects 2023, 14, 893. [Google Scholar] [CrossRef]
- Polilov, A.A.; Beutel, R.G. Developmental stages of the hooded beetle Sericoderus lateralis (Coleoptera: Corylophidae) with comments on the phylogenetic position and effects of miniaturization. Arthropod. Struct. Dev. 2010, 39, 52–69. [Google Scholar] [CrossRef]
- Ge, S.Q.; Hua, Y.; Ren, J.; Ślipiński, A.; Heming, B.; Beutel, R.G.; Yang, X.K.; Wipfler, B. Transformation of head structures during the metamorphosis of Chrysomela populi (Coleoptera: Chrysomelidae). Arthropod. Syst. Phylogeny 2015, 73, 129–152. [Google Scholar] [CrossRef]
- Raś, M.; Iwan, D.; Kaminski, M.J. The tracheal system in post-embryonic development of holometabolous insects: A case study using the mealworm beetle. J. Anat. 2018, 232, 997–1015. [Google Scholar] [CrossRef]
- Vommaro, M.L.; Donato, S.; Caputo, S.; Agostino, R.G.; Montali, A.; Tettamanti, G.; Giglio, A. Anatomical changes of Tenebrio molitor and Tribolium castaneum during complete metamorphosis. Cell Tissue Res. 2024, 396, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.G.; Xiao, Y.Q.; Zhang, C.W.; Liu, Y.; Zhou, H.; Li, F. Micro-CT data of complete metamorphosis process in Harmonia axyridis. Sci. Data 2024, 11, 557. [Google Scholar] [CrossRef]
- Dyar, H.G. The number of molts of lepidopterous larvae. Psyche A J. Entomol. 1890, 5, 420–422. [Google Scholar] [CrossRef]
- Hammack, L.; Ellsbury, M.M.; Roehrdanz, R.L.; Pikul, J.R. Larval sampling and instar determination in field populations of northern and western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2003, 96, 1153–1159. [Google Scholar] [CrossRef]
- Panzavolta, T. Instar determination for Pissodes castaneus (Coleoptera: Curculionidae) using head capsule widths and lengths. Environ. Entomol. 2014, 36, 1054–1058. [Google Scholar] [CrossRef]
- Bleiker, K.P.; Régnière, J. Determining the instar of mountain pine beetle (Coleoptera: Curculionidae) larvae by the width of their head capsules. Can. Entomol. 2015, 147, 635–640. [Google Scholar] [CrossRef]
- Li, Z.W.; He, L.H.; Xia, J.; Ma, L.; Zeng, A.P. Determination of larval instars of the camellia weevil, Curculio chinensis (Coleoptera: Curculionidae). Acta Entomol. Sin. 2015, 58, 181–189. (In Chinese) [Google Scholar]
- Cao, X.M.; Dou, W.; Deng, Y.X.; Wang, J.J. Research progress on biological and ecological characteristics and control methods of Callosobruchus maculatus. Plant Quar. 2008, 22, 41–44. (In Chinese) [Google Scholar]
- Ruan, Y.Y.; Zhang, M.N.; Kundrata, R.; Qiu, L.; Ge, S.Q.; Yang, X.K.; Chen, X.Q.; Jiang, S.H. Functional morphology of the thorax of the click beetle Campsosternus auratus (Coleoptera, Elateridae), with an emphasis on its jumping mechanism. Insects 2022, 13, 248. [Google Scholar] [CrossRef]
- Friedrich, F.; Beutel, R.G. The thorax of Zorotypus (Hexapoda, Zoraptera) and a new nomenclature for the musculature of Neoptera. Arthropod. Struct. Dev. 2008, 37, 29–54. [Google Scholar] [CrossRef]
- Robertson, C.W. The metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J. Morphol. 1936, 59, 351–399. [Google Scholar] [CrossRef]
- Schulman, V.K.; Dobi, K.C.; Baylies, M.K. Morphogenesis of the somatic musculature in Drosophila melanogaster. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 313–334. [Google Scholar] [CrossRef] [PubMed]
- Hartenstein, V. Atlas of Drosophila development. In The Development of Drosophila melanogaster; Bate, M., Arias, A.M., Eds.; Cold Spring Harbor Press: New York, NY, USA, 1993; Volume 2, pp. 1–53. [Google Scholar]







| Stages | Parameters | |||||
|---|---|---|---|---|---|---|
| Voltage (kV) | Current (μA) | Pixel Size (μm) | Voxel Size (μm3) | Image Size | Optical Magnification | |
| L1 | 60 | 133 | 0.8456 | 0.6046 | 1003 × 1003 | 9.8225: “10x” |
| L2 | 1.5118 | 3.4552 | 1012 × 1012 | 3.9687: “4x” | ||
| L3 | 2.0158 | 8.1911 | 1012 × 1012 | 3.9687: “4x” | ||
| L4 | 2.0661 | 8.8197 | 992 × 1005 | 3.9687: “4x” | ||
| PP | 2.0920 | 9.1555 | 980 × 1003 | 9.8225: “10x” | ||
| PI | 1.9607 | 7.5376 | 980 × 1003 | 9.8225: “10x” | ||
| PM | 1.9685 | 7.6279 | 972 × 1003 | 9.8225: “10x” | ||
| PL | 1.9607 | 7.5376 | 980 × 1003 | 9.8225: “10x” | ||
| A | 2.2522 | 11.4240 | 988 × 1012 | 3.9687: “4x” | ||
| Larval Instar | Sample Size | Mean ± SD (µm) | Range (µm) | Coefficient of Variation | Misclassification Probability | ||
|---|---|---|---|---|---|---|---|
| I as I − 1 | I as I + 1 | Total | |||||
| L1 | 51 | 58.86 ± 3.76 | 51.35–74.05 | 0.0638 | - | 0.0000 | 0.0000 |
| L2 | 64 | 115.98 ± 9.04 | 83.92–134.88 | 0.0779 | 0.0000 | 0.0021 | 0.0021 |
| L3 | 38 | 169.57 ± 11.83 | 140.57–193.91 | 0.0697 | 0.0036 | 0.0124 | 0.0160 |
| L4 and prepupa | 73 | 229.15 ± 13.01 | 196.71–264.06 | 0.0568 | 0.0022 | - | 0.0022 |
| L1 | L2 | L3 | L4 | Prepupa | |
|---|---|---|---|---|---|
| Length | 204.75 | 495.55 | 781.08 | 1060.61 | 1154.03 |
| Width | 334.09 | 819.70 | 1194.10 | 1528.95 | 1523.12 |
| Height | 328.15 | 706.39 | 898.46 | 1331.37 | 1435.07 |
| Muscles | Origin | Insertion |
|---|---|---|
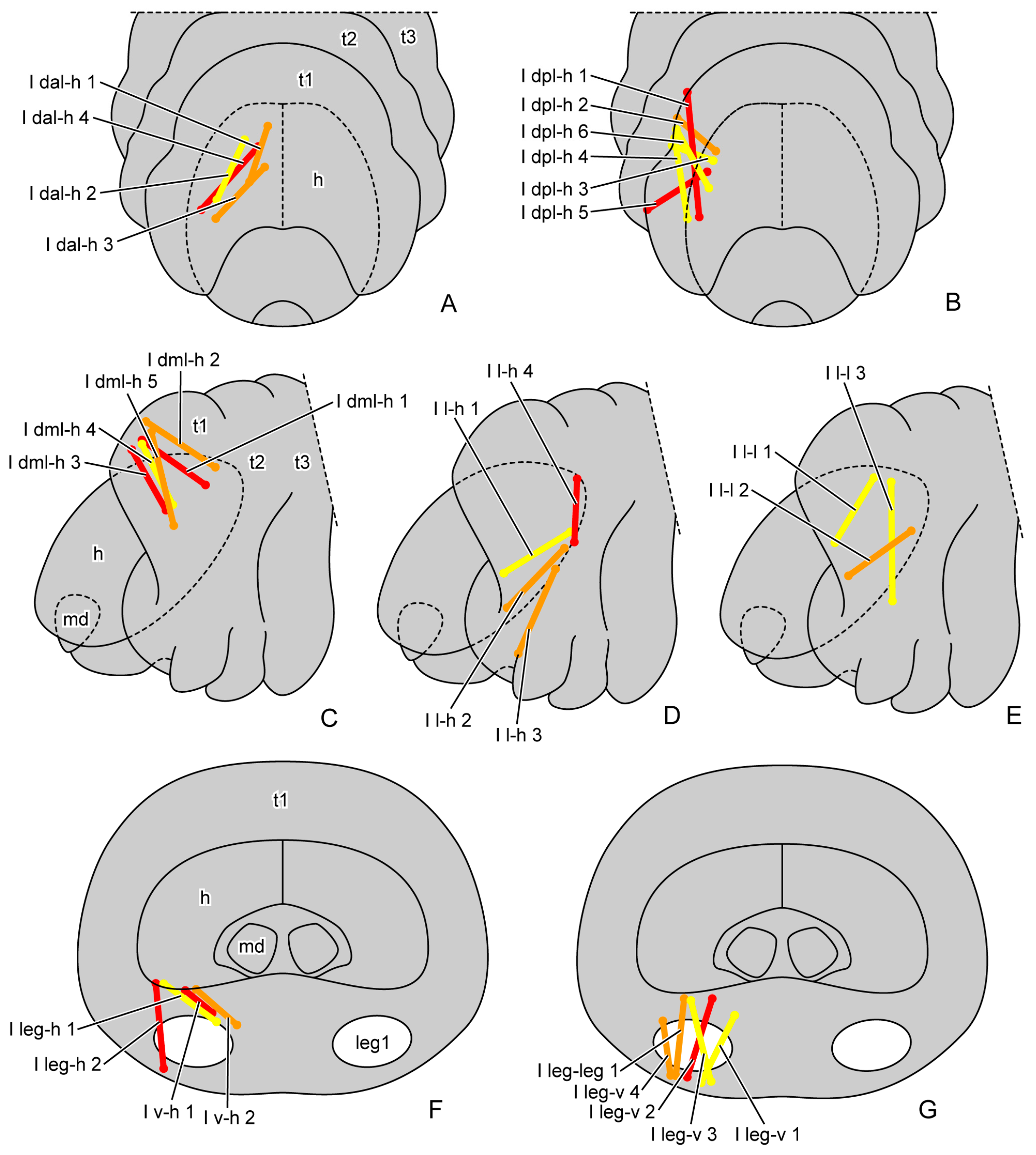
| I dam-h | antero-median area of prothoracic dorsal region | head |
| I dal-h | antero-lateral area of prothoracic dorsal region | head |
| I dml-h | meso-lateral area of prothoracic dorsal region | head |
| I dpl-h | postero-lateral area of prothoracic dorsal region | head |
| I dpm-h | postero-median area of prothoracic dorsal region | head |
| I d-d | prothoracic dorsal region | prothoracic dorsal region |
| I l-h | prothoracic lateral region | head |
| I l-l | prothoracic lateral region | prothoracic lateral region |
| I l-leg | prothoracic lateral region | basal rim of proleg |
| I v-h | prothoracic ventral region | head |
| I leg-h | basal rim of proleg | head |
| I leg-v | basal rim of proleg | prothoracic ventral region |
| I leg-leg | basal rim of proleg | basal rim of proleg |
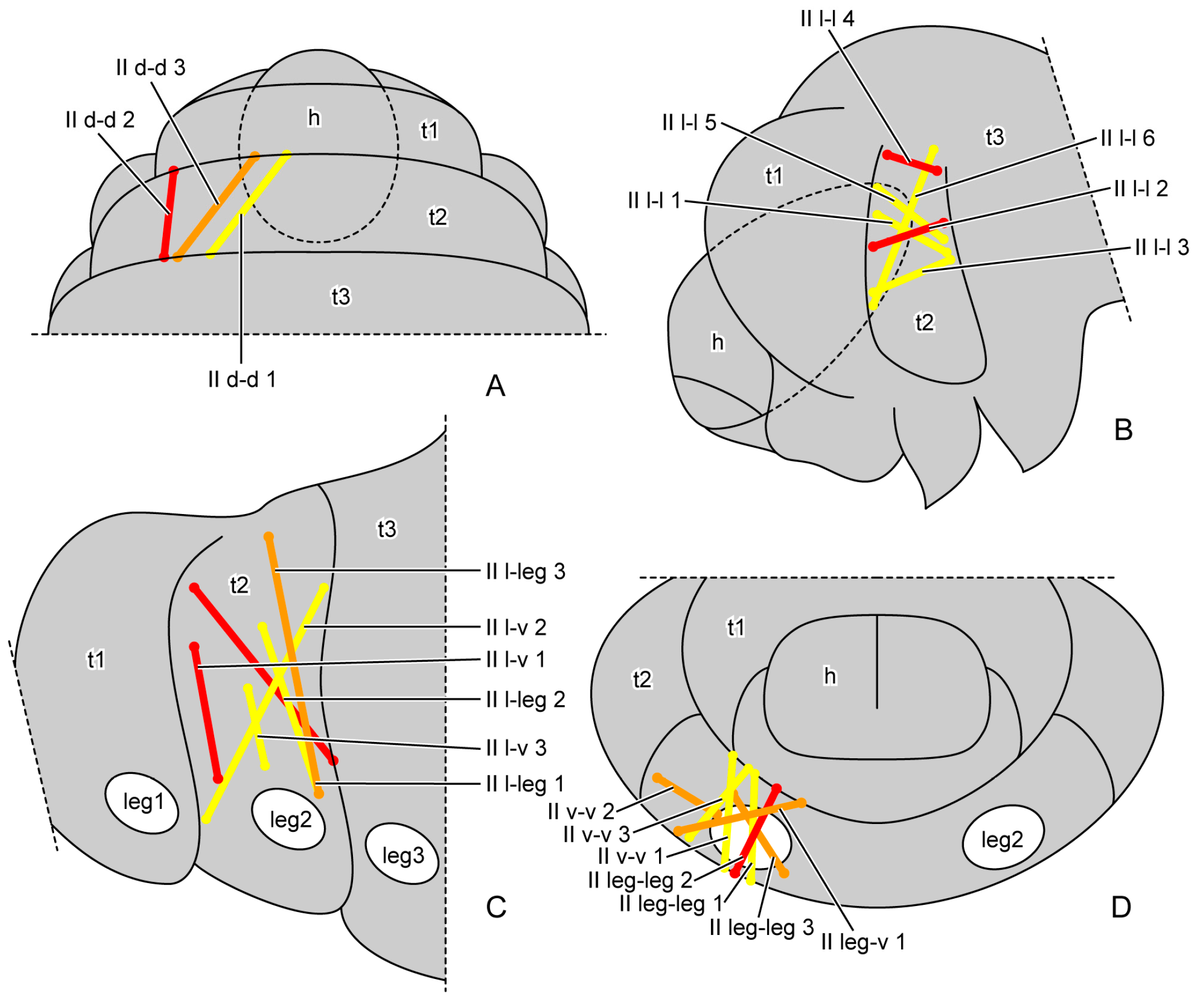
| II d-d | mesothoracic dorsal region | mesothoracic dorsal region |
| II d-l | mesothoracic dorsal region | mesothoracic lateral region |
| II l-l | mesothoracic lateral region | mesothoracic lateral region |
| II l-v | mesothoracic lateral region | mesothoracic ventral region |
| II l-leg | mesothoracic lateral region | basal rim of midleg |
| II v-v | mesothoracic ventral region | mesothoracic ventral region |
| II leg-v | basal rim of midleg | mesothoracic ventral region |
| II leg-leg | basal rim of midleg | basal rim of midleg |
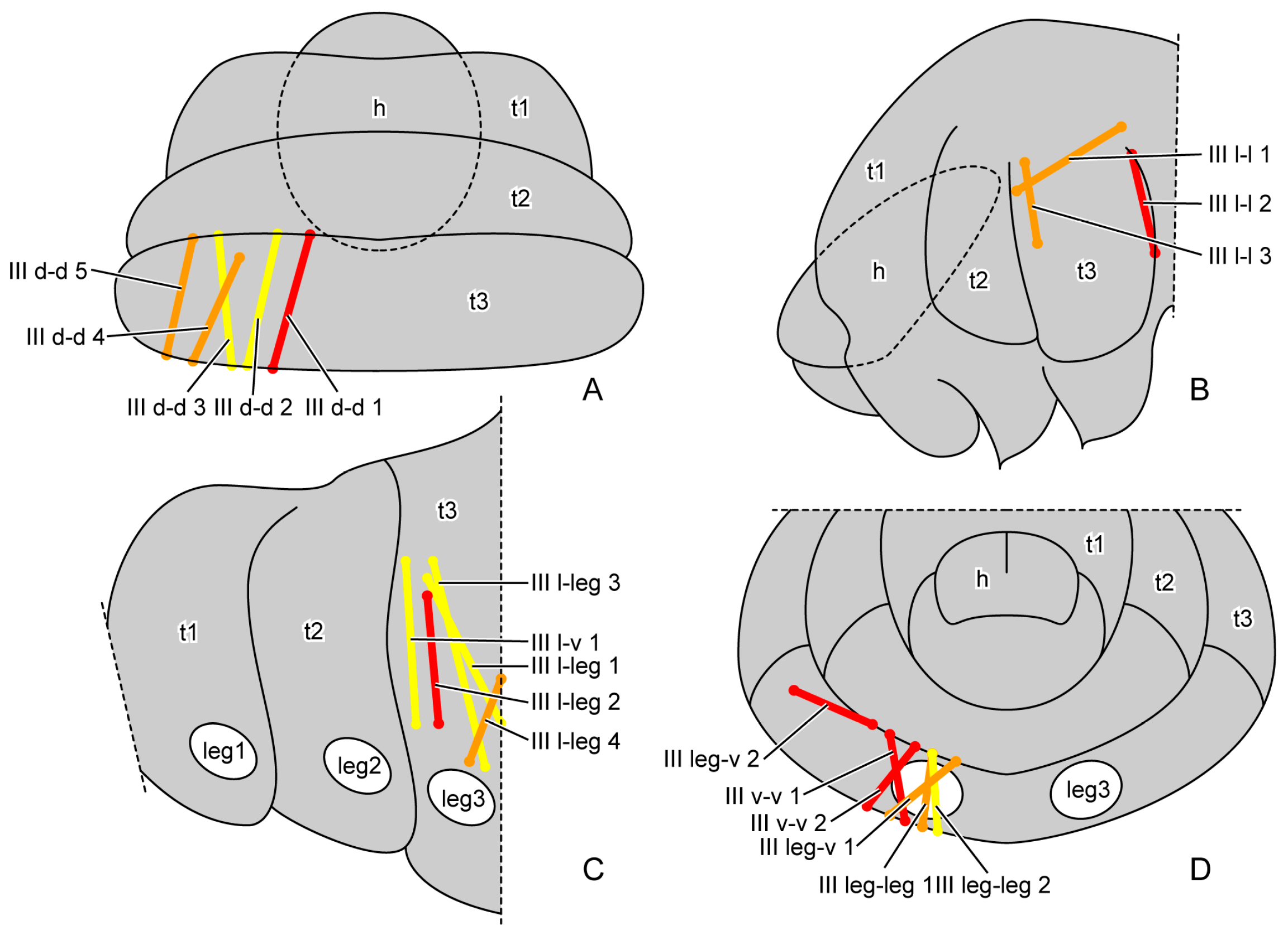
| III d-d | metathoracic dorsal region | metathoracic dorsal region |
| III d-l | metathoracic dorsal region | metathoracic lateral region |
| III d-v | metathoracic dorsal region | metathoracic ventral region |
| III l-l | metathoracic lateral region | metathoracic lateral region |
| III l-v | metathoracic lateral region | metathoracic ventral region |
| III l-leg | metathoracic lateral region | basal rim of hindleg |
| III v-v | metathoracic ventral region | metathoracic ventral region |
| III leg-v | basal rim of hindleg | metathoracic ventral region |
| III leg-leg | basal rim of hindleg | basal rim of hindleg |
| T Iv-IIl | prothoracic ventral region | mesothoracic lateral region |
| T Iv-IIv | prothoracic ventral region | mesothoracic ventral region |
| T IIl-h | mesothoracic lateral region | head |
| T IId-IIId | mesothoracic dorsal region | metathoracic dorsal region |
| T IId-IIIv | mesothoracic dorsal region | metathoracic ventral region |
| T IIl-IIIv | mesothoracic lateral region | metathoracic ventral region |
| T IIv-h | mesothoracic ventral region | head |
| T IIv-IIIl | mesothoracic ventral region | mesothoracic lateral region |
| T IIv-IIIv | mesothoracic ventral region | metathoracic ventral region |
| T IIId-At | metathoracic dorsal region | abdomen |
| T IIIl-h | metathoracic lateral region | head |
| T IIIv-h | metathoracic ventral region | head |
| Muscles | 1st Instar | 2nd Instar | 3rd Instar | 4th Instar | Prepupa |
|---|---|---|---|---|---|
| Prothorax | |||||
| I dam-h 1 | + | + | + | − | − |
| I dam-h 2 | − | + | + | + | − |
| I dal-h 1 | + | + | + | + | + |
| I dal-h 2 | + | + | + | + | − |
| I dal-h 3 | − | + | + | + | + |
| I dal-h 4 | − | + | + | + | + |
| I dml-h 1 | + | + | + | + | − |
| I dml-h 2 | + | + | + | − | − |
| I dml-h 3 | − | + | + | − | − |
| I dml-h 4 | − | + | + | − | − |
| I dml-h 5 | − | + | − | + | − |
| I dpl-h 1 | + | + | + | + | + |
| I dpl-h 2 | + | + | + | + | + |
| I dpl-h 3 | + | + | + | + | + |
| I dpl-h 4 | − | + | + | + | + |
| I dpl-h 5 | − | + | + | + | + |
| I dpl-h 6 | − | + | − | + | + |
| I dpm-h 1 | + | + | + | + | + |
| I d-d 1 | + | − | − | − | − |
| I l-h 1 | + | + | + | + | + |
| I l-h 2 | + | + | + | − | + |
| I l-h 3 | + | + | + | + | − |
| I l-h 4 | − | + | − | − | − |
| I l-l 1 | − | + | + | − | − |
| I l-l 2 | − | + | + | + | + |
| I l-l 3 | − | + | + | − | + |
| I l-leg 1 | + | + | + | − | − |
| I v-h 1 | − | + | + | + | − |
| I v-h 2 | − | − | + | + | − |
| I leg-h 1 | + | + | + | + | − |
| I leg-h 2 | + | + | + | + | − |
| I leg-v 1 | − | + | + | + | + |
| I leg-v 2 | − | − | − | + | − |
| I leg-v 3 | − | − | − | + | − |
| I leg-v 4 | − | − | − | + | − |
| I leg-leg 1 | + | + | + | + | − |
| Mesothorax | |||||
| II d-d 1 | − | + | − | + | − |
| II d-d 2 | − | + | + | + | − |
| II d-d 3 | − | + | + | + | + |
| II d-l 1 | − | + | − | − | − |
| II l-l 1 | − | + | + | + | − |
| II l-l 2 | − | + | + | − | − |
| II l-l 3 | − | + | + | + | − |
| II l-l 4 | − | − | + | − | − |
| II l-l 5 | − | − | + | − | − |
| II l-l 6 | − | − | + | + | − |
| II l-v 1 | − | + | + | + | + |
| II l-v 2 | − | + | − | − | − |
| II l-v 3 | − | − | + | + | + |
| II l-leg 1 | − | + | + | − | + |
| II l-leg 2 | − | − | − | − | + |
| II l-leg 3 | − | − | − | − | + |
| II v-v 1 | − | + | + | + | + |
| II v-v 2 | − | + | + | − | + |
| II v-v 3 | − | − | − | + | − |
| II leg-v 1 | − | − | − | + | − |
| II leg-leg 1 | − | + | + | + | − |
| II leg-leg 2 | − | − | + | + | − |
| II leg-leg 3 | − | − | − | + | − |
| Metathorax | |||||
| III d-d 1 | − | + | + | + | − |
| III d-d 2 | − | + | − | + | − |
| III d-d 3 | − | + | + | + | + |
| III d-d 4 | − | + | + | + | + |
| III d-d 5 | − | + | + | + | + |
| III d-l 1 | − | + | + | + | + |
| III d-l 2 | − | − | + | + | − |
| III d-v 1 | − | − | − | − | + |
| III d-v 2 | − | − | − | − | + |
| III d-v 3 | − | − | − | − | + |
| III l-l 1 | − | + | + | + | − |
| III l-l 2 | − | − | + | − | + |
| III l-l 3 | − | − | − | − | + |
| III l-v 1 | − | − | − | − | + |
| III l-leg 1 | − | + | + | + | + |
| III l-leg 2 | − | + | + | + | + |
| III l-leg 3 | − | − | + | + | + |
| III l-leg 4 | − | − | − | − | + |
| III v-v 1 | − | − | + | + | − |
| III v-v 2 | − | − | + | + | + |
| III v-leg 1 | − | + | + | + | − |
| III v-leg 2 | − | + | + | + | − |
| III leg-leg 1 | − | + | + | + | − |
| III leg-leg 2 | − | − | + | + | − |
| Intersegmental muscles | |||||
| T Iv-IIl 1 | − | − | + | − | − |
| T Iv-IIv 1 | − | + | − | − | − |
| T Iv-IIv 2 | − | + | − | − | − |
| T IId-IIId 1 | − | + | − | − | − |
| T IId-IIIl 1 | − | + | − | − | − |
| T IId-IIIv 1 | − | + | − | − | − |
| T IIl-h 1 | + | + | + | + | + |
| T IIl-h 2 | + | − | + | + | − |
| T IIl-IIIv 1 | − | + | − | − | − |
| T IIv-h 1 | + | + | + | + | + |
| T IIv-IIIl 1 | − | − | + | − | − |
| T IIv-IIIv 1 | − | + | + | − | − |
| T IIId-At 1 | − | + | − | − | − |
| T IIIl-h 1 | − | + | + | + | + |
| T IIIv-h 1 | − | + | + | + | + |
| PI | PM | PL | A | |
|---|---|---|---|---|
| Length | 1046.37 | 1333.79 | 1280.20 | 1390.40 |
| Width | 1102.97 | 1230.87 | 1289.23 | 1334.74 |
| Height | 962.00 | 1326.50 | 1264.24 | 1324.20 |
| Muscles | Initial Pupa | Middle Pupa | Late Pupa | Adult |
|---|---|---|---|---|
| Prothorax | ||||
| Idlm1 | − | + | + | + |
| Idlm2 | − | + | + | + |
| Idlm3 | − | − | + | + |
| Idlm5 | − | + | + | + |
| Idlm6 | − | − | + | − |
| Idvm2 | − | − | + | + |
| Idvm6 | − | − | + | + |
| Idvm7 | − | + | + | + |
| Idvm8 | − | − | + | + |
| Idvm9 | − | − | − | + |
| Idvm10 | − | − | − | + |
| Idvm18 | − | + | + | + |
| Itpm3 | − | + | + | + |
| Itpm4 | − | − | − | + |
| Itpm5 | − | + | + | + |
| Itpm6 | − | + | + | + |
| Ipcm6 | − | + | + | + |
| Ivlm1 | − | − | + | + |
| Ivlm3 | − | − | + | + |
| Ivlm7 | − | + | + | + |
| Iscm4 | − | − | + | + |
| Mesothorax | ||||
| IIdlm1 | − | + | + | + |
| IIdlm2 | − | + | + | + |
| IIdvm1 | − | + | + | + |
| IIdvm2 | − | − | + | + |
| IItpm1 | − | − | + | + |
| IItpm4 | − | − | + | + |
| IIspm2 | − | − | + | + |
| IIspm7 | − | − | + | + |
| IIpcm4 | − | + | + | + |
| IIvlm3 | − | + | + | + |
| IIscm1 | − | − | + | + |
| IIscm2 | − | + | + | + |
| IIscm6 | − | + | + | + |
| Metathorax | ||||
| IIIdlm1 | − | + | + | + |
| IIIdlm2 | − | + | + | + |
| IIIdvm1 | + | + | + | + |
| IIIdvm2 | + | + | + | + |
| IIIdvm4 | + | + | + | + |
| IIIdvm5 | − | + | + | + |
| IIIdvm8 | − | + | + | + |
| IIItpm1 | − | + | + | + |
| IIItpm3 | − | + | + | + |
| IIItpm7 | − | + | + | + |
| IIItpm8 | − | − | − | + |
| IIItpm9 | − | + | + | + |
| IIItpm10 | − | − | − | + |
| IIIspm1 | + | + | + | + |
| IIIpcm3 | + | + | + | + |
| IIIpcm4 | + | + | + | + |
| IIIscm1 | + | + | + | + |
| IIIscm2 | − | − | − | + |
| IIIscm3 | − | + | + | + |
| IIIscm4 | − | + | + | + |
| IIIscm6 | − | + | + | + |
| Muscles of Prepupa | Muscles of Initial Pupa | ||
|---|---|---|---|
| III d-v 1 | O (=origin): antero-lateral area of metathoracic dorsal region I (=insertion): antero-lateral area of metathoracic ventral region | IIIdvm1 | O: antero-lateral area of metanotum I: antero-median area of metasternum |
| III d-v 2 | O: antero-lateral area of metathoracic dorsal region I: antero-lateral area of metathoracic ventral region | IIIdvm2 | O: latero-median area of metanotum I: latero-median area of metasternum |
| III d-v 3 | O: postero-lateral area of metathoracic dorsal region I: postero-lateral area of metathoracic ventral region | IIIdvm4 | O: antero-median area of metapleuron I: postero-lateral area of metasternum |
| III l-v 1 | O: antero-dorsal area of metathoracic lateral region I: antero-lateral area of metathoracic ventral region | IIIspm1 | O: antero-median area of metapleuron I: latero-median area of metasternum |
| III l-l 3 | O: antero-dorsal area of metathoracic lateral region I: antero-median area of metathoracic lateral region | IIIpcm3 | O: antero-median area of metapleuron I: latero-ventral margin of metacoxal rim |
| III l-leg 4 | O: postero-ventral area of metathoracic lateral region I: antero-lateral margin of basal rim of hindleg | IIIpcm4 | O: postero-median area of metapleuron I: dorso-lateral margin of metacoxal rim |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, X.; Zhang, L.; Wang, X.; Zhang, X.; Zong, L.; Li, W.; Huang, Z.; Liu, X.; Ge, S. Transformation of Internal Thoracic Structures of Callobruchus maculatus (Coleoptera: Bruchidae) from Larva to Adult. Insects 2025, 16, 324. https://doi.org/10.3390/insects16030324
Liu S, Liu X, Zhang L, Wang X, Zhang X, Zong L, Li W, Huang Z, Liu X, Ge S. Transformation of Internal Thoracic Structures of Callobruchus maculatus (Coleoptera: Bruchidae) from Larva to Adult. Insects. 2025; 16(3):324. https://doi.org/10.3390/insects16030324
Chicago/Turabian StyleLiu, Sipei, Xiaokun Liu, Lijie Zhang, Xieshuang Wang, Xinying Zhang, Le Zong, Wenjie Li, Zhengzhong Huang, Xin Liu, and Siqin Ge. 2025. "Transformation of Internal Thoracic Structures of Callobruchus maculatus (Coleoptera: Bruchidae) from Larva to Adult" Insects 16, no. 3: 324. https://doi.org/10.3390/insects16030324
APA StyleLiu, S., Liu, X., Zhang, L., Wang, X., Zhang, X., Zong, L., Li, W., Huang, Z., Liu, X., & Ge, S. (2025). Transformation of Internal Thoracic Structures of Callobruchus maculatus (Coleoptera: Bruchidae) from Larva to Adult. Insects, 16(3), 324. https://doi.org/10.3390/insects16030324







