1. Introduction
The western flower thrips,
Frankliniella occidentalis, is polyphagous and causes damage to high-value crops including hot peppers [
1]. The insect pest also transmits plant viruses to crops, causing devastating economic damage [
2]. Due to frequent use of chemical insecticides, this species has developed insecticide resistance [
3]. Moreover, its ability to hide in flowers or crevices makes it difficult to effectively control by insecticide sprays [
4]. Alternatively, non-chemical control techniques have been developed and implemented to suppress the outbreaks of
F. occidentalis. For example, aggregation pheromone has been used in sticky traps for mass-trapping [
5,
6]. In addition to chemical cues, visual signals during day time are used to locate hosts and facilitate diurnal feeding and mating rhythmicity [
7,
8]. Four circadian clock genes (Period (
Per), Timeless (
TIM), Doubletime (
DBT), and Clock (
CLK)) are expressed in
F. occidentalis and associated with the diel rhythmicity [
8].
Insect feeding behavior is functionally associated with expression of a foraging gene encoding a cGMP-dependent protein kinase (
PKG) [
9]. Upon activation by cGMP, PKG phosphorylates a number of biologically important targets associated with the regulation of muscle contraction, metabolism, and gene expression [
10]. Variation of
PKG expression is implicated in the plasticity of insect behaviors [
11]. Manipulation of its expression level and subsequent kinetic activity influences alternative feeding behaviors of the fruit fly,
Drosophila melanogaster; the two patterns are referred to as sitters and rovers [
12]. The expression levels also drive division of labor systems across diverse social species [
13,
14]. These suggest a functional role of PKG in controlling insect behaviors. In mammals, the circadian clock is entrained by signals mediated through PKG activity, which is induced by cGMP up-regulated by nitric oxide (NO) which catalyzes the phosphorylation of TIM [
15]. This suggests that PKG influences the circadian clock of
F. occidentalis. However, the functional link between PKG and circadian clock function remained elusive in insects.
This study investigated the physiological role of PKG in the diurnal behavior of the thrips, F. occidentalis, by regulating expression of the clock genes. To address this hypothesis, PKG gene expression in F. occidentalis was monitored over a 24-h period in a 12:12 LD cycle. To test the functional link, PKG expression was suppressed by its specific RNA interference (RNAi) and the resulting changes of the clock gene expressions were analyzed. In addition, any subtle alteration of the thrips behavior was assessed by mathematical parameters extracted from continuous automated 24-h monitoring of movement tracks.
2. Materials and Methods
2.1. Insect Rearing
Both larvae and adults of F. occidentalis were obtained from the Department of Crop Protection, National Institute of Agricultural Sciences (Jeonju, Republic of Korea), and maintained at conditions of 25 ± 1 °C temperature, 60 ± 5% relative humidity, and a 14:10 h (LD) light cycle. Newly germinated beans (Phaseolus coccineus L.) were supplied for feeding and oviposition. Eggs that were newly laid on the beans in adult colonies were transferred to a breeding dish (SPL Life Science, Seoul, Republic of Korea). After 3 days, by which point most larvae had hatched, new beans were supplied every day. Under the laboratory conditions, larvae underwent two instars (L1–L2) and were distinct from prepupae or pupae that developed wing pads.
2.2. Bioinformatics Analysis
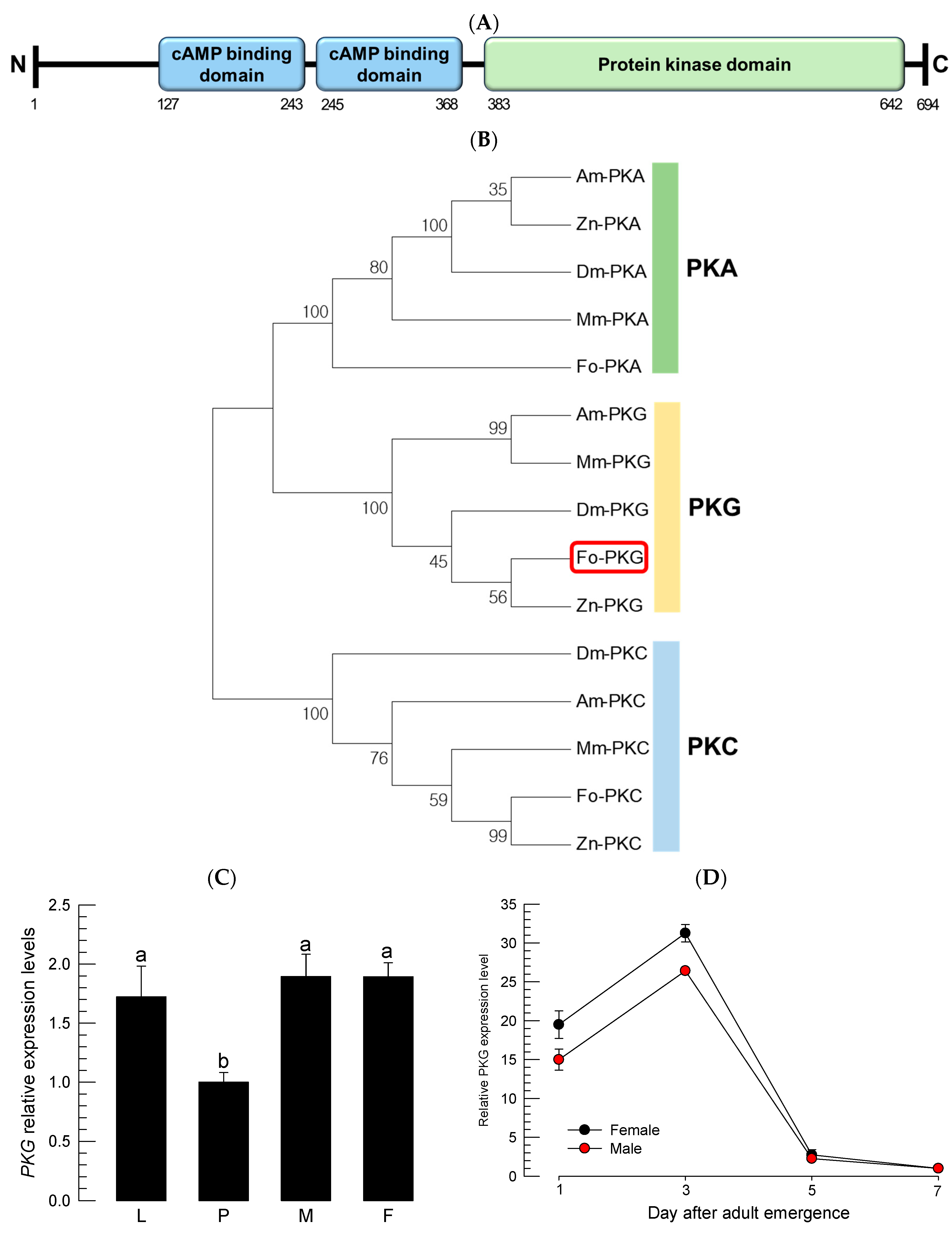
DNA and amino acid sequences including the
PKG gene of
F. occidentalis (
Fo-PKG) were obtained from NCBI (National Center for Biotechnology Information:
https://blast.ncbi.nlm.nih.gov, accessed on 1 October 2024) with accession numbers. MEGA6.0 was used to construct a phylogenetic tree through clustering using Maximum likelihood, where the evolutionary distances were computed using the Poisson correction method. Bootstrapping values were obtained with 1000 trials to support branching and clustering. Protein domains were predicted using a program searching the conserved domain (
https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi accessed on 1 October 2024), and InterPro (
https://www.ebi.ac.uk/interpro/ accessed on 1 October 2024). The N-terminal signal peptide was determined using the SignalP 5.0 server (
https://services.healthtech.dtu.dk/service.SignalP-5.0/ accessed on 1 October 2024). The resulting domains were drawn by Biorender (
https://biorender.com/ accessed on 1 October 2024). The protein–protein interaction map was generated by using STRING 12.0 (
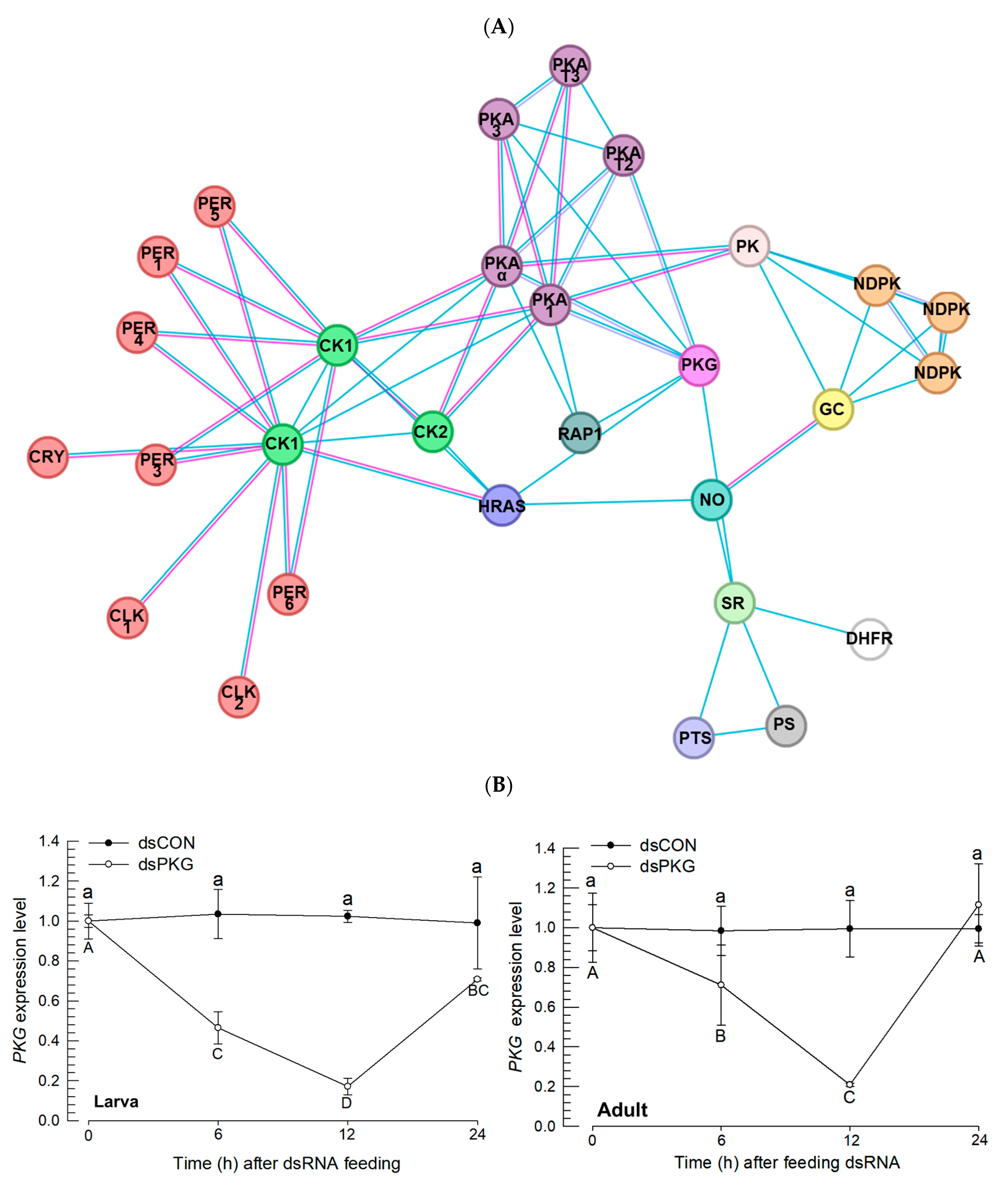
https://string-db.org accessed on 1 October 2024).
2.3. RNA Extraction and cDNA Preparation
Total RNAs were extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. For each RNA extraction, 25 females were macerated using 500 μL of Trizol reagent. Following the RNA extraction, RNA was resuspended in 30 μL of diethylpyrocarbonate (DEPC)-treated water and quantified using a spectrophotometer (Nanodrop, Thermo Fisher Scientific, Wilmington, DE, USA). For cDNA synthesis, 400 ng of RNA was used in each sample with RT oligo dT premix (Intron Biotechnology, Seoul, Republic of Korea) containing oligo dT primer. A reaction mixture consisted of 2 μL of RNA extract and 18 μL of DEPC-treated water and was run according to the manufacturer’s instructions. The resulting cDNA samples were kept at −20 °C before being used for experimentation.
2.4. RT-PCR and RT-qPCR
RT-PCR used the cDNA and amplified
Fo-PKG and two clock genes with a Taq polymerase (GeneALL, Seoul, Republic of Korea). A reaction mixture for PCR consisted of 2.5 μL of dNTP (each 10 pmol), 2.5 μL of 10× Taq buffer, 2 μL of forward and reverse primers (10 pmol/μL,
Table S1), 0.5 μL of Taq polymerase, 1 μL of cDNA, and 16.5 μL of distilled deionized water. The PCR conditions began with an initial denaturation at 95 °C for 5 min, which was followed by 35 amplification cycles consisting of 95 °C for 1 min, 50~55 °C for 30 sec, and 72 °C for 1 min. At the end of the amplification cycle, an additional extension was performed at 72 °C for 5 min. The PCR product was confirmed by 1% agarose gel electrophoresis. The qPCR used a Step One Plus Real-Time PCR System (Applied Biosystem, Waltham, MA, USA) under the guidelines of Bustin et al. [
16]. A sample of qPCR reaction (20 µL) contained 10 µL of Power SYBR Green PCR Master Mix (Toyobo, Osaka, Japan), 3 µL of cDNA template (100 ng), and 1 µL (10 pmol) each of the forward and reverse primers (
Table S1). After an initial heat treatment at 95 °C for 2 min, qPCR was performed with 40 cycles of denaturation at 95 °C for 30 s, annealing at 50−55 °C for 30 s, and extension at 72 °C for 30 s. The transcript levels of elongation factor 1 (EF1) were used as a reference for the normalization of each test sample. Quantitative analysis was conducted using the comparative CT (2
−ΔΔCT) method [
17]. All experiments were independently replicated three times.
2.5. RNA Interference (RNAi) Treatment and Subsequent Behavior Assays
Template DNA was amplified with gene-specific primers (
Table S1) containing T7 promoter sequence (5′-TAATACGACTCACTATAGGGAGA-3′) at the 5′ end. The PCR conditions were as described above. After confirming the PCR product, the resulting PCR product was used to synthesize double-stranded RNA (dsRNA) encoding Fo-PKG using T7 RNA polymerase with NTP mixture at 37 °C for 3 h (MEGA script RNAi kit, Ambion, Austin, TX, USA). dsRNA (2 μg/10 μL) specific to
Fo-PKG (‘dsPKG’) was mixed with a transfection reagent Metafectene PRO (Biontex, Plannegg, Germany) at a 1:1 (
v/
v) ratio and incubated at 25 °C for 30 min to form liposomes, and the resulting mixture was supplied with beans to
F. occidentalis. Beans were coated with the dsRNA mixture of 10 μL per 20 thrips and fed for 12 h. Control dsRNA (‘dsCON’) was prepared according to the method outlined by Vatanparast et al. [
18].
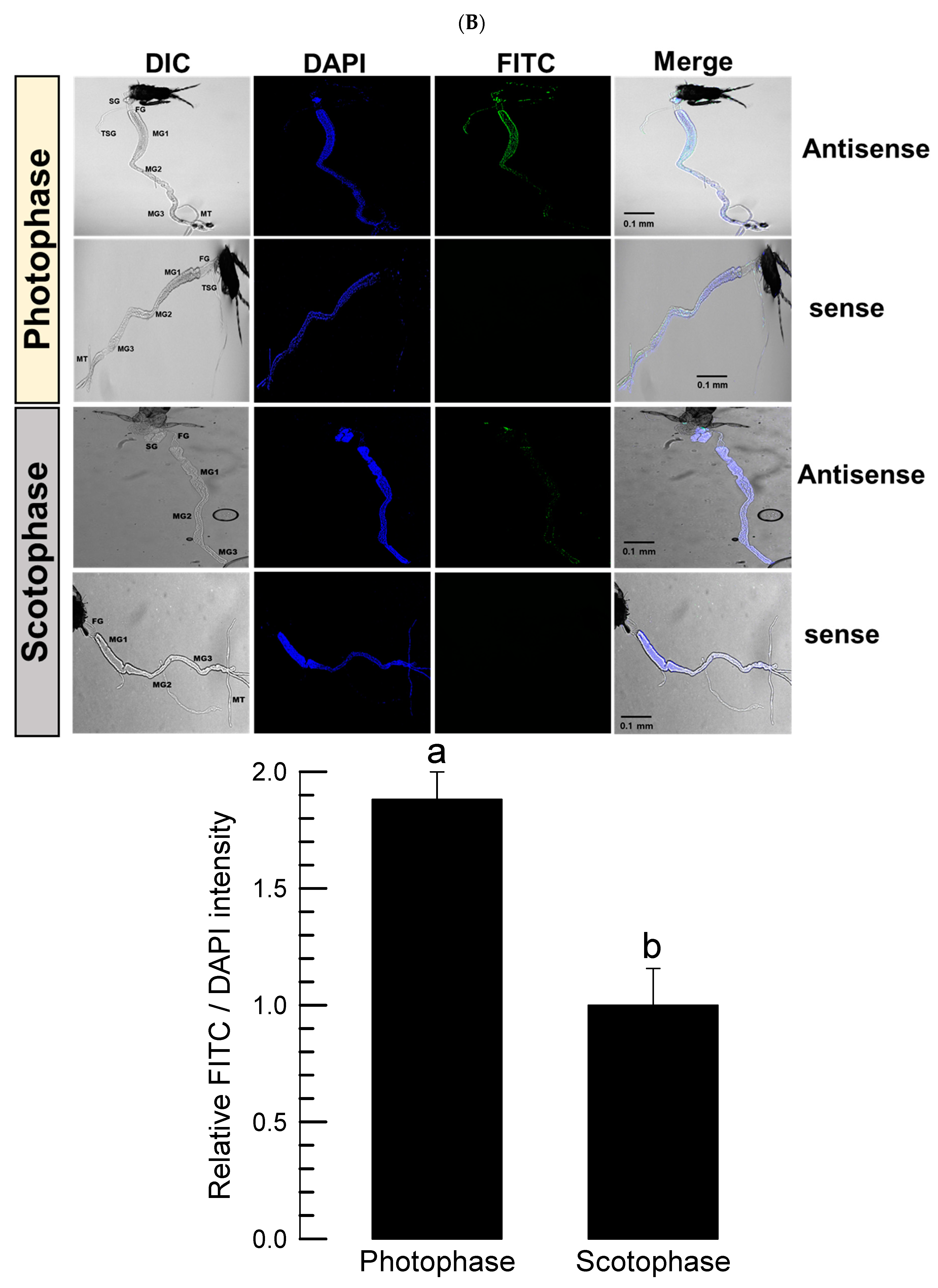
2.6. Fluorescence In Situ Hybridization (FISH) Assay
Adult thrips were tested using FISH assay to detect the expression pattern of Fo-PKG. The guts of thrips were dissected upon a sterilized glass slide and then treated with 4% paraformaldehyde for 1 h at room temperature. The guts were permeabilized with 1% Triton X-100 in PBS for 2 h at room temperature after being cleaned with 1× PBS. After that, the gut was washed with 1× PBS, rinsed with 2× SSC, and incubated for 1 h at 42 °C in a dark, humid environment with 25 μL of pre-hybridization solution (2 μL of yeast tRNA, 2 μL of 20× SSC, 4 μL of dextran sulfate, 2.5 μL of 10% SDS, and 14.5 μL of deionized distilled water). The buffer was then replaced with hybridization buffer (5 μL of deionized formamide and 1 μL of oligonucleotide (10 pmol) tagged with fluorescein in 19 μL of the pre-hybridization buffer). The DNA oligonucleotide probes were tagged with fluorescein amidite (FAM) at the 5′ end and purified using high-performance liquid chromatography at Bioneer (Daejeon, Korea). To identify Fo-PKG mRNA, an antisense probe (5′-FAM-TTT-TCAGTCACATTGTGTACGTT-3′) that is complementary to the target mRNA and a negative dsCON sense probe (5′-FAM-AAA-AACGTACACAATGTGACTGA-3′) were produced at a concentration of 10 pmol. The slides were then sealed with an RNase-free coverslip and left in a humid room at 42 °C for 16~18 h. Following hybridization, the gut was incubated with 4× SSC containing 1% Triton X-100 at room temperature for 5 min, and it was rinsed twice with 4× SSC for 10 min each. The gut samples were washed three times with 4× SSC and then incubated at 37 °C with a 1% solution of anti-rabbit-FITC conjugated antibody (Thermo Fisher Scientific) in PBS for 30 min under darkness. The guts were incubated with 4x SSC for 10 min, and then with 2× SSC for 10 min. The samples were viewed under a fluorescence microscope (DM2500, Leica, Wetzlar, Germany) at 200× magnification after adding a drop of PBS/glycerol (1:1, v/v) and incubating at room temperature for 15 min.
2.7. Behavior Monitoring and Automatic Digitization
Individual adult female thrips at 2–3 days after emergence were observed continuously for 24 h in each trial. The detailed observation system is provided in
Supplementary information. The observation system was installed on an optic fiber microscope (Camscope
®, AST-ICS305B, Sometech Vision, Seoul, Republic of Korea) consisting of observation arena, camera, computer, and light (
Figure S1A). The observation arena (6 mm in diameter and 1.7 mm in depth) was made of dental modelling wax [
19] and was divided into food-provision (2.5 mm radius), intermediate (0.5 mm width) and edge (1 mm width) areas (
Figure S1B). According to measurements of 20 adult females, the average length, width and height were 1.64 ± 0.07 mm, 0.29 ± 0.03 mm and 0.25 ± 0.01 mm, respectively. According to preliminary experiments, the tested females moved around over distances about three times of either the width or height of their bodies along the edge area; 1 mm was therefore determined as the width of the edge area. The intermediate area was defined as the area between the food-provision area and the edge area. The parameters were measured in the whole observation arena, while durations (%) were separately measured for different micro-areas including the food-provision, intermediate and edge areas (See
Figure S1). Within the food-provision area, a particle (2 mm in diameter and 1 mm in height) of fresh bean just after germination was provided as food in the center. Water was provided for the food continuously to keep the food fresh during the observation period (
Figure S1C).
The temperature was 23.6 ± 2.0 °C and humidity was 55.3 ± 10.0% in the observation room. Photophase (14 L) and scotophase (10D) were provided using white and red LEDs, respectively. In order not to cause compound effects between transplant of test females and light phase change, the test females were introduced to the observation arena 2 h before the start of photophase for acclimation. Digital observation was conducted continuously for 24 h just after light-on in the light cycle.
Behavioral tracks were recorded with an image resolution of 1920 × 1080 with 15.88 frames per second (fps). Movement was recognized by a convolutional neural network, YOLOv8 [
20] continuously in photo- and scotophases as examples shown in
Figure S1D. One second was defined as the observation time unit to obtain movement parameters and duration rates in percentage (durations (%)) in this study, although time intervals of less than 1 s (e.g., 0.25 s) have been used in similar research to monitor the movement of insects (e.g.,
Drosophila melanogaster) responding to external stimuli (toxins) [
21,
22,
23]. Measuring the parameters at a time interval of 1 s was sufficient to present the movement and positioning status over the entire 24 h period. The time unit in 1 s also reduced the computational time.
Speed, locomotory rate, and direction change rate (DCR) were recorded for movement parameters, while durations (%) in different micro-areas were obtained to indicate where test animals stayed within the observation arena as time progressed. Whereas speed was obtained over the entire observation period including the time without movement, locomotory rate was defined as the mean speed only when the test individuals moved. DCR was calculated as the angle change (without considering direction) after one unit time (1 s). Duration of staying at the food-provision (edge) area was measured as the total period while the digitized body center was located either within or on the border of food-provision (edge) area. The rest of the period was regarded as duration in the intermediate area. The parameters and durations (%) were measured in each time unit first, and averaged in each hour defined as a light phase (i.e., P1-P14 for photophase, and S1-S10 for scotophase).
2.8. Statistical Analysis
Percent data for genetic analyses were arcsine-transformed, and the subsequent transformed data were confirmed to follow a normal distribution using PROC UNIVARIATE of the SAS program [
24]. Data obtained from the feeding or mating test were subjected to a one-way analysis of variance (ANOVA) using PROC GLM of the SAS program. The means were compared using the least significant difference (LSD) test at a Type I error of 0.05.
For behavioral monitoring, the mean of each trial (four trials for dsCON and three trials for dsPKG) was measured initially. Subsequently, means and standard deviations (SDs) of the trial means were obtained again as representative values of parameters and durations (%), according to the central limit theorem [
25,
26]. Two aspects of statistical difference were considered in this study: difference between dsCON and dsPKG at each light phase, and difference among light phases within each strain. Behavioral data in this study had a property of measurement dependence. Since observations were continuously conducted throughout the whole observation period, movement across light phases was dependently observed (i.e., observed continuously). In this study, measurements in different light phases were considered as independent because this is the initial phase of a behavioral study to confirm physiological effects, and an extensive experimental design would be required for analyzing repeated measurements, possibly with a large number of trials.
For analyzing multiple comparisons among light phases, each pair was selected separately and compared with each other, assuming measurements were independent. Considering high variability in behavioral data, the Mann–Whitney U test (or Wilcoxon–Mann–Whitney test) [
27] was adopted for analyzing nonparametric data (rank) while the Kolmogorov–Smirnov test [
28] was additionally adopted for analyzing parametric data (mean) for two-sample tests (four and three trials for dsCON and dsPKG, respectively, in each light phase). The Kolmogorov–Smirnov test could be effectively applied to the data not following the Gaussian distribution [
28].
The software was obtained from ‘scipy.stats’, a Python package (V1.14.1). By combining two test results, a statistical differentiation score was devised to indicate a possibility of quantitative separation between photo- and scotophases. Scores 1 and 2 were given to probability of alpha error less than 0.10 and 0.05 for each test, respectively. Then, the scores for the two tests were summed to present overall statistical differentiation with a maximum of 4 and minimum of 1. In comparing statistical difference between dsCON and dsPKG in each light phase, the t-Test was applied to parameters and durations (%) between dsCON and dsPKG directly in each light phase (four and three trials for dsCON and dsPKG, respectively, in each light phase) with one-tail analysis under the condition of heteroscedasticity, considering difference in variance between two treatments.
4. Discussion
The continuous monitoring of the thrips behavior showed a diurnal rhythmicity with relatively high activity during photophase and relatively low activity during scotophase. This kind of diel pattern is consistent with circadian regulation mediated through two oscillatory loops: the Per/TIM oscillatory loop and the CRY loop [
29,
30]. In each of these loops, CLK/CYC acts as a transcriptional activator that promotes
Per and
TIM, or
CRY transcription. The product proteins Per and TIM, or CRY are thought to provide negative feedback to inhibit the transcriptional activator. In
D. melanogaster,
CRY is expressed in specific clock neurons in the brain [
31]. CRY is then activated by blue light and catalyzes TIM degradation through its protease activity, at which point the circadian clock is reset [
32,
33]. In
F. occidentalis,
Per and
CLK genes exhibited diel patterns with relatively high expressions during photophase and relatively low expressions during scotophase. This supports the circadian rhythmicity of thrips behavior controlled by clock genes, because any interruption of the clock gene expressions significantly altered the diel rhythmicity [
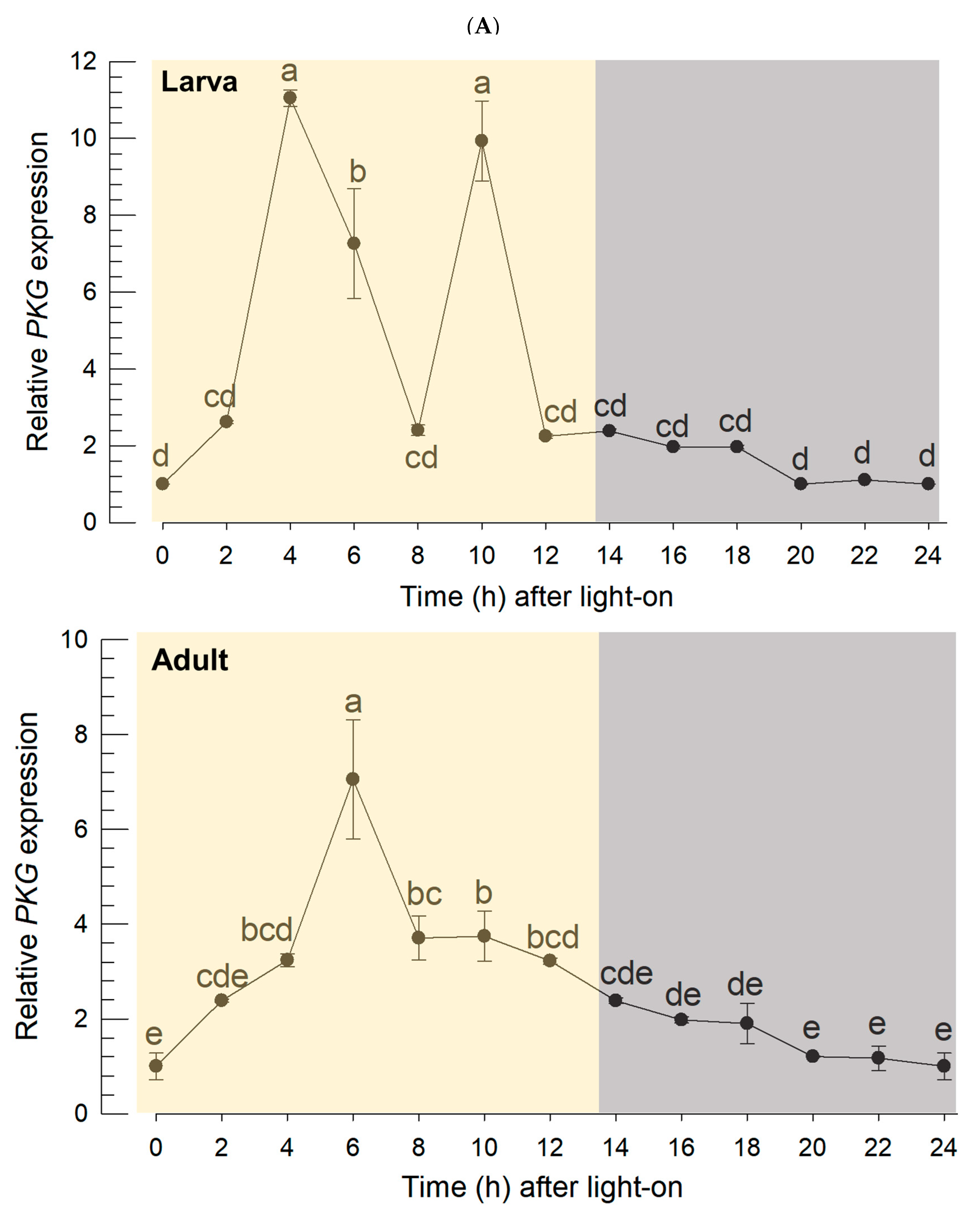
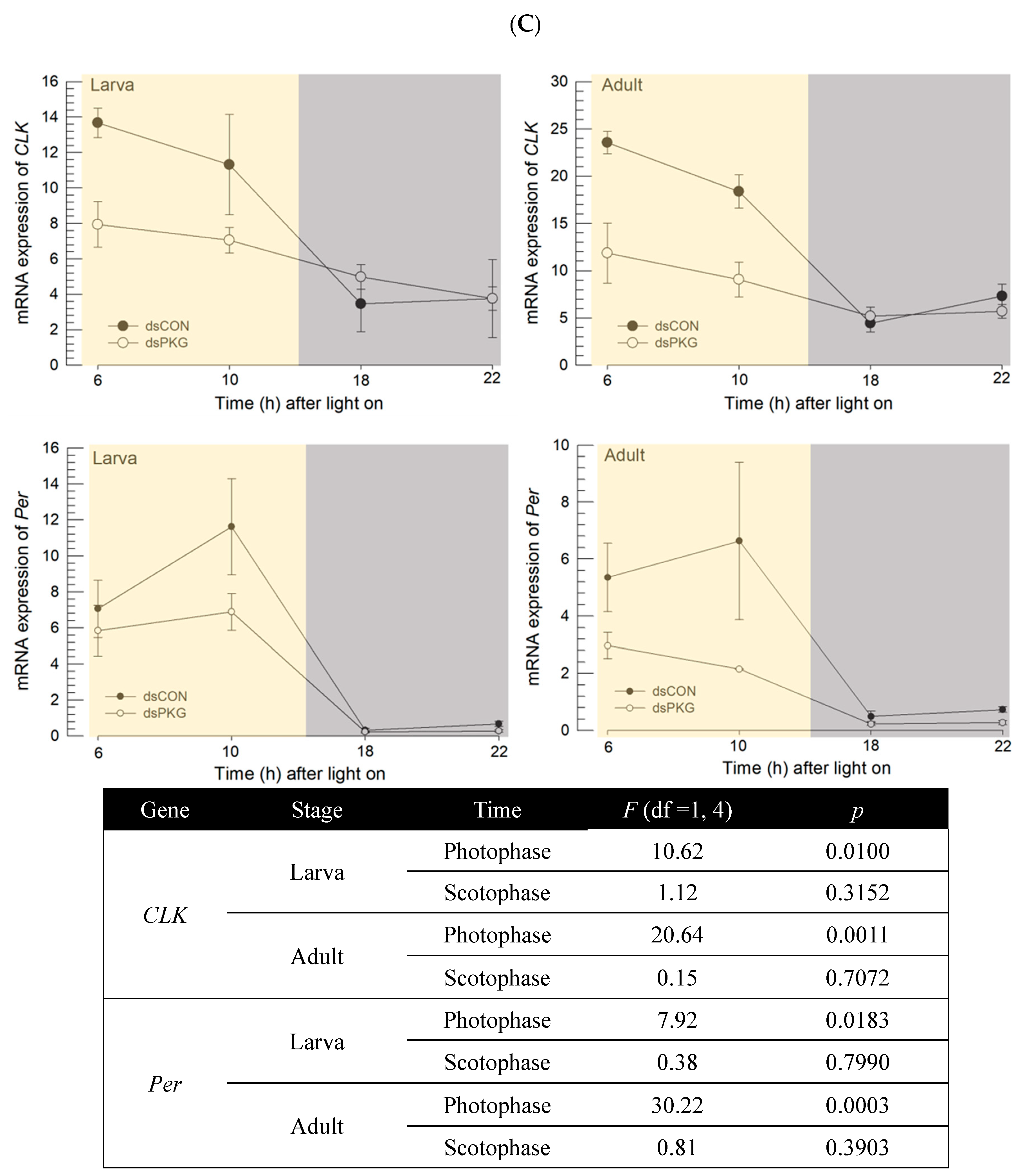
8]. Interestingly, this current study showed that
PKG expression followed this diel pattern, suggesting a functional association of its expression with clock gene expression. The expression pattern was visualized in the intestine by FISH. Even though the circadian clock is mainly controlled by the brain, the peripheral tissues should be adjusted to the rhythm. This study focused on feeding behavior to assess the circadian rhythm. The diel pattern of feeding behavior is controlled by central (brain) and peripheral (fat body and gut) signals because any mismatch between these signals would lead to significant decrease of feeding activity in
Drosophila [
34]. This supports the diel patterning of PKG expression in the intestine of thrips.
RNAi specific to
PKG expression led to alterations in the clock gene expressions during photophase by preventing up-regulation of the clock genes.
PKG expression is associated with division of labor in the honey bee,
Apis mellifera, in which specific expression in the mushroom bodies of the brain, subesophageal ganglion, and corpora allata is associated with foraging behavior to collect pollens and nectars [
9]. The role of
PKG expression in labor division in social insects was also found in a fire ant,
Solenopsis invicta, in which RNAi specific to
PKG reduced the locomotory activity and facilitated the behavioral change from foragers to nurses [
35]. PKG activity and locomotory activity has been well established in
Drosophila, in which flies are discriminated into sitters with low PKG activity or rovers with high PKG activity [
36]. These suggest that
PKG expression is associated with high locomotory activities of
F. occidentalis during photophase. Thus, the RNAi specific to
PKG expression resulted in reduced early-life development and adult fecundity, presumably by inhibiting feeding and ovipositional behavior (
Figure S2). In addition, the suppression of
PKG expression led to the suppression of the clock gene expressions during photophase, supporting the functional association of
PKG expression with circadian rhythmicity. In mammals, the suppression of clock gene expressions led to the suppression of PKG protein level [
37]. This suggests that alterations of the clock gene expressions indirectly influence the reduced
PKG mRNA level caused by RNAi in
F. occidentalis. It is also noteworthy that there are at least four isoforms of PKG in the
F. occidentalis genome: 176 (XP_052119469.1), 417 (XP_052132331.1), 694 (XP_026272945.1), and 1,010 (XP_026272942.1) amino acid residues. In this study, we analyzed only PKG with the 694 amino acid length isoform. Thus, the other isoforms should be analyzed in their independent roles. In addition, our current study showed altered expressions of two circadian genes by manipulating
PKG expression, but did not show control of the
PKG gene expression by circadian gene expression. Thus, it is still unclear whether the PKG gene’s effects on clock genes are causal or incidental with respect to the changes in behavior. This needs to be clarified in subsequent research.
Automatic individual recognition of live individuals and continuous parameter extraction demonstrated an impact on movement behavior of the thrips caused by PKG and associated clock genes. Diel rhythm in adult females with RNAi specific to PKG expression was substantially affected, without showing much variation in the activity parameters and durations of stay at different areas of the observation arena, whereas the dsCON females showed clear diel difference.
The continuous detection of behaviors over the entire period of 24 h effectively characterized diel difference between dsCON and dsPKG. Not only differences in movement parameters but also differences in durations (%) in the micro-areas in the observation arena were observed between dsCON and dsPKG (see
Table 1). It is noted that total speed was high (0.13 mm/s) in dsCON compared with dsPKG (0.07 mm/s), indicating a higher level of energy consumption by dsCON.
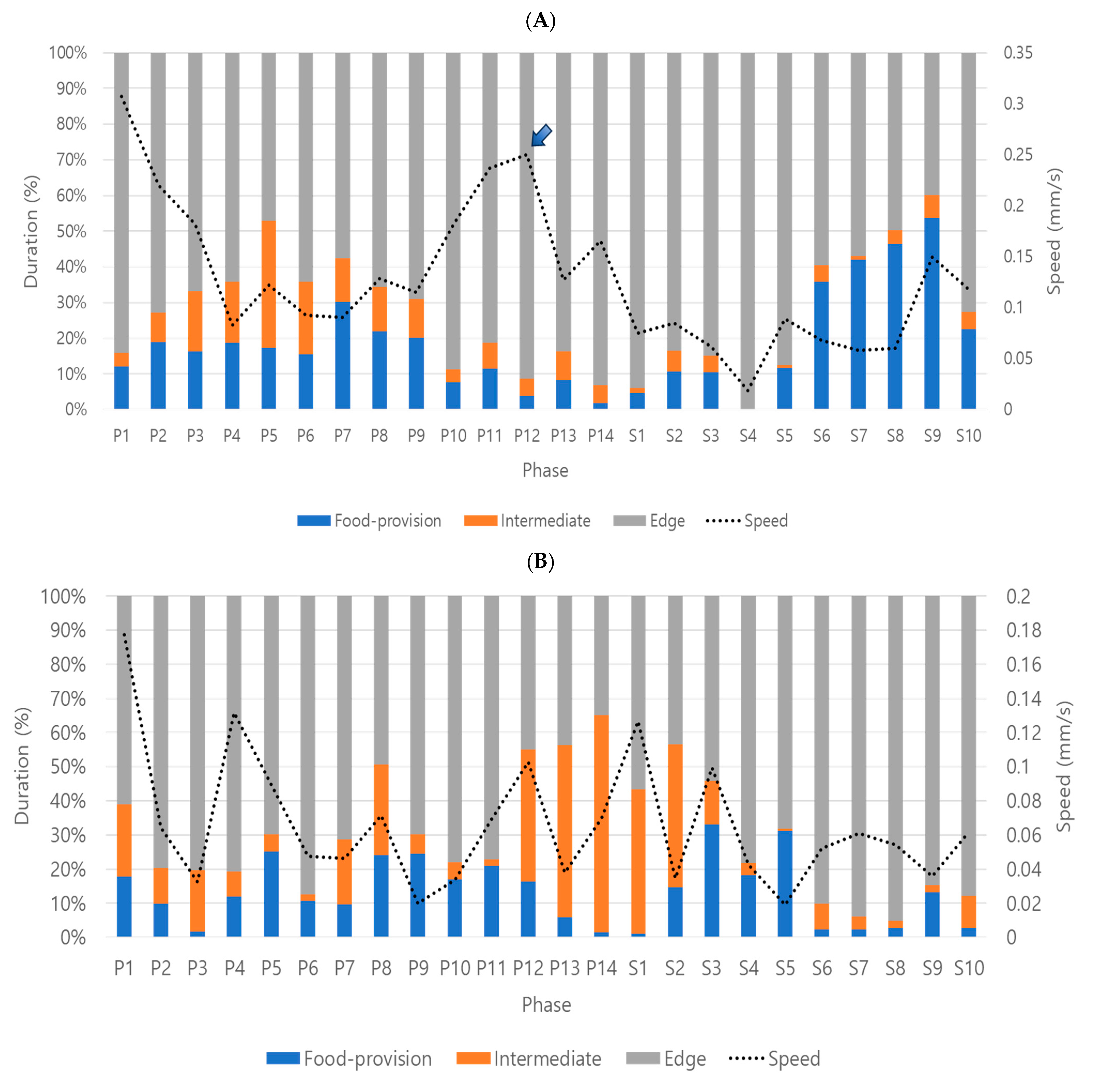
Behavior profiles were produced in three stages by superimposing speed over durations (%) on continuously observed data (see
Figure 4). Overall, behavior profiles were effectively characterized by sequential stages, activity followed by feeding and visiting of other micro-areas in dsCON-females, and corresponding behavior disruptions after dsPKG suppression (see
Figure 4A,B). Currently, however, the causes of behavior profile changes are unknown. More studies are warranted regarding physiological and genetic aspects along with diverse experimental conditions in the future.
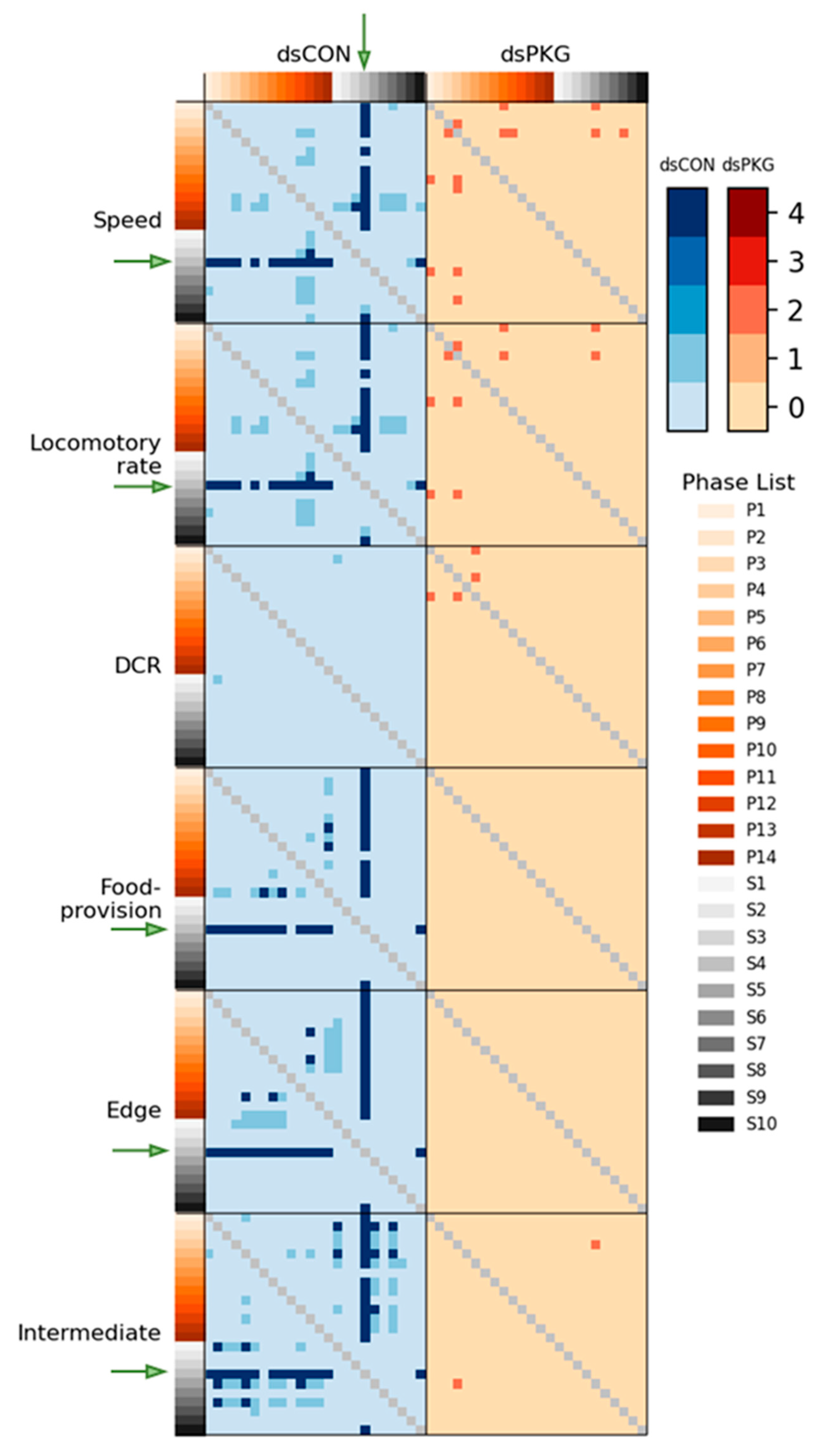
Although the means and SDs were highly variable in both movement parameters and durations (%), statistical significances according to Mann–Whitney U and Kolmogorov–Smirnov tests were found between photo- and scotophases in dsCON in both movement parameters and durations (%) in different micro-areas, whereas the difference was not observed in dsPKG (see
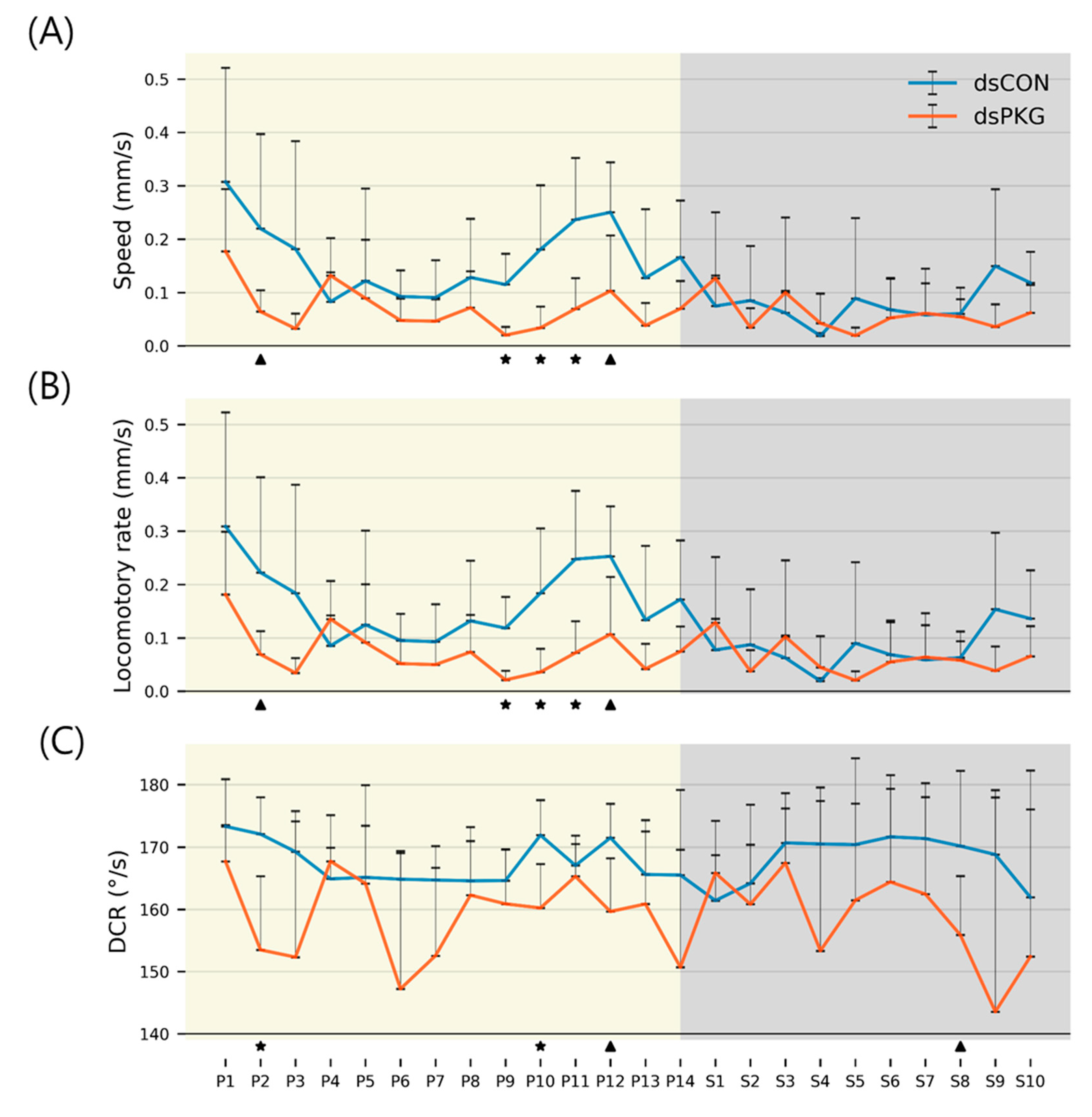
Figure 7). It is noted that a period in scotophase, S4, mainly had statistical differentiations with most periods in photophase in both parameters and duration rates (%) in dsCON, confirming coincidence in movement parameters and durations (%) in behavioral changes. Phase S4, in mid-scotophase, had the minimum speed (0.19 mm/s) and locomotory rate (0.19 mm/s) along with minimal SD ranges (see
Figure 5A,B). Similarly, durations (%) in S4 were either maximal in the edge area or minimal in the food-provision and intermediate areas, while SDs were commonly minimal in this phase (see
Figure 6). The distinctive differences in mean values between light phases, concurrently with minimal range in SDs, contributed to presenting statistical significance originating from S4 in light phases.
Statistical significance of parameters was also examined specifically between dsCON and dsPKG in each light phase according to
t-Test (See
Section 2). The speed and locomotory rate showed significant differences mainly in three light phases, P9–P11, between dsCON and dsPKG (see
Figure 5A,B). These differences were contrasted with the case of statistical differences within each treatment where S4 was different from photophase, as stated above (see
Figure 7). The results suggested two notable areas of behavioral change: the late photophase showed greater differences when comparing dsCON and dsPKG effects in each light phase, while light phase S4 showed greater differences after dsPKG suppression in relation to other light phases. Since high variability existed in the behavioral data, results need to be confirmed with more investigations through integrative approaches linking behavior, physiology and genetics along with more trials.
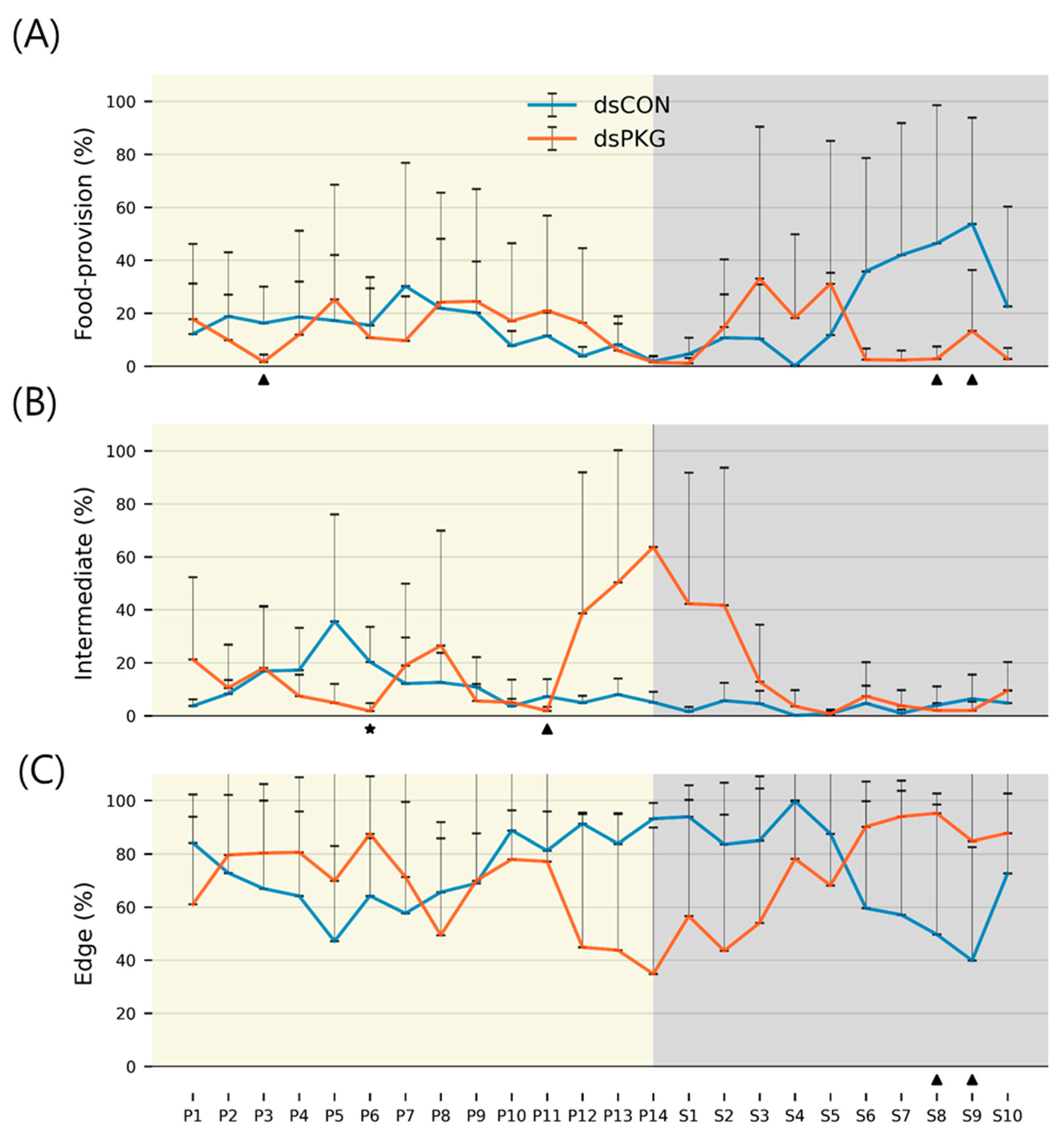
It is noted that behavioral changes were more obvious in the intermediate area. Duration of stay in this area increased to 16.7% for dsPKG whilst remaining low, at 8.3%, for dsCON (see
Table 1). In addition, the tested females spent longer durations in the intermediate area in photophase (11.9%) compared with scotophase (3.3%) in dsCON (see
Table 2). It is also noteworthy that the tested females stayed a long time in the edge area (70.0~73.3%) compared with other micro-areas in both treatments (e.g.,
Table 1). This may indicate that the tendency to stay near boundary would persist after PKG modulation.
Durations (%) in the intermediate area were extremely high during P12~S2 compared with other light phases in dsPKG (see
Figure 6B). However, no distinctive statistical differentiation was observed among light phases in duration (%) in the intermediate area in dsPKG (
Figure 7). This would be due to the extremely high levels of SDs of durations (%) observed in this period, P12~S2 (
Figure 6B). Further physiological and genetic investigations are required along with more trials to confirm if diel differences would exist in durations (%) in the intermediate area in dsPKG.
During P1, very high speed was observed in both treatments (see
Figure 5A). This would suggest high activity after feeding in the previous stage in photophase (see
Figure 4A), as discussed above. But the speed was also high in P1 for dsPKG, although feeding did not occur in the previous stage for dsPKG (see
Figure 4B). This may be because the starting location for the test individuals was the edge area to secure minimum disturbance to test females in transferring them from the stock to the observation arena. Although the test females were acclimated for 2 h before observation (see
Section 2.7.
Behavior-monitoring and automatic digitization), they may have stayed longer and have been alerted to the new environment in the edge area in the initial phase of observation. More investigations are thus required in the future regarding examinations of mechanisms causing high speed in P1 in dsPKG or effect of acclimation on initial behavior in the observation arena.
In this study, measurements in different light phases were considered as independent for statistical analyses (see
Section 2.8. Statistical analysis). When one-way repeated ANOVA was applied to dependent measurements of parameters and durations (%), statistical differences were not observed between light phases. This would be partly due to the low number of trials and partly due to the heterogenous conditions of the tested females. In the future, a more extensive experimental design will be needed including an increase in trial numbers and increase in physiological homogeneity of test females (e.g., precise range in age) as well. In this study, parameters in different micro-areas were not separately measured as time progressed. Some empirical results were presented including the speed change in the edge area (
Figure 4). Future research will be required to quantitatively investigate the coupled dependence, micro-areas in space and light phases in time, throughout the observation period, possibly along with an increase in the number of trials.
With continuous and concurrent observation of movement activity and durations of stay in areas of the observation arena, the computational analysis of response behaviors supported physiological evidence of dsPKG suppression effects, demonstrating changes in behavioral status according to instantaneous movement parameters and durations (%) in different micro-areas, and revealing consecutive behavioral changes as well. Further study relating molecular and physiological mechanisms to behavior is warranted in the future to illustrate genetic functioning in an integrated manner.