Significant Differences in the Effects of Pine Wilt Disease Invasion on Plant Diversity in Natural and Planted Forests
Simple Summary
Abstract
1. Introduction
- How does plant diversity change at the three scales of national, natural, and planted forests in China following PWD invasion, and are there significant spatial variations? Studies have shown that PWD invasion can cause serious disruption of local pine resources in the short term, leading to a loss in plant diversity [13,16,21]. However, with the subsequent adoption of a series of conservation measures, such as logging of affected wood, the succession from pure forest to broad-leaved forest has been accelerated, the community structure has been improved, and the plant diversity may recover [28]. Therefore, we hypothesized that plant diversity at the three scales of national, natural, and planted forests in China would show a trend of continuous loss in the early stage and gradual recovery in the later stage. Because of the differences in the intensity of protection measures in response to PWD invasion in different areas of China [29], the study guessed that there might be significant spatial differences in the changes in plant diversity. The findings of this study will quantify the multi-scale effects of PWD invasion on plant diversity, reveal the multi-scale dynamic patterns of plant diversity after PWD invasion, and provide a scientific and theoretical basis for local governments and managers to develop effective pest management practices and plant diversity restoration measures.
- Is there a significant difference in the loss rate of plant diversity between natural and planted forests after PWD invasion? If so, which rate of loss is faster? High species diversity in host communities may promote pest invasions by providing more ecological niches (facilitation effect), but it may also diminish invasion success because of low host dominance (dilution effect) [30]. Natural forests usually have higher plant diversity than planted forests, so we guess that PWD is less likely to occur in natural forests than in planted forests, and that the difference in invasion likelihood leads to a significant difference in the loss rate of plant diversity between the two following PWD invasions, and that the loss rate is slower in natural forests than in planted forests. If natural forests show greater resilience to invasions, priority should be given to protecting high-diversity natural forests as buffer zones against biological invasions to provide a basis for differentiated prevention and control.
- Is there a significant difference in the rate of recovery of plant diversity between natural and planted forests in the late stage of diversity recovery after PWD invasion? If it exists, which of the two recovers faster? Natural regeneration of forests is considered to be the most effective method to restore biodiversity [31,32], and natural forests, as naturally regenerated forests, have more obvious natural regeneration compared to planted forests, so we conjecture that there is a significant difference in the rate of restoration of plant diversity in natural forests and planted forests after the PWD invasion, and the natural forests recovered faster than planted forests. The results of this study can directly guide the choice of management strategies, and if the recovery rate of natural forests is significantly superior, then the near-natural recovery mode should be preferred to reduce unnecessary afforestation inputs.
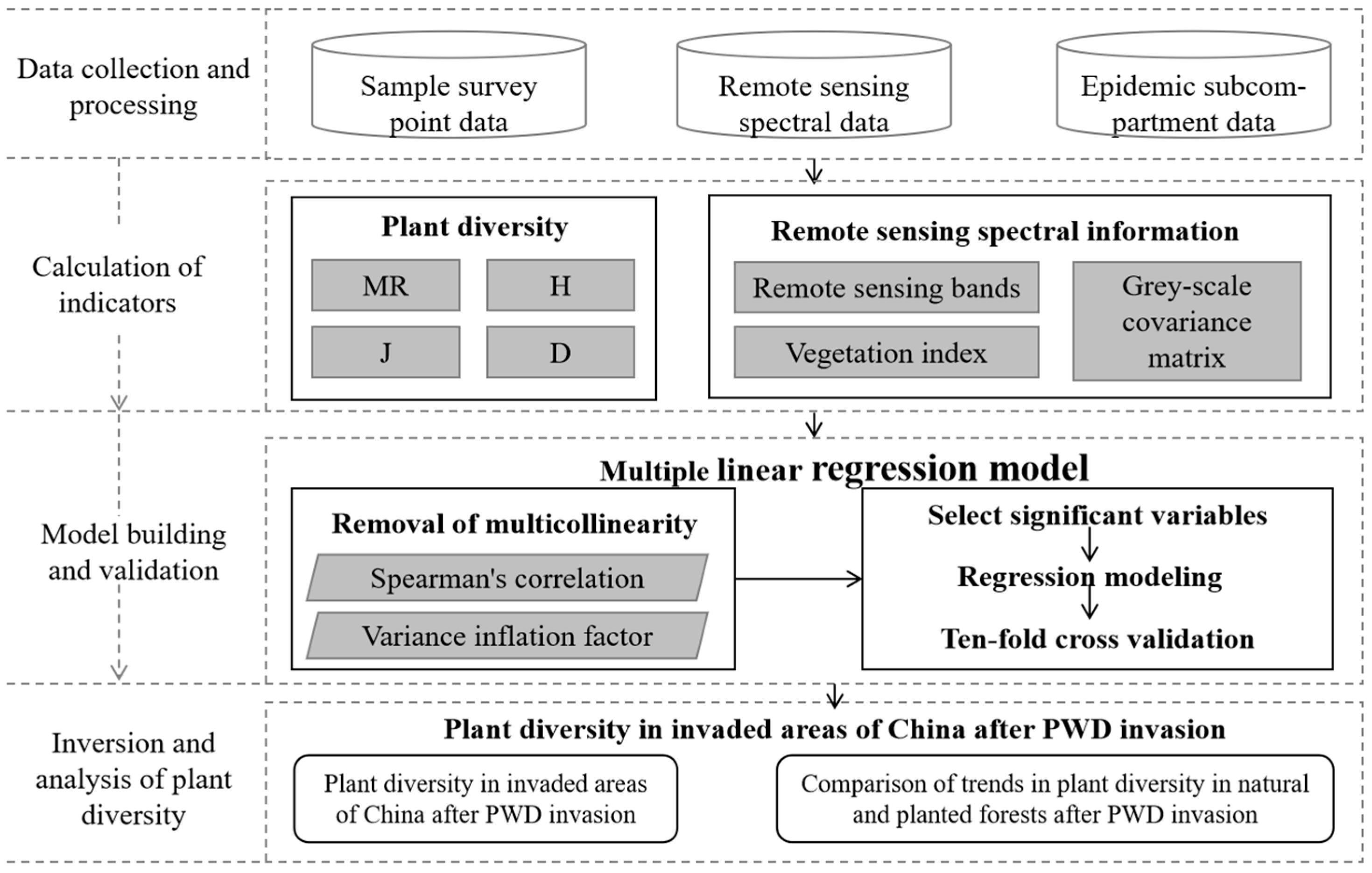
2. Materials and Methods
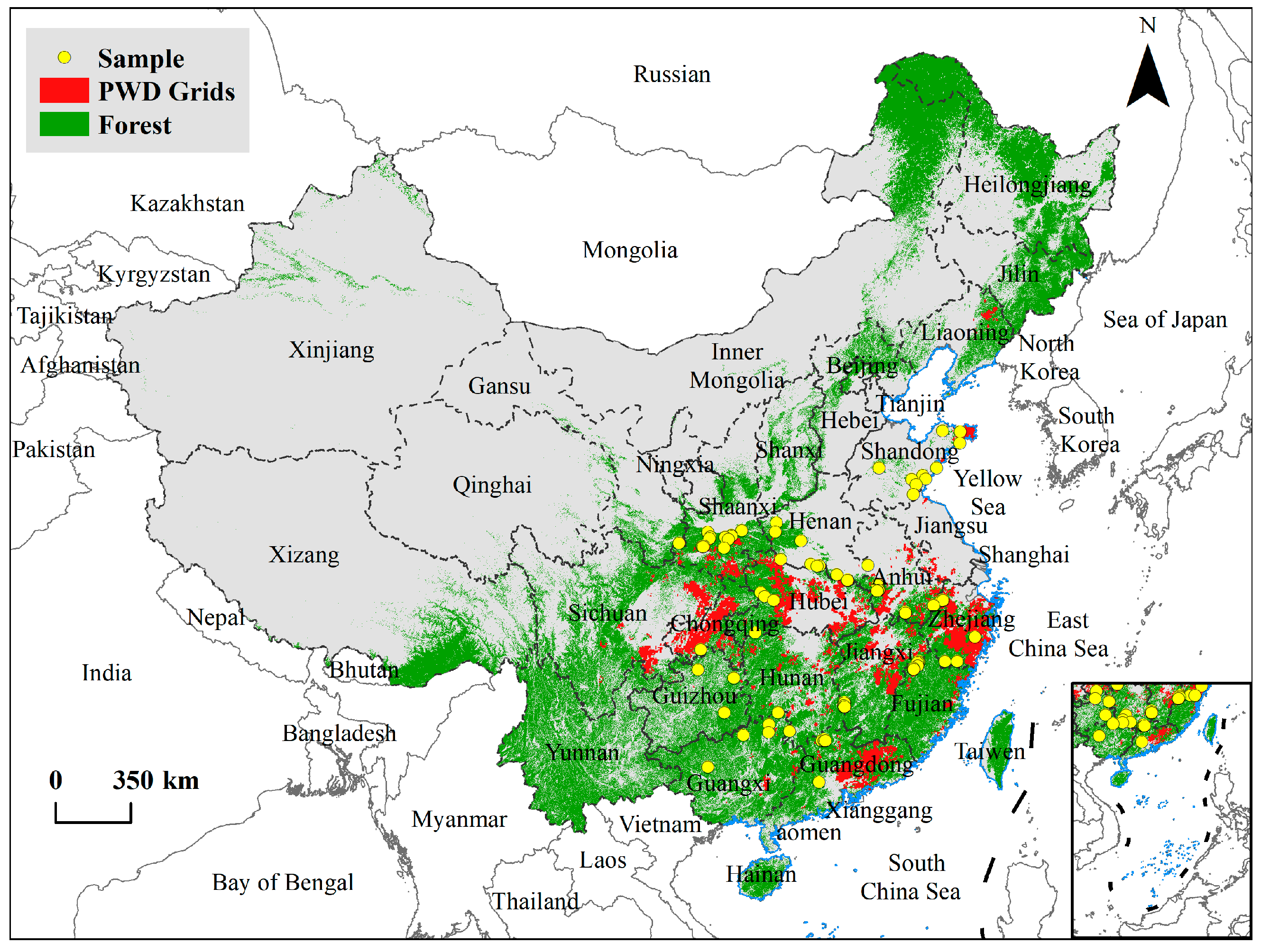
2.1. PWD Occurring Data
2.2. Plant Diversity Data
2.3. Remote Sensed Data
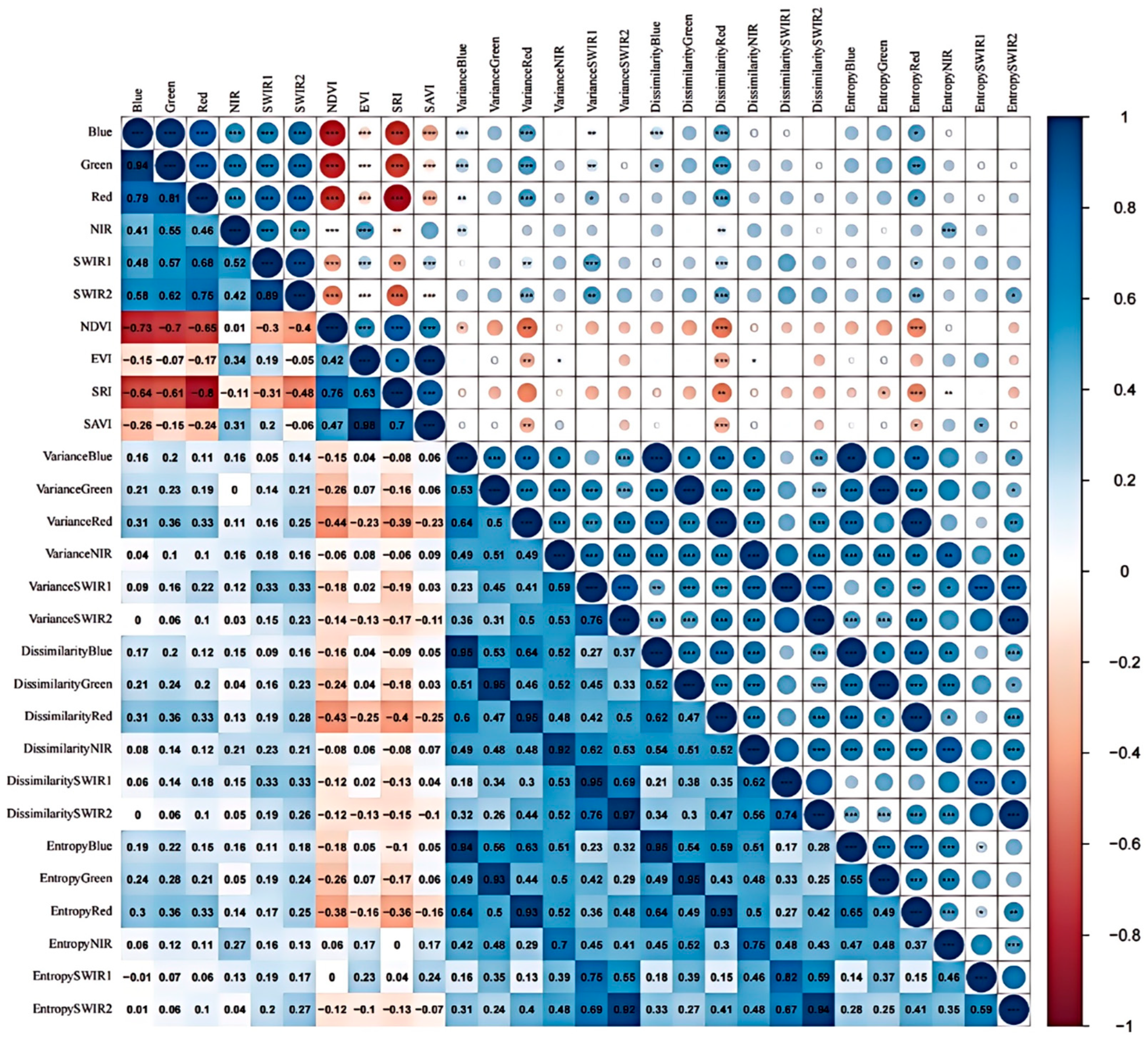
2.4. Relationship Between Remote Sensing Metrics and Plant Diversity
2.5. Impact of PWD Invasion on the Plant Diversity
3. Results
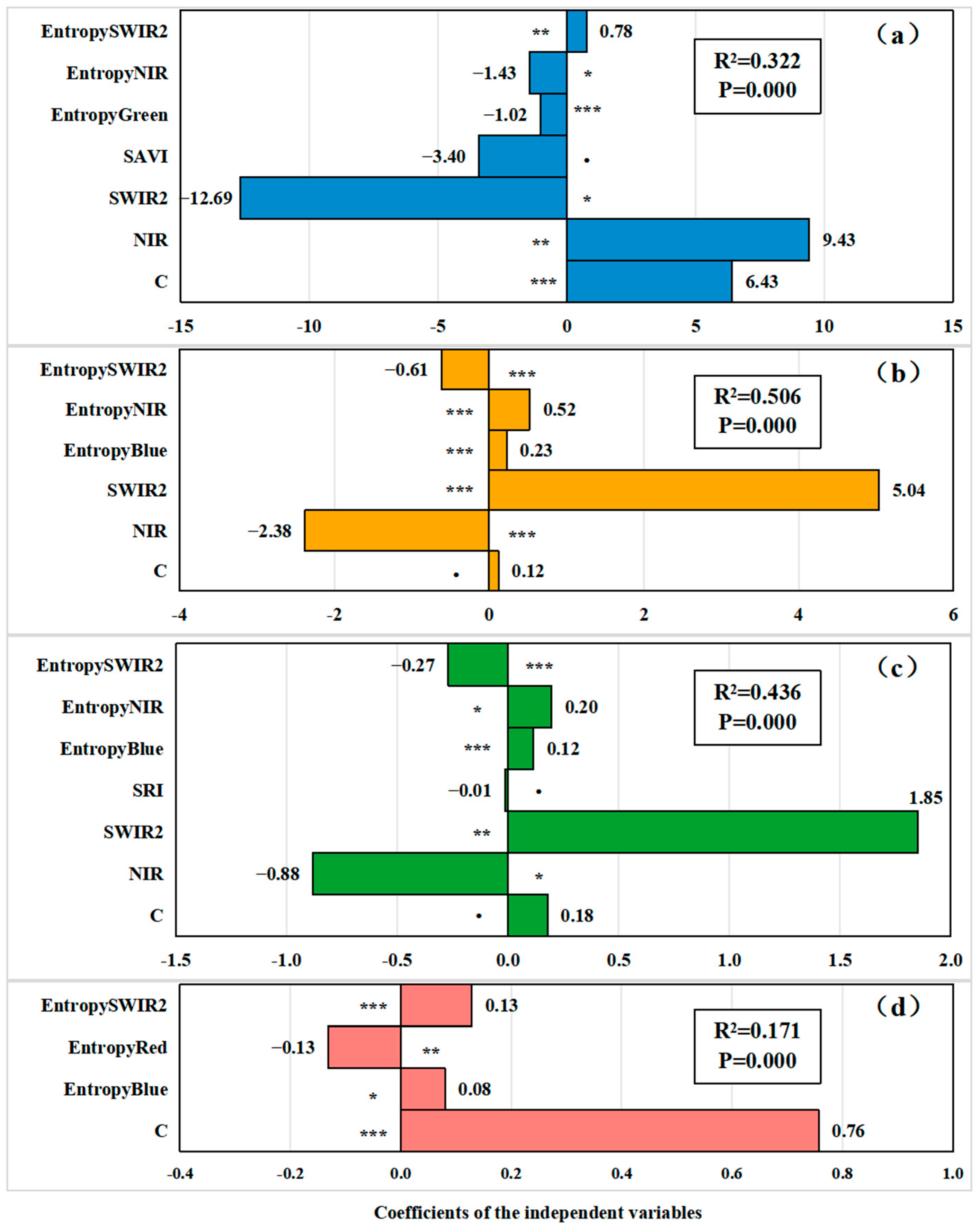
3.1. Relationship Between Remote Sensing Spectral Features and Plant Species Diversity
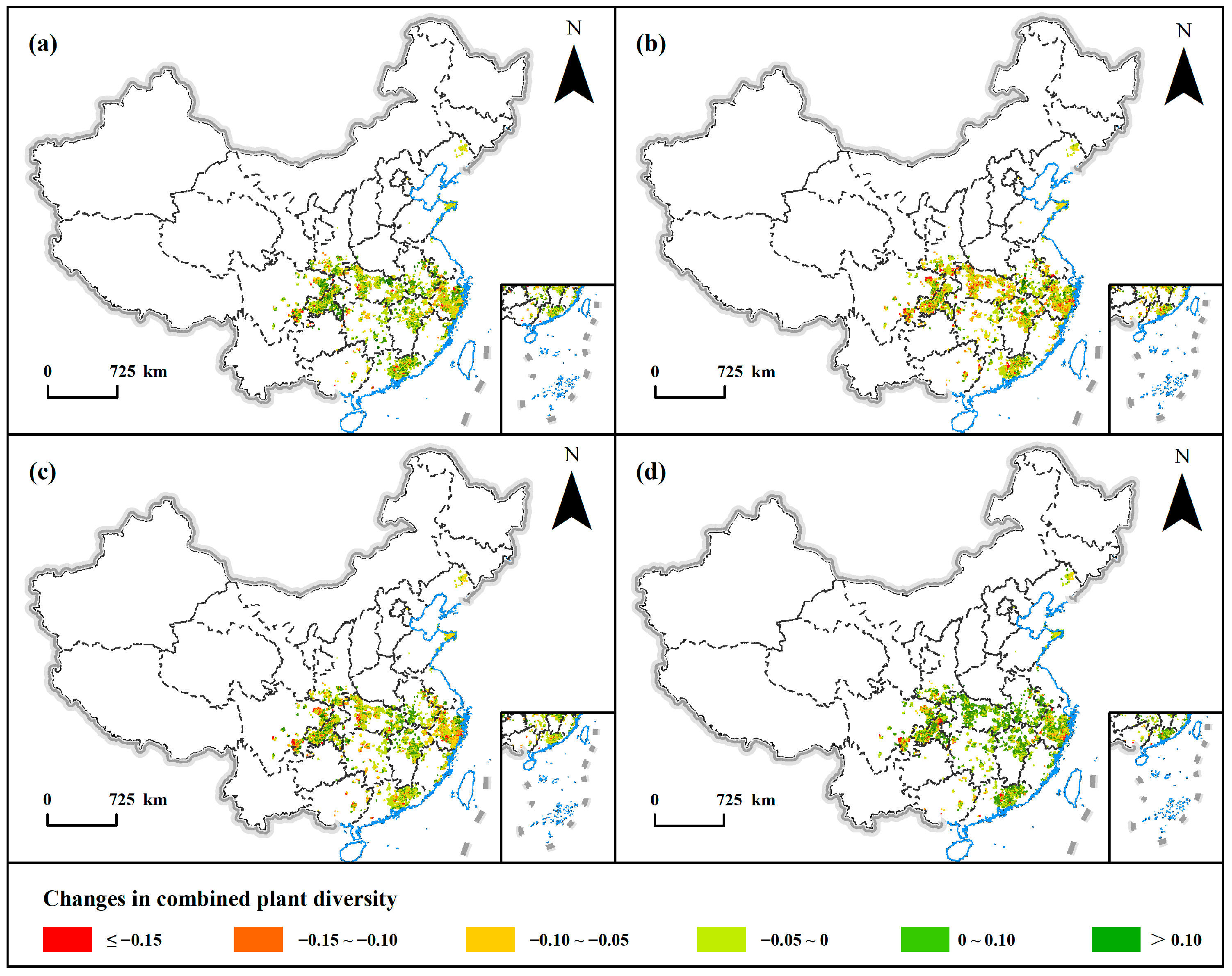
3.2. Changes of Plant Diversity with PWD Invasion
3.3. Different Effects of PWD Invasion on Plant Diversity Between Natural and Planted Forests
4. Discussion
4.1. Continued Short-Term Loss and Gradual Recovery of Plant Diversity in China After PWD Invasion
4.2. Planted Forests Lose Plant Diversity Faster than Natural Forests After PWD Invasion
4.3. Plant Diversity in Natural Forests Recovered More Rapidly than in Planted Forests After PWD Invasion
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PWD | pine wilt disease |
| PWN | pine wilt nematode |
| MR | Margalef’s richness index |
| H | Shannon’s diversity index |
| D | Simpson’s dominance index |
| J | Pielou’s evenness index |
| GLCM | Gray-level covariance matrix |
| VIF | Variance Inflation Factor |
| R2 | the coefficient of determination |
| RMSE | root mean square error |
| MAE | mean absolute error |
| ComDiv | combined plant diversity index |
Appendix A

| Variables | Equation/Spectral Bands | |
|---|---|---|
| Vegetation indices | Normalized Difference Vegetation Index (NDVI) | =(NIR − RED)/(NIR + RED) |
| Enhanced Vegetation Index (EVI) | =2.5 × (NIR − RED)/(NIR + 6.0 × RED − 7.5 × BLUE + 1.0) | |
| Simple Ratio Index (SRI) | =NIR/RED | |
| Soil Adjusted Vegetation index (SAVI) | =(NIR − RED)/(NIR + RED + L) × (1 + L), L = 0.5 | |
| Landsat spectral bands | Blue band | 0.45–0.52 µm |
| Green band | 0.52–0.60 µm | |
| Red band | 0.63–0.69 µm | |
| Near-infrared band | 0.76–0.90 µm | |
| Shortwave infrared band-1 | 1.55–1.75 µm | |
| Shortwave infrared band-2 | 2.08–2.35 µm | |
| Gray-level co-occurrence matrix textural layers | Variance | |
| Dissimilarity | ||
| Entropy | ||
| No. | Independent Variable | No. | Independent Variable |
|---|---|---|---|
| 1 | Blue | 15 | VarianceSWIR1 |
| 2 | Green | 16 | VarianceSWIR2 |
| 3 | Red | 17 | DissimilarityBlue |
| 4 | NIR | 18 | DissimilarityGreen |
| 5 | SWIR1 | 19 | DissimilarityRed |
| 6 | SWIR2 | 20 | DissimilarityNIR |
| 7 | NDVI | 21 | DissimilaritySWIR1 |
| 8 | EVI | 22 | DissimilaritySWIR2 |
| 9 | SRI | 23 | EntropyBlue |
| 10 | SAVI | 24 | EntropyGreen |
| 11 | VarianceBlue | 25 | EntropyRed |
| 12 | VarianceGreen | 26 | EntropyNIR |
| 13 | VarianceRed | 27 | EntropySWIR1 |
| 14 | VarianceNIR | 28 | EntropySWIR2 |
| RMSE | R2 | MAE | |
|---|---|---|---|
| MR | 2.081 | 0.300 | 1.644 |
| H | 0.496 | 0.493 | 0.374 |
| J | 0.265 | 0.441 | 0.207 |
| D | 0.238 | 0.239 | 0.172 |
| Diversity Change | Duration of Invasion | |||
|---|---|---|---|---|
| One Year | Two Years | Three Years | Four Years | |
| ≤0.15 | 214 (2.17%) | 552 (5.6%) | 382 (3.88%) | 258 (2.62%) |
| −0.15~−0.10 | 643 (6.52%) | 1214 (12.32%) | 962 (9.76%) | 500 (5.07%) |
| −0.10~−0.05 | 2066 (20.96%) | 2967 (30.1%) | 2473 (25.09%) | 1343 (13.63%) |
| −0.05~0 | 3950 (40.08%) | 3487 (35.38%) | 3690 (37.44%) | 3102 (31.47%) |
| 0~0.1 | 2723 (27.63%) | 1464 (14.85%) | 2181 (22.13%) | 4161 (42.22%) |
| >0.1 | 260 (2.64%) | 172 (1.75%) | 168 (1.7%) | 492 (4.99%) |
References
- Cardinale, B.; Duffy, J.; Gonzalez, A.; Hooper, D.; Perrings, C.; Venail, P.; Narwani, A.; Tilman, D.; Wardle, D.; Kinzig, A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.; Fetzer, I.; Bennett, E.; Biggs, R.; Carpenter, S.; Vries, W.; de Wit, C.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Niu, B.; Fu, G. Response of plant diversity and soil microbial diversity to warming and increased precipitation in alpine grasslands on the Qinghai-Xizang Plateau—A review. Sci. Total Environ. 2024, 912, 168878. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Q.; Wang, S.; Yang, G.; Xue, S. A global meta-analysis of the impacts of exotic plant species invasion on plant diversity and soil properties. Sci. Total Environ. 2022, 810, 152286. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef]
- Čerevková, A.; Mota, M.; Vieira, P. Bursaphelenchus xylophilus (Steiner & Buhrer, 1934) Nickle 1970—Pinewood nematode: A threat to European forests. For. J. 2014, 60, 125–129. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguín, O. Detection of Bursaphelenchus xylophilus, Causal Agent of Pine Wilt Disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.X.; Li, X.D.; Fang, G.F.; Liu, D.Q. The interaction of environmental factors increases the risk of spatiotemporal transmission of pine wilt disease. Ecol. Indic. 2021, 133, 108394. [Google Scholar] [CrossRef]
- Zhao, H.; Xian, X.; Yang, N.; Guo, J.; Zhao, L.; Shi, J.; Liu, W. Risk assessment framework for pine wilt disease: Estimating the introduction pathways and multispecies interactions among the pine wood nematode, its insect vectors, and hosts in China. Sci. Total Environ. 2023, 905, 167075. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.Q.; Huang, J.X.; Li, X.D.; Sun, H.; Fang, G.F. A multi-point aggregation trend of the outbreak of pine wilt disease in China over the past 20 years. For. Ecol. Manag. 2022, 505, 119890. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhou, Y.T.; Sun, H.; Wang, Y.; Xu, Z.T.; Li, X.D. Occurrence of major forest pests in China in 2022 and trend prediction in 2023. For. Pest Dis. 2023, 42, 51–54. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Yao, H. Current status of pine wilt disease infected areas in China and management countermeasures. For. Pest Dis. 2019, 38, 45–46. (In Chinese) [Google Scholar] [CrossRef]
- Proença, D.N.; Francisco, R.; Kublik, S.; Schöler, A.; Vestergaard, G.; Schloter, M.; Morais, P.V. The Microbiome of Endophytic, Wood Colonizing Bacteria from Pine Trees as Affected by Pine Wilt Disease. Sci. Rep. 2017, 7, 4205. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.J.; Li, Y.L.; Qu, Z.L.; Zhang, Y.M.; Liu, B.; Liu, K.; Gao, Z.W.; Dong, L.N.; Sun, H. Fungal community structure shifts in litter degradation along forest succession induced by pine wilt disease. Microbiol. Res. 2024, 280, 127588. [Google Scholar] [CrossRef]
- Futai, K. Pine Wood Nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Zhao, L.L.; Mota, M.; Vieira, P.; Butcher, R.A.; Sun, J.H. Interspecific communication between pinewood nematode, its insect vector, and associated microbes. Trends Parasitol. 2014, 30, 299–308. [Google Scholar] [CrossRef]
- Zhao, L.L.; Zhang, X.X.; Wei, Y.A.; Zhou, J.; Zhang, W.; Qin, P.J.; Chinta, S.; Kong, X.B.; Liu, Y.P.; Yu, H.Y.; et al. Ascarosides coordinate the dispersal of a plant-parasitic nematode with the metamorphosis of its vector beetle. Nat. Commun. 2016, 7, 12341. [Google Scholar] [CrossRef]
- Seidl, R.; Klonner, G.; Rammer, W.; Essl, F.; Moreno, A.; Neumann, M.; Dullinger, S. Invasive alien pests threaten the carbon stored in Europe’s forests. Nat. Commun. 2018, 9, 1626. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.N. A review of regional biodiversity studies under the effect of pine wilt disease. Bot. Res. 2022, 11, 103–107. (In Chinese) [Google Scholar] [CrossRef]
- Wang, G.M.; Zhao, Y.; Chen, B.; Lu, Z.; Qiu, H.S.; Shi, J. Natural Succession and Characteristics of Pine Community after the Invasion of Bursaphelenchus xylophilus in Zhoushan lslands of Zhejiang Province. Sci. Silvae Sin. 2011, 47, 124–132. (In Chinese) [Google Scholar]
- Zhang, Y.; Luo, Y.Q.; Shi, J.; Tu, Q.; Ren, L.L.; Wang, Z. Impact of invasion of Bursaphelenchus xylophilus on the diversity of pine vegetation community in Fuyang, Zhejiang Province. Ecol. Sci. 2009, 28, 487–494. (In Chinese) [Google Scholar]
- Zhang, H.F.; Chen, S.Y.; Liu, G.; Wang, Y.X. Effects of sanitation cutting pine wilt diseased trees on the stand structure of pure Pinus massoniana plantation. J. Zhejiang A F Univ. 2020, 37, 745–751. (In Chinese) [Google Scholar]
- Hua, F.; Bruijnzeel, L.; Meli, P.; Martin, P.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Peña-Arancibia, J.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liu, J.L.; Xu, X.H.; Jin, Y.; Lu, G. Community phylogenetic diversityand structure of pinewood nematode infected Masson pine forests. For. Pest Dis. 2015, 34, 37–41. (In Chinese) [Google Scholar]
- Shi, J.; Luo, Y.Q.; Wu, H.W.; Yan, X.S.; Chen, W.P.; Jiang, P. Effects of pine wood nematode invasion on plant community functioning in Sargasso pine forests. J. Beijing For. Univ. 2007, 29, 114–120. (In Chinese) [Google Scholar] [CrossRef]
- Dong, Y.Q.; Yan, H.; Pan, J.L.; Zhang, Y.Z.; Guan, Z.H.; Yan, J. Prevention and control measures of pine wilt disease in China. For. Pest Dis. 2022, 41, 1–8. (In Chinese) [Google Scholar] [CrossRef]
- Guo, Q.F.; Fei, S.L.; Potter, K.M.; Liebhold, A.M.; Wen, J. Tree diversity regulates forest pest invasion. Proc. Natl. Acad. Sci. USA 2019, 116, 7382–7386. [Google Scholar] [CrossRef]
- Chazdon, R. Beyond Deforestation: Restoring Forests and Ecosystem Services on Degraded Lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Wheeler, C.; Mitchard, E.T.A.; Koch, A. Regenerate natural forests to store carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef]
- Yang, J.; Huang, X. The 30 m annual land cover dataset and its dynamics in China from 1990 to 2019. Earth Syst. Sci. Data 2021, 13, 3907–3925. [Google Scholar] [CrossRef]
- Zhu, R.; Xiu, D.; Xue, R.; Teng, S. How did the diversity of woody plants in urban forests vary with the urban-rural gradient in Qingdao, China? Heliyon 2024, 10, e36901. [Google Scholar] [CrossRef]
- Shannon, C.E. The mathematical theory of communication. Bell Labs Tech. J. 1950, 3, 31–32. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Madonsela, S.; Cho, M.A.; Ramoelo, A.; Mutanga, O. Remote sensing of species diversity using Landsat 8 spectral variables. ISPRS J. Photogramm. 2017, 133, 116–127. [Google Scholar] [CrossRef]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Yamanaka, T.; Roques, A.; Augustin, S.; Chown, S.L.; Brockerhoff, E.G.; Pysek, P. Plant diversity drives global patterns of insect invasions. Sci. Rep. 2018, 8, 12095. [Google Scholar] [CrossRef] [PubMed]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The Consequence of Tree Pests and Diseases for Ecosystem Services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; McBride, R.C.; Shurin, J.B.; Bever, J.D.; Crews, T.E.; Tilman, G.D. Crop diversification can contribute to disease risk control in sustainable biofuels production. Front. Ecol. Environ. 2015, 13, 561–567. [Google Scholar] [CrossRef]
- Chytrý, M.; Jarosik, V.; Pyšek, P.; Hájek, O.; Knollova, I.; Tichý, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef]
- Clark, G.F.; Johnston, E.L. Temporal change in the diversity-invasibility relationship in the presence of a disturbance regime. Ecol. Lett. 2011, 14, 52–57. [Google Scholar] [CrossRef]
- Delavaux, C.S.; Crowther, T.W.; Zohner, C.M.; Robmann, N.M.; Lauber, T.; van den Hoogen, J.; Kuebbing, S.; Liang, J.J.; de-Miguel, S.; Nabuurs, G.J.; et al. Native diversity buffers against severity of non-native tree invasions. Nature 2023, 621, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Liebhold, A.M.; Brockerhoff, E.G.; Nuñez, M.A. Biological invasions in forest ecosystems: A global problem requiring international and multidisciplinary integration. Biol. Invasions 2017, 19, 3073–3077. [Google Scholar] [CrossRef]
- Rottstock, T.; Joshi, J.; Kummer, V.; Fischer, M. Higher plant diversity promotes higher diversity of fungal pathogens, while it decreases pathogen infection per plant. Ecology 2014, 95, 1907–1917. [Google Scholar] [CrossRef]
- Watts, A.G.; Saura, S.; Jardine, C.; Leighton, P.; Werden, L.; Fortin, M.J. Host functional connectivity and the spread potential of Lyme disease. Landsc. Ecol. 2018, 33, 1925–1938. [Google Scholar] [CrossRef]
- Guo, Q.F.; Ren, H. Productivity as related to diversity and age in planted versus natural forests. Glob. Ecol. Biogeogr. 2014, 23, 1461–1471. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Jedlicka, J.A.; Bothwell, S.G.; Moreno, C.R. Effects of Natural Enemy Biodiversity on the Suppression of Arthropod Herbivores in Terrestrial Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 573–592. [Google Scholar] [CrossRef]
- Fridley, J.D.; Stachowicz, J.J.; Naeem, S.; Sax, D.F.; Seabloom, E.W.; Smith, M.D.; Stohlgren, T.J.; Tilman, D.; Von Holle, B. The invasion paradox: Reconciling pattern and process in species invasions. Ecology 2007, 88, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.; Matzke, N.; Tomiya, S.; Wogan, G.; Swartz, B.; Quental, T.; Marshall, C.; McGuire, J.; Lindsey, E.; Maguire, K.C.; et al. Has the Earth’s Sixth Mass Extinction Already Arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Crouzeilles, R.; Ferreira, M.S.; Chazdon, R.L.; Lindenmayer, D.B.; Sansevero, J.B.B.; Monteiro, L.; Iribarrem, A.; Latawiec, A.E.; Strassburg, B.B.N. Ecological restoration success is higher for natural regeneration than for active restoration in tropical forests. Sci. Adv. 2017, 3, e1701345. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Peres, C.A.; Dent, D.; Sheil, D.; Lugo, A.E.; Lamb, D.; Stork, N.E.; Miller, S.E. The Potential for Species Conservation in Tropical Secondary Forests. Conserv. Biol. 2009, 23, 1406–1417. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Fu, B.J.; Stafford-Smith, M.; Wang, S.; Zhao, W.W. Improve forest restoration initiatives to meet Sustainable Development Goal 15. Nat. Ecol. Evol. 2021, 5, 10–13. [Google Scholar] [CrossRef]
- Gao, J.; Ji, Y.H.; Zhang, X. Net primary productivity exhibits a stronger climatic response in planted versus natural forests. For. Ecol. Manag. 2023, 529, 120722. [Google Scholar] [CrossRef]

| Species Diversity Index | Equation | Reference |
|---|---|---|
| Margalef’s richness index | [34] | |
| Shannon index | [35] | |
| Simpson index | [36] | |
| Pielou evenness index | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Huang, J.; Tang, Z.; Zhao, J.; Mo, X. Significant Differences in the Effects of Pine Wilt Disease Invasion on Plant Diversity in Natural and Planted Forests. Insects 2025, 16, 295. https://doi.org/10.3390/insects16030295
Zhang Z, Huang J, Tang Z, Zhao J, Mo X. Significant Differences in the Effects of Pine Wilt Disease Invasion on Plant Diversity in Natural and Planted Forests. Insects. 2025; 16(3):295. https://doi.org/10.3390/insects16030295
Chicago/Turabian StyleZhang, Zijing, Jixia Huang, Zhiyao Tang, Junhao Zhao, and Xiumei Mo. 2025. "Significant Differences in the Effects of Pine Wilt Disease Invasion on Plant Diversity in Natural and Planted Forests" Insects 16, no. 3: 295. https://doi.org/10.3390/insects16030295
APA StyleZhang, Z., Huang, J., Tang, Z., Zhao, J., & Mo, X. (2025). Significant Differences in the Effects of Pine Wilt Disease Invasion on Plant Diversity in Natural and Planted Forests. Insects, 16(3), 295. https://doi.org/10.3390/insects16030295






