Forecasting the Impact of Climate Change on Apis dorsata (Fabricius, 1793) Habitat and Distribution in Pakistan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Occurrence/Presence Data
2.3. Thinning and Partition Occurrence Data Preprocessing of Occurrence/Presence Data
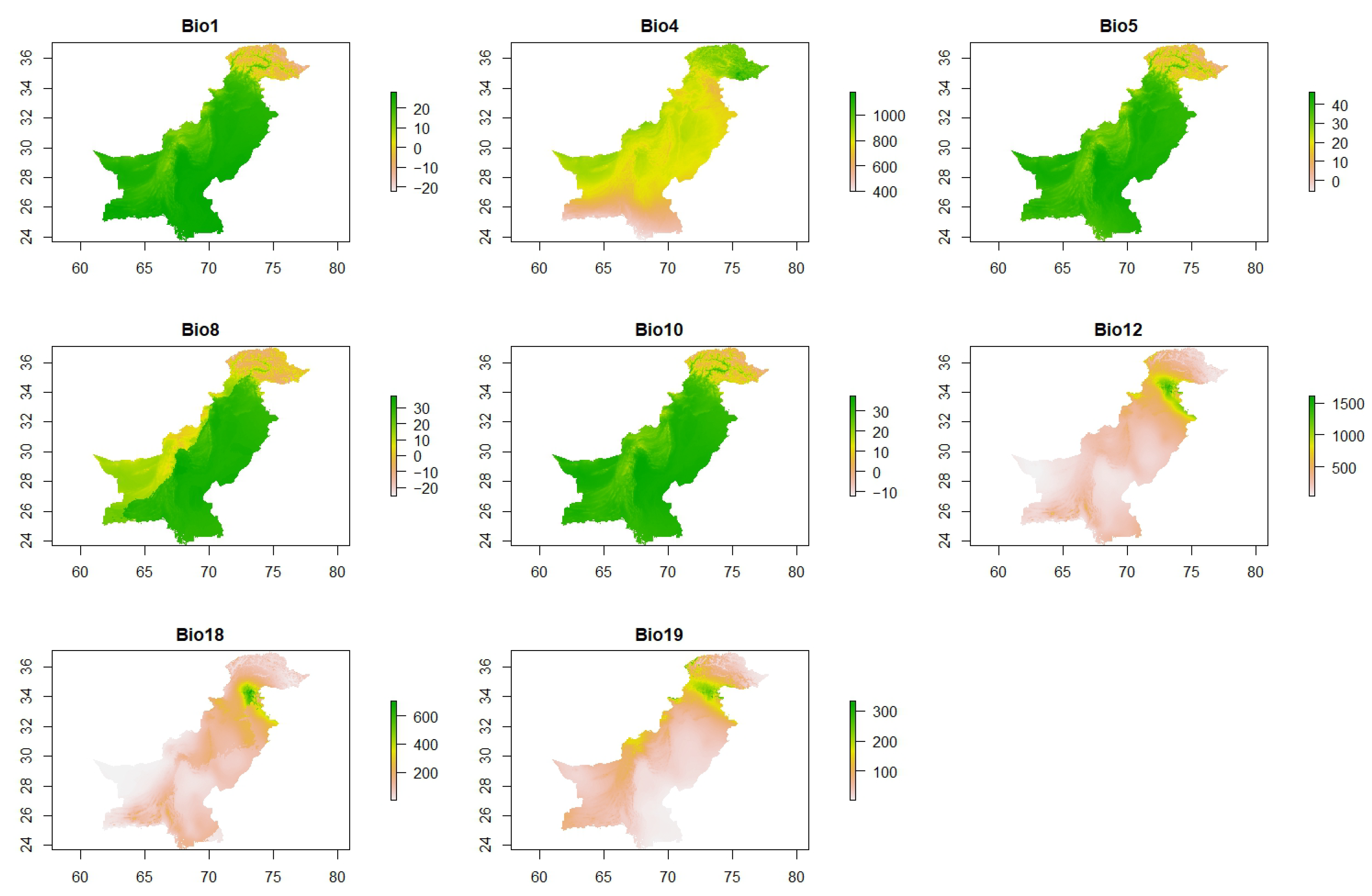
2.4. Selection and Processing of Environmental Variables
2.5. Construction of Maximum Entropy (MaxEnt) Model
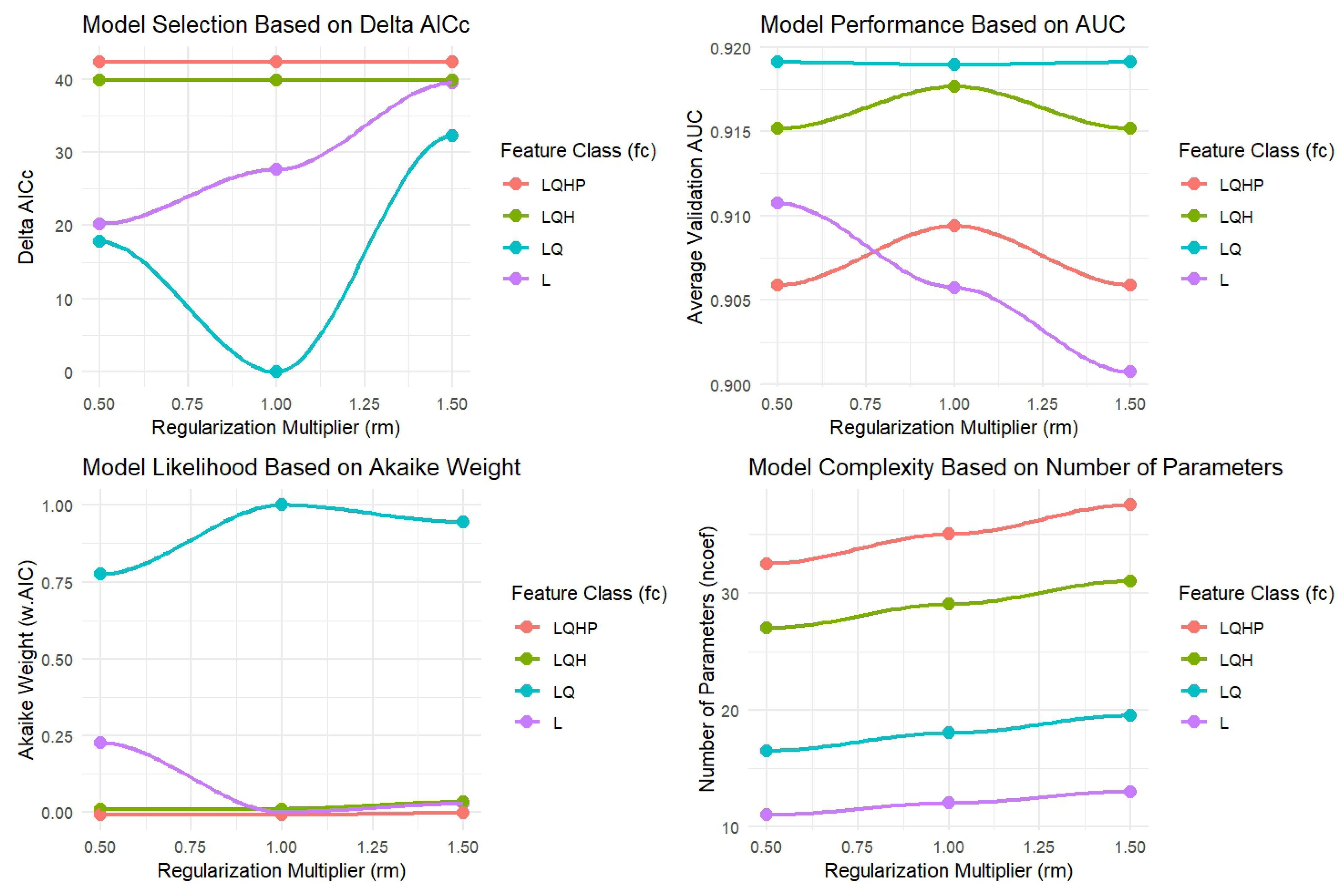
2.6. Model Evaluation and Selection Process
2.7. Future Projection Data
2.8. Division of Potential Suitable Growing Areas for Apis dorsata
3. Results
3.1. Model Performance and Selection
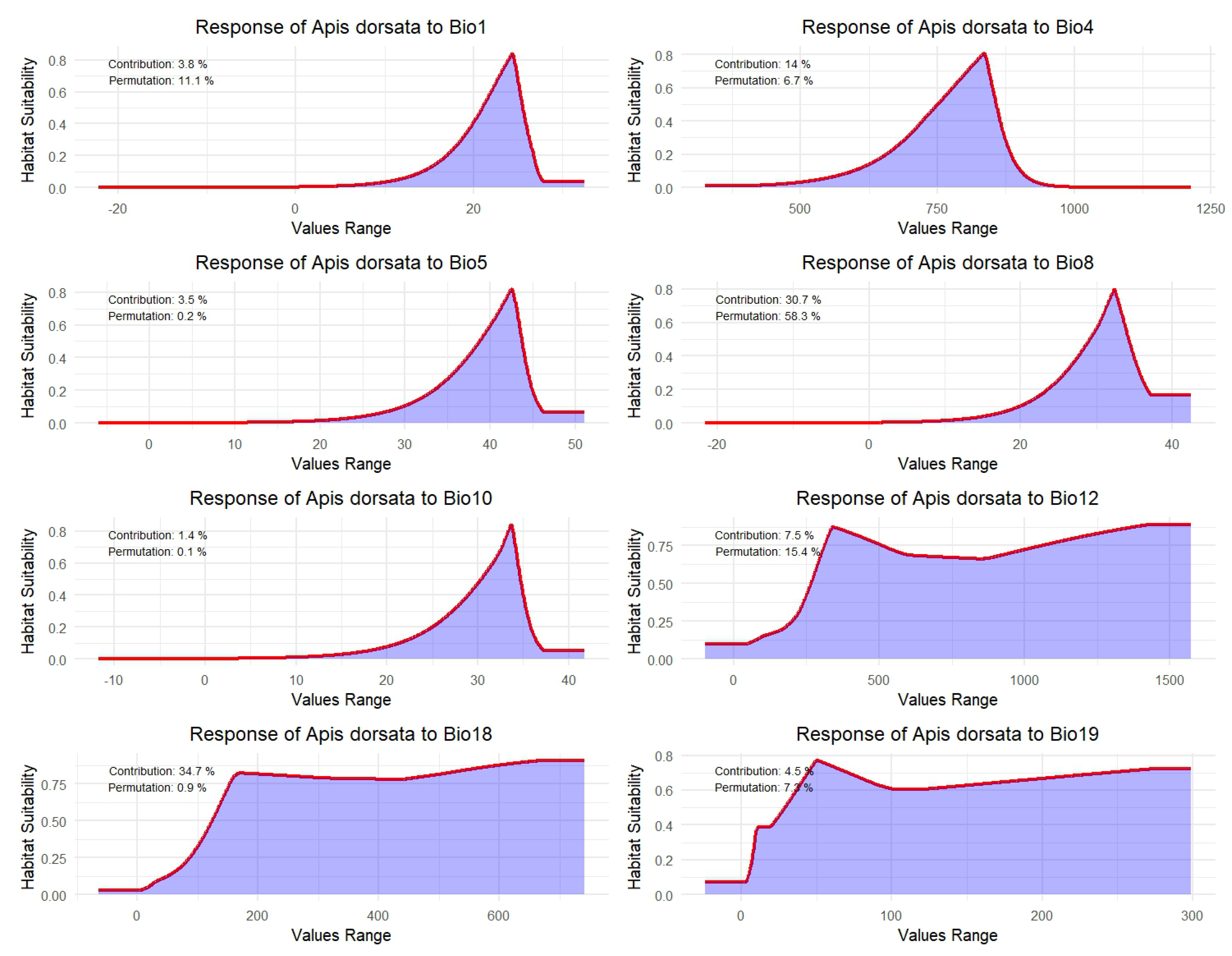
3.2. Response of A. dorsata Habitat Suitability to Key Bioclimatic Variables
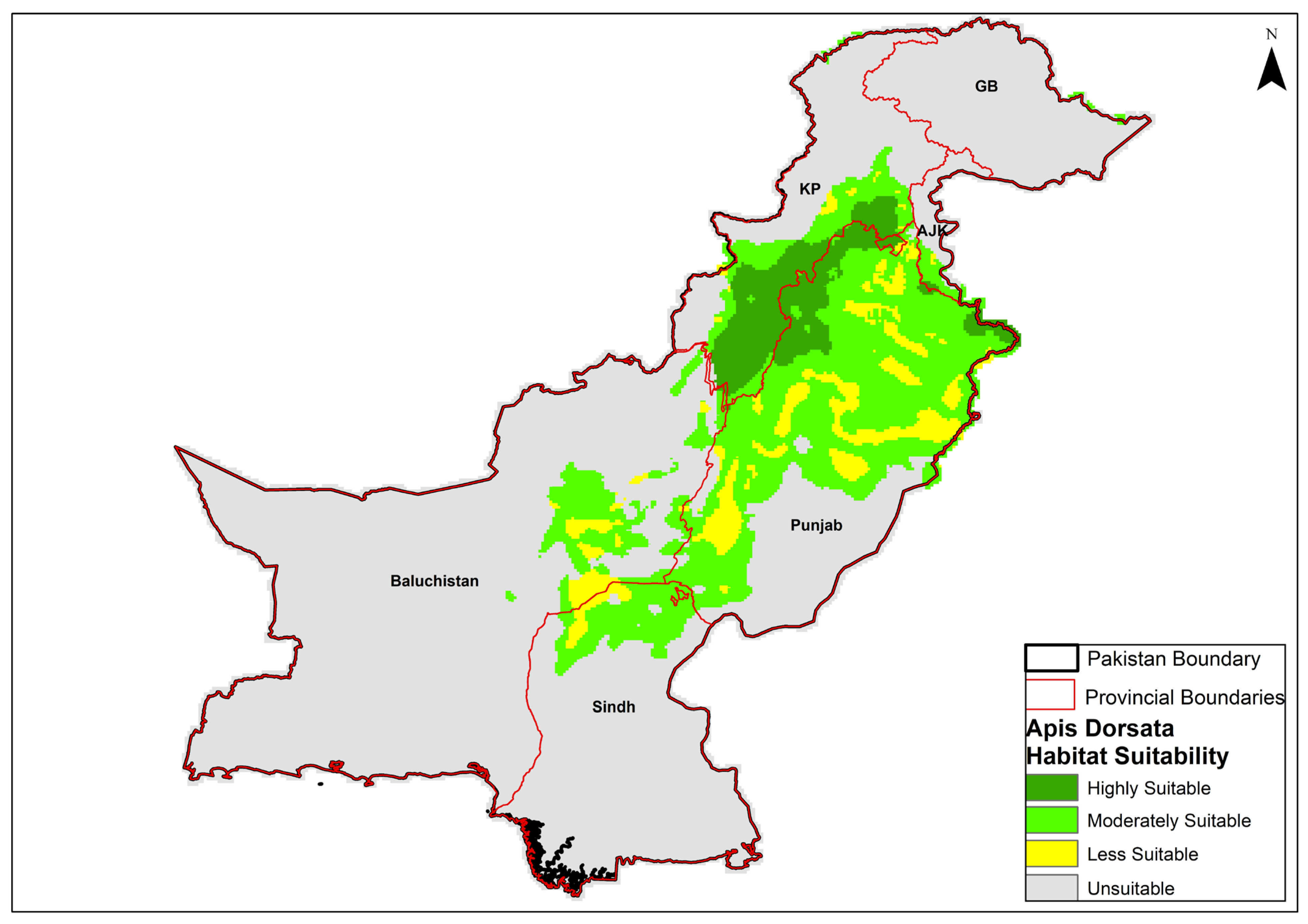
3.3. Species Habitat Under Current Environmental Conditions
3.4. Quantification and Distribution of Future Suitable Habitat of Apis dorsata
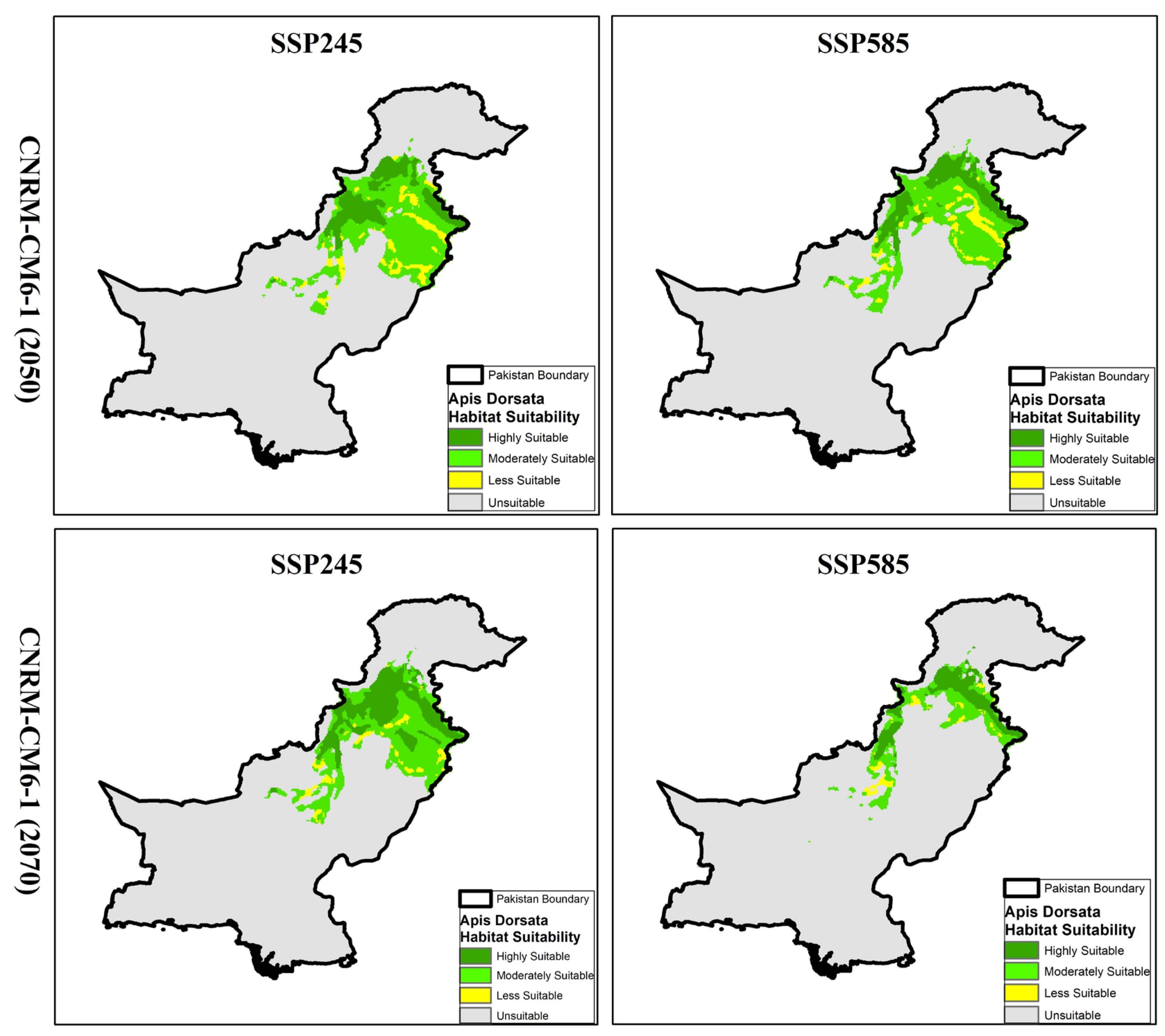
3.5. Habitat Projections and Distribution of A. dorsata Under CNRM-CM6-1 Model
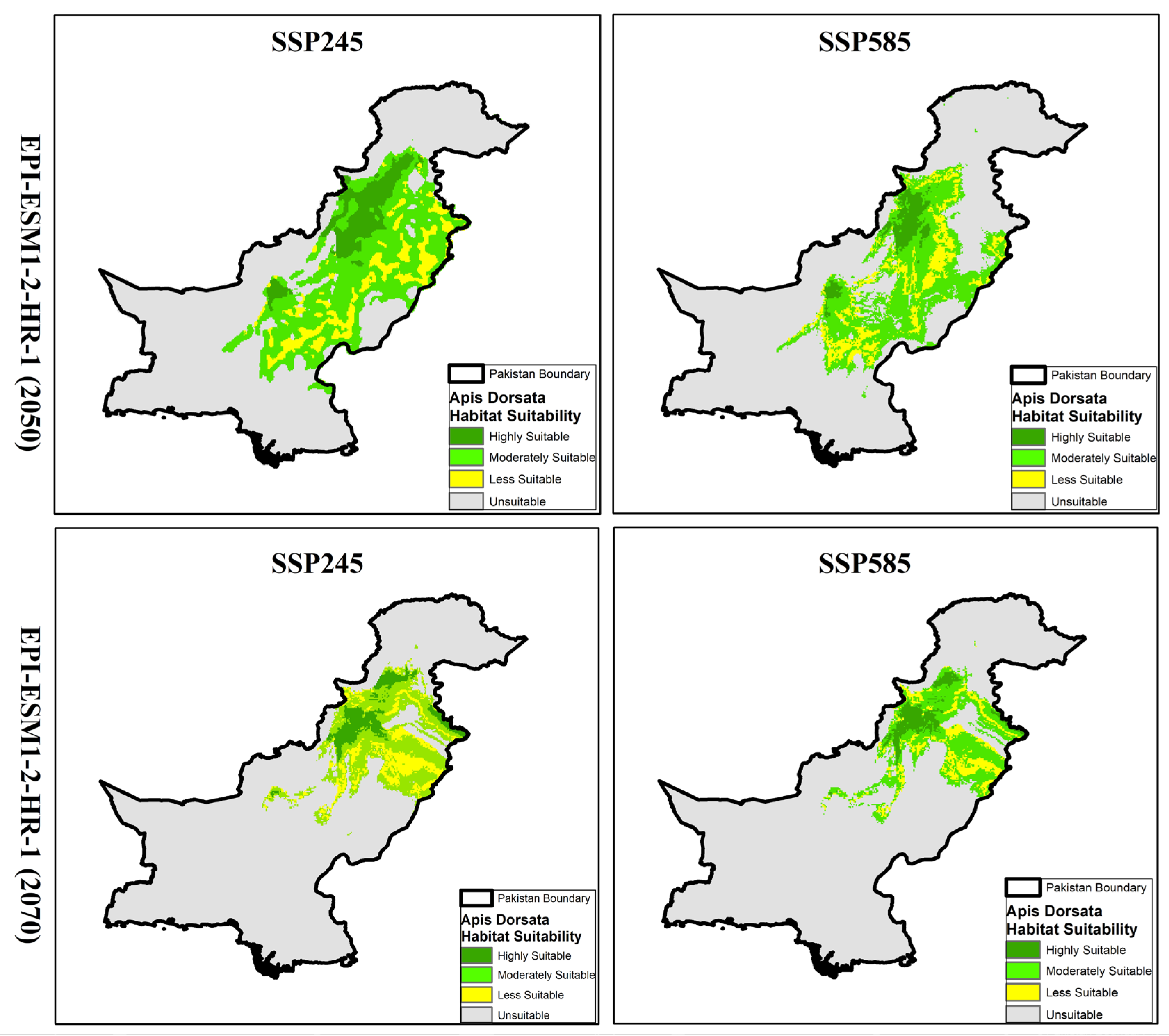
3.6. Habitat Projections and Distribution of A. dorsata Under EPI-ESM1-2-HR-1 Model
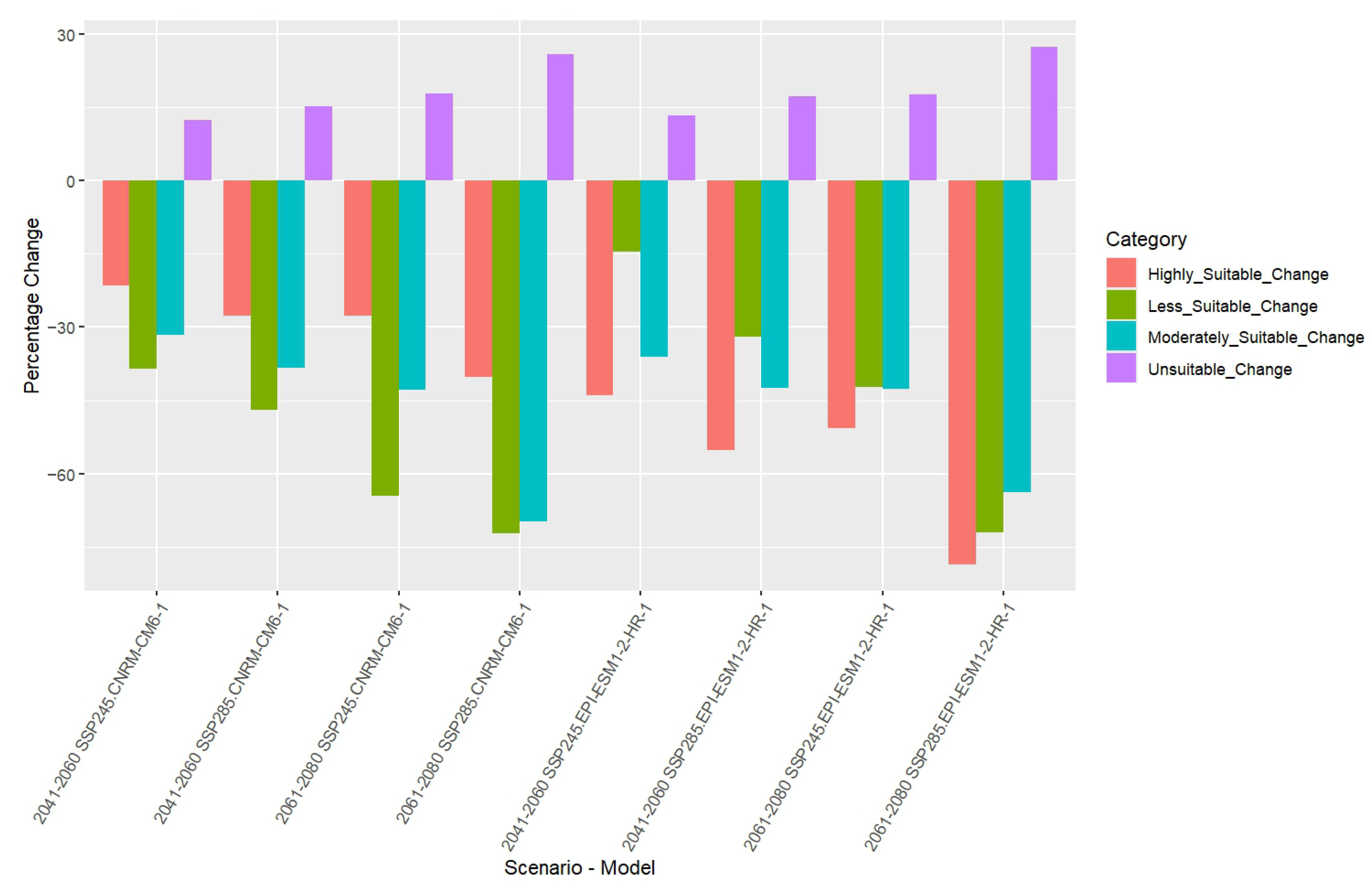
3.7. Percentage Change in Habitat Suitability Categories
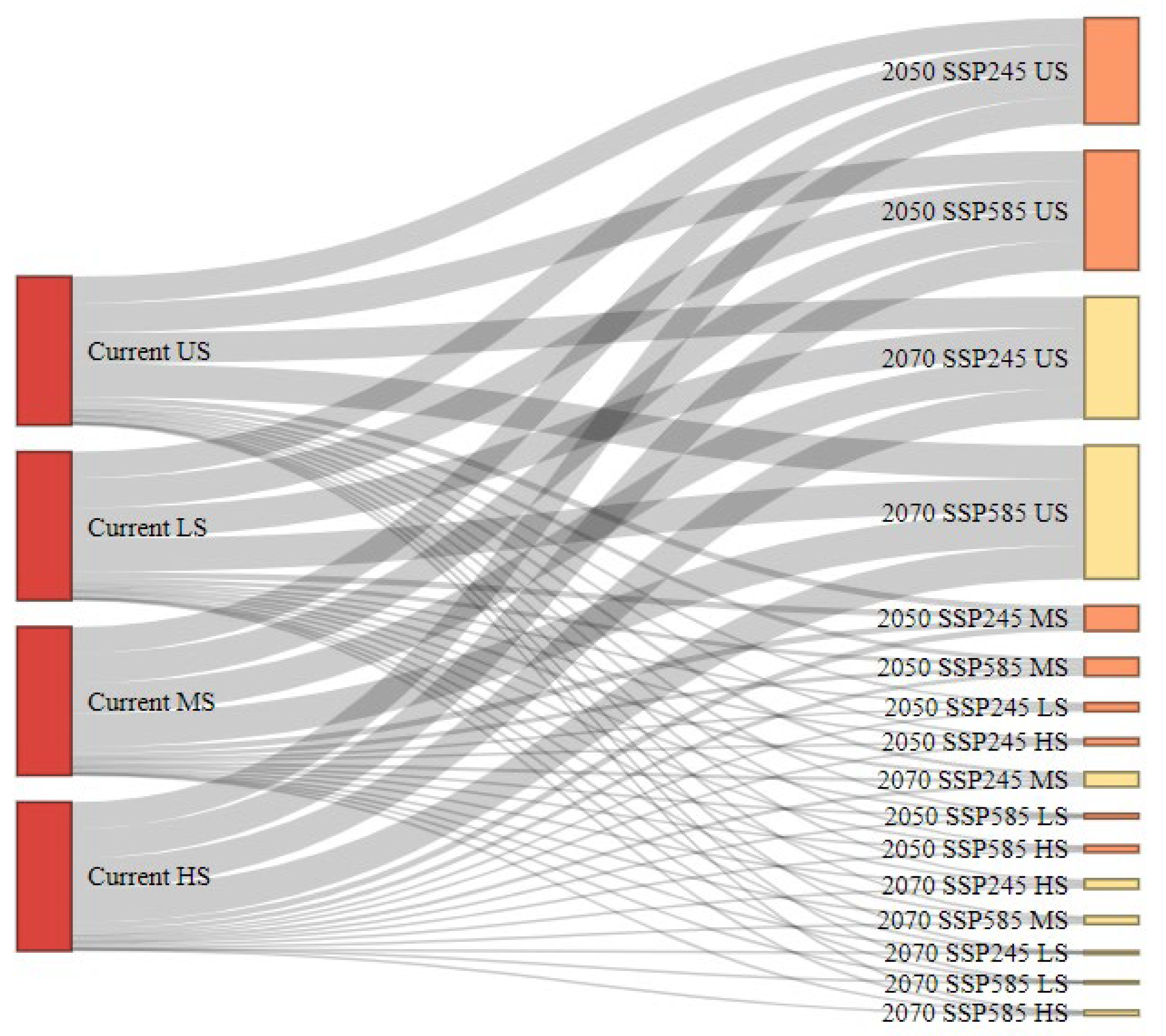
3.8. Habitat Transition Pathways
4. Discussion
Conservation and Management Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.M. Climate change is impacting nutritional security from seafood. Nat. Clim. Change 2023, 13, 1166–1167. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Biaou, S.; Gouwakinnou, G.N.; Noulèkoun, F.; Salako, K.V.; Kpoviwanou, J.M.R.H.; Houehanou, T.D.; Biaou, H.S.S. Incorporating intraspecific variation into species distribution models improves climate change analyses of a widespread West African tree species (Pterocarpus erinaceus Poir, Fabaceae). Glob. Ecol. Conserv. 2023, 45, e02538. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Xu, C. Study on Taiwania cryptomerioides under climate change: MaxEnt modeling for predicting the potential geographical distribution. Glob. Ecol. Conserv. 2020, 24, e01313. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 2006, 87, 2773–2786. [Google Scholar] [CrossRef]
- Barrett, M.A.; Brown, J.L.; Junge, R.E.; Yoder, A.D. Climate change, predictive modeling and lemur health: Assessing impacts of changing climate on health and conservation in Madagascar. Biol. Conserv. 2013, 157, 409–422. [Google Scholar] [CrossRef]
- Kellermann, V.; Van Heerwaarden, B.; Sgrò, C.M.; Hoffmann, A.A. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 2009, 325, 1244–1246. [Google Scholar] [CrossRef]
- Abolmaali, S.M.-R.; Tarkesh, M.; Bashari, H. MaxEnt modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cuena-Lombraña, A.; Fois, M.; Fenu, G.; Cogoni, D.; Bacchetta, G. The impact of climatic variations on the reproductive success of Gentiana lutea L. in a Mediterranean mountain area. Int. J. Biometeorol. 2018, 62, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Bar-Massada, A.; Ives, A.R.; Butsic, V. A mathematical partitioning of the effects of habitat loss and habitat degradation on species abundance. Landsc. Ecol. 2019, 34, 9–15. [Google Scholar] [CrossRef]
- Wiegand, T.; Revilla, E.; Moloney, K.A. Effects of habitat loss and fragmentation on population dynamics. Conserv. Biol. 2005, 19, 108–121. [Google Scholar] [CrossRef]
- Bogich, T.L.; Barker, G.M.; Mahlfeld, K.; Climo, F.; Green, R.; Balmford, A. Fragmentation, grazing and the species–area relationship. Ecography 2012, 35, 224–231. [Google Scholar] [CrossRef]
- Heinrichs, J.A.; Bender, D.J.; Schumaker, N.H. Habitat degradation and loss as key drivers of regional population extinction. Ecol. Model. 2016, 335, 64–73. [Google Scholar] [CrossRef]
- Moraitis, M.L.; Valavanis, V.D.; Karakassis, I. Modelling the effects of climate change on the distribution of benthic indicator species in the Eastern Mediterranean Sea. Sci. Total Environ. 2019, 667, 16–24. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Wilson, K.L.; Skinner, M.A.; Lotze, H.K. Projected 21st-century distribution of canopy-forming seaweeds in the Northwest Atlantic with climate change. Divers. Distrib. 2019, 25, 582–602. [Google Scholar] [CrossRef]
- Aguayo, J.; Elegbede, F.; Husson, C.; Saintonge, F.X.; Marçais, B. Modeling climate impact on an emerging disease, the Phytophthora alni-induced alder decline. Glob. Change Biol. 2014, 20, 3209–3221. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Halstead, N.T.; Raffel, T.R. Modelling the future distribution of the amphibian chytrid fungus: The influence of climate and human-associated factors. J. Appl. Ecol. 2011, 48, 174–176. [Google Scholar] [CrossRef]
- Kinezaki, N.; Kawasaki, K.; Shigesada, N. The effect of the spatial configuration of habitat fragmentation on invasive spread. Theor. Popul. Biol. 2010, 78, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.R.; Deb, P.; Singha, H.; Chakdar, B.; Medhi, M. Predicting the probable distribution and threat of invasive Mimosa diplotricha Suavalle and Mikania micrantha Kunth in a protected tropical grassland. Ecol. Eng. 2016, 97, 23–31. [Google Scholar] [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Michalski, F.; Peres, C.A. Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biol. Conserv. 2005, 124, 383–396. [Google Scholar] [CrossRef]
- Sih, A.; Jonsson, B.G.; Luikart, G. Habitat loss: Ecological, evolutionary and genetic consequences. Trends Ecol. Evol. 2000, 15, 132–134. [Google Scholar] [CrossRef]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Spooner, F.E.; Pearson, R.G.; Freeman, R. Rapid warming is associated with population decline among terrestrial birds and mammals globally. Glob. Change Biol. 2018, 24, 4521–4531. [Google Scholar] [CrossRef]
- Soroye, P.; Newbold, T.; Kerr, J. Climate change contributes to widespread declines among bumble bees across continents. Science 2020, 367, 685–688. [Google Scholar] [CrossRef]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kaloveloni, A.; Petanidou, T. Assessing climate change impacts on Island bees: The Aegean Archipelago. Biology 2022, 11, 552. [Google Scholar] [CrossRef]
- Eickermann, M.; Junk, J.; Rapisarda, C. Climate change and insects. Insects 2023, 14, 678. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Chikowore, G.; Nyamukondiwa, C.; Mudereri, B.T.; Khan, Z.R.; Chidawanyika, F. Biogeography of cereal stemborers and their natural enemies: Forecasting pest management efficacy under changing climate. Pest Manag. Sci. 2022, 78, 4446–4457. [Google Scholar] [CrossRef]
- Srivastava, V.; Liang, W.; Keena, M.A.; Roe, A.D.; Hamelin, R.C.; Griess, V.C. Assessing niche shifts and conservatism by comparing the native and post-invasion niches of major forest invasive species. Insects 2020, 11, 479. [Google Scholar] [CrossRef]
- Matias, D.M.S.; Leventon, J.; Rau, A.-L.; Borgemeister, C.; von Wehrden, H. A review of ecosystem service benefits from wild bees across social contexts. Ambio 2017, 46, 456–467. [Google Scholar] [CrossRef]
- Papa, G.; Maier, R.; Durazzo, A.; Lucarini, M.; Karabagias, I.K.; Plutino, M.; Bianchetto, E.; Aromolo, R.; Pignatti, G.; Ambrogio, A. The honey bee Apis mellifera: An insect at the interface between human and ecosystem health. Biology 2022, 11, 233. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Yoo, M.-S.; Lee, H.-S.; Truong, A.-T.; Youn, S.-Y.; Lee, S.-J.; Kim, J.; Cho, Y.S. First detection and prevalence of Apis mellifera filamentous virus in Apis mellifera and Varroa destructor in the Republic of Korea. Sci. Rep. 2024, 14, 14105. [Google Scholar] [CrossRef]
- Gustafson, D.I. Climate change: A crop protection challenge for the twenty-first century. Pest Manag. Sci. 2011, 67, 691–696. [Google Scholar] [CrossRef]
- Zou, Y.; Ge, X.; Guo, S.; Zhou, Y.; Wang, T.; Zong, S. Impacts of climate change and host plant availability on the global distribution of Brontispa longissima (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2020, 76, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Abro, Z.; Kassie, M.; Tiku, H.A.; Taye, B.; Ayele, Z.A.; Ayalew, W. The impact of beekeeping on household income: Evidence from north-western Ethiopia. Heliyon 2022, 8, e09492. [Google Scholar] [CrossRef] [PubMed]
- Basa, B.; Belay, W.; Tilahun, A.; Teshale, A. Review on medicinal value of honeybee products: Apitherapy. Adv. Biol. Res. 2016, 10, 236–247. [Google Scholar]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical prospects of bee products: Special focus on anticancer, antibacterial, antiviral, and antiparasitic properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef]
- Salatino, A. Perspectives for uses of propolis in therapy against infectious diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef]
- Kishan Tej, M.; Aruna, R.; Mishra, G.; Srinivasan, M. Beekeeping in India. Ind. Entomol. 2017, 35–66. [Google Scholar] [CrossRef]
- Fakrudin, B.; Ugalat, J.; Lakshmidevamma, T.; Kumar, C.; Rakesh, K.; Thimmarayappa, R. Genetic Diversity of Apis dorsata and Apis laboriosa. In Role of Giant Honeybees in Natural and Agricultural Systems; CRC Press: Boca Raton, FL, USA, 2023; pp. 62–77. [Google Scholar]
- Migdał, P.; Murawska, A.; Berbeć, E.; Zarębski, K.; Ratajczak, N.; Roman, A.; Latarowski, K. Biochemical Indicators and Mortality in Honey Bee (Apis mellifera) Workers after Oral Exposure to Plant Protection Products and Their Mixtures. Agriculture 2023, 14, 5. [Google Scholar] [CrossRef]
- Rahimi, E.; Jung, C. Global trends in climate suitability of bees: Ups and downs in a warming world. Insects 2024, 15, 127. [Google Scholar] [CrossRef]
- Czekońska, K.; Łopuch, S.; Miścicki, S. The effect of meteorological and environmental variables on food collection by honey bees (Apis mellifera). Ecol. Indic. 2023, 156, 111140. [Google Scholar] [CrossRef]
- Landaverde, R.; Rodriguez, M.T.; Parrella, J.A. Honey production and climate change: Beekeepers’ perceptions, farm adaptation strategies, and information needs. Insects 2023, 14, 493. [Google Scholar] [CrossRef]
- Novelli, S.; Vercelli, M.; Ferracini, C. An easy mixed-method analysis tool to support rural development strategy decision-making for beekeeping. Land 2021, 10, 675. [Google Scholar] [CrossRef]
- Ruttner, F.; Ruttner, F. Apis dorsata Fabricius 1793: 328. In Biogeography and Taxonomy of Honeybees; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1988; pp. 103–119. [Google Scholar]
- Renner, I.W.; Warton, D.I. Equivalence of MAXENT and Poisson point process models for species distribution modeling in ecology. Biometrics 2013, 69, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K. Taxonomy and distribution of different honeybee species. In Beekeeping for Poverty Alleviation and Livelihood Security; Springer: Dordrecht, The Netherlands, 2014; pp. 63–103. [Google Scholar]
- Haldhar, S.; Nidhi, C.; Singh, K.; Devi, A. Honeybees diversity, pollination, entrepreneurship and beekeeping scenario in NEH region of India. J. Agric. Ecol. 2021, 12, 27–43. [Google Scholar] [CrossRef]
- Khan, K.A.; Ansari, M.J.; Al-Ghamdi, A.; Sharma, D.; Ali, H. Biodiversity and relative abundance of different honeybee species (Hymenoptera: Apidae) in Murree-Punjab, Pakistan. J. Entomol. Zool. Stud. 2014, 2, 324–327. [Google Scholar]
- Kitnya, N.; Prabhudev, M.; Bhatta, C.P.; Pham, T.H.; Nidup, T.; Megu, K.; Chakravorty, J.; Brockmann, A.; Otis, G.W. Geographical distribution of the giant honey bee Apis laboriosa Smith, 1871 (Hymenoptera, Apidae). ZooKeys 2020, 951, 67. [Google Scholar] [CrossRef]
- Mustafa, G.; Iqbal, A.; Arshad Javid, W.A.; Ahmad, N.; Saleem, M.; Farooq, M.; Farooq, M.; Hussain, S.; Ali, A.; Khalid, M. Morphological and genetic characterization of various Apis species captured from selected sites of Punjab. Feb-Fresenius Environ. Bull. 2022, 31, 11259. [Google Scholar]
- Bashir, S.; Malik, M.F.; Hussain, M. Spatiotemporal occurrence of beehives of genus Apis in Northern Punjab and Azad Jammu and Kashmir, Pakistan. Kuwait J. Sci. 2023, 50, 40–46. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Evcin, O.; Kucuk, O.; Akturk, E. Habitat suitability model with maximum entropy approach for European roe deer (Capreolus capreolus) in the Black Sea Region. Environ. Monit. Assess. 2019, 191, 669. [Google Scholar] [CrossRef]
- Friedman, K.; Shimony, A. Jaynes’s maximum entropy prescription and probability theory. J. Stat. Phys. 1971, 3, 381–384. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.; Li, J.; Peterson, A.T.; Graham, C.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Aryal, A.; Shrestha, U.B.; Ji, W.; Ale, S.B.; Shrestha, S.; Ingty, T.; Maraseni, T.; Cockfield, G.; Raubenheimer, D. Predicting the distributions of predator (snow leopard) and prey (blue sheep) under climate change in the Himalaya. Ecol. Evol. 2016, 6, 4065–4075. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.D.S.; Nevin, O.T.; Smith, D.; Convery, I. Environmental niche overlap between snow leopard and four prey species in Kazakhstan. Ecol. Inform. 2018, 48, 97–103. [Google Scholar] [CrossRef]
- Sony, R.; Sen, S.; Kumar, S.; Sen, M.; Jayahari, K. Niche models inform the effects of climate change on the endangered Nilgiri Tahr (Nilgiritragus hylocrius) populations in the southern Western Ghats, India. Ecol. Eng. 2018, 120, 355–363. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, Z.; Tang, S. Impacts of climate change on distributions and diversity of ungulates on the Tibetan Plateau. Ecol. Appl. 2015, 25, 24–38. [Google Scholar] [CrossRef]
- Khan, B.; Ablimit, A.; Khan, G.; Jasra, A.W.; Ali, H.; Ali, R.; Ahmad, E.; Ismail, M. Abundance, distribution and conservation status of Siberian ibex, Marco Polo and Blue sheep in Karakoram-Pamir mountain area. J. King Saud Univ.-Sci. 2016, 28, 216–225. [Google Scholar] [CrossRef]
- Khan, T.U.; Ullah, I.; Hu, Y.; Liang, J.; Ahmad, S.; Omifolaji, J.K.; Hu, H. Assessment of Suitable Habitat of the Demoiselle Crane (Anthropoides virgo) in the Wake of Climate Change: A Study of Its Wintering Refugees in Pakistan. Animals 2024, 14, 1453. [Google Scholar] [CrossRef]
- Irshad, M.; Stephen, E. Value of insect pollinators to agriculture of Pakistan. Int. J. Agron. Agric. Res 2013, 3, 14–21. [Google Scholar]
- Ghani, U.; Nawaz, A.; Mustafa, T.; Muneer, S.; Ghafar, A.; Akbar, S.; Bibi, S.; Aziz, M.; Aslam, A. Challenges and Threats for Pollinator Conservation. In Advances in Insect Pollination Technology in Sustainable Agriculture; IK International Pvt. Ltd.: Delhi, India, 2023. [Google Scholar] [CrossRef]
- Usman, M.; Hasnain, M.; Banaras, S.; Akram, M.; Abbas, Q.; Shah, J.A.; Tabasum, S.; Shah, S.A.; Raza, A.; Khan, M.N. Potential emerging constraints and management strategies of different honeybee species in Pakistan: A review. CABI Rev. 2022. [Google Scholar] [CrossRef]
- Eckstein, D.; Künzel, V.; Schäfer, L. Global Climate Risk Index 2019. Who Suffers Most from Extreme Weather Events? Weather-Related Loss Events in 2017 and 1998 to 2017; Germanwatch: Bonn, Germany, 2017. [Google Scholar]
- Eckstein, D.; Künzel, V.; Schäfer, L.; Winges, M. Global Climate Risk Index 2020; Germanwatch: Bonn, Germany, 2019; pp. 1–50. [Google Scholar]
- Rehman, A.; Jingdong, L.; Du, Y.; Khatoon, R.; Wagan, S.A.; Nisar, S.K. Flood disaster in Pakistan and its impact on agriculture growth (a review). Environ. Dev. Econ. 2016, 6, 39–42. [Google Scholar]
- Looney, R. Economic impacts of the floods in Pakistan. In Pakistan in National and Regional Change; Routledge: London, UK, 2016; pp. 53–69. [Google Scholar]
- Ahmad, Z.; Hafeez, M.; Ahmad, I. Hydrology of mountainous areas in the upper Indus Basin, Northern Pakistan with the perspective of climate change. Environ. Monit. Assess. 2012, 184, 5255–5274. [Google Scholar] [CrossRef]
- Ashraf, A.; Naz, R.; Roohi, R. Glacial lake outburst flood hazards in Hindukush, Karakoram and Himalayan Ranges of Pakistan: Implications and risk analysis. Geomat. Nat. Hazards Risk 2012, 3, 113–132. [Google Scholar] [CrossRef]
- Joshi, S.; Jasra, W.; Ismail, M.; Shrestha, R.; Yi, S.; Wu, N. Herders’ perceptions of and responses to climate change in Northern Pakistan. Environ. Manag. 2013, 52, 639–648. [Google Scholar] [CrossRef]
- Tahir, A.A.; Chevallier, P.; Arnaud, Y.; Ashraf, M.; Bhatti, M.T. Snow cover trend and hydrological characteristics of the Astore River basin (Western Himalayas) and its comparison to the Hunza basin (Karakoram region). Sci. Total Environ. 2015, 505, 748–761. [Google Scholar] [CrossRef]
- Khan, T.U.; Ahmad, S. Protect Pakistan’s otters. Science 2024, 384, 519. [Google Scholar] [CrossRef]
- Kulkarni, A.; Patwardhan, S.; Kumar, K.K.; Ashok, K.; Krishnan, R. Projected climate change in the Hindu Kush–Himalayan region by using the high-resolution regional climate model PRECIS. Mt. Res. Dev. 2013, 33, 142–151. [Google Scholar] [CrossRef]
- Xu, J.; Grumbine, R.E.; Shrestha, A.; Eriksson, M.; Yang, X.; Wang, Y.; Wilkes, A. The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conserv. Biol. 2009, 23, 520–530. [Google Scholar] [CrossRef]
- Ali, Z.; Khan, A. Captive Breeding and Multiple Clutching Techniques of Captive Cranes in Bannu and Lakki Marwat, NWFP; The Ministry of Environment’s Pakistan Wetlands Programme: Bannu, Pakistan, 2007; p. 42. [Google Scholar]
- Baig, M.B.; Al-Subaiee, F.S. Biodiversity in Pakistan: Key issues. Biodiversity 2009, 10, 20–29. [Google Scholar] [CrossRef]
- White, P.C.; Jennings, N.V.; Renwick, A.R.; Barker, N.H. Questionnaires in ecology: A review of past use and recommendations for best practice. J. Appl. Ecol. 2005, 42, 421–430. [Google Scholar] [CrossRef]
- Shima, A.L.; Berger, L.; Skerratt, L.F. Conservation and health of Lumholtz’s tree-kangaroo (Dendrolagus lumholtzi). Aust. Mammal. 2018, 41, 57–64. [Google Scholar] [CrossRef]
- Lunney, D.; Matthews, A. The contribution of the community to defining the distribution of a vulnerable species, the spotted-tailed quoll, Dasyurus maculatus. Wildl. Res. 2001, 28, 537–545. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Beauchamp, G.; Jiang, Z. Flock size and human disturbance affect vigilance of endangered red-crowned cranes (Grus japonensis). Biol. Conserv. 2011, 144, 101–105. [Google Scholar] [CrossRef]
- Said, F.; Jalal, F.; Imtiaz, M.; Khan, M.A.; Hussain, S. Foraging behavior of the giant honey bee, Apis dorsata F. (Hymenoptera: Apidae) in sunflower (Helianthus annuus L.) at Peshawar District of Pakistan. Pure Appl. Biol. 2018, 7, 1115–1121. [Google Scholar] [CrossRef]
- Akram, A.; Sohail, A.; Masud, T.; Latif, A.; Tariq, S.; Butt, S.J.; Hassan, I. Physico-chemical and antimicrobial assessment of honey of Apis dorsata from different geographical regions of Pakistan. Int. J. Agric. Sci. Res. 2014, 3, 25–30. [Google Scholar]
- Sajid, M.; Haider, Z.; Awan, Q.T.; Ahmad, B.; Naz, S.; Khan, J.; Khan, N.A.; Sharif, S.; Qamer, S. Bio-chemical analysis of honey made by three, Apis florea, Apis mellifera and Apis dorsata’s honeybee species from Punjab region. Pure Appl. Biol. (PAB) 2023, 12, 1017–1024. [Google Scholar] [CrossRef]
- Ahmad, R. Methods to control migration by Apis dorsata colonies in Pakistan. Bee World 1989, 70, 160–162. [Google Scholar] [CrossRef]
- Kousar, R.; Qamer, S. Physciochemical variations in the honey produced by Apis dorsata from Punjab, Pakistan. Pure Appl. Biol. (PAB) 2017, 6, 733–739. [Google Scholar] [CrossRef]
- Sajjad, A.; Maqsood, S.; Abbasi, A.; Awais, M.; Rafiq, S.; Rafique, M.K.; Iqra, R.; Haq, I.U. Comparison of wild honeybees in the pollination of strawberries in Bahawalpur, Pakistan: Comparación de abejas silvestres en la polinización de frutilla en Bahawalpur, Pakistán. Rev. Soc. Entomológica Argent. 2023, 82, 1–8. [Google Scholar] [CrossRef]
- Iftikhar, F.; Masood, M.A.; Waghchoure, E.S. Comparison of Apis cerana, Apis dorsata, Apis florea and Apis mellifera honey from different areas of Pakistan. Asian J. Exp. Biol. Sci. 2011, 2, 399–403. [Google Scholar]
- Qamer, S.; Al-Abbadi, A.A.; Sajid, M.; Asad, F.; Khan, M.F.; Khan, N.A.; Sthanadar, A.A.; Akhtar, M.N.; Mahmoud, A.H.; Mohammed, O.B. Genetic analysis of honey bee, Apis dorsata populations using random amplified polymorphic DNA (RAPD) markers. J. King Saud Univ.-Sci. 2021, 33, 101218. [Google Scholar] [CrossRef]
- Farooqi, M.A.; Akhtar, S.; Arshad, M.; Aslam, M.N.; Rafay, M. Detection of insecticide residues in honey of Apis dorsata F. from Southern Punjab, Pakistan. Pak. J. Zool. 2017, 49, 1761–1766. [Google Scholar] [CrossRef]
- Mustafa, G.; Iqbal, A.; Javid, A.; Manzoor, M.; Aslam, S.; Ali, A.; Azam, S.M.; Khalid, M.; Farooq, M.; Al Naggar, Y. Antibacterial properties of Apis dorsata honey against some bacterial pathogens. Saudi J. Biol. Sci. 2022, 29, 730–734. [Google Scholar] [CrossRef]
- Perveen, N.; Ahmad, M. Toxicity of some insecticides to the haemocytes of giant honeybee, Apis dorsata F. under laboratory conditions. Saudi J. Biol. Sci. 2017, 24, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.; Aziz, M.A.; Naeem, M.; Ahmed, M.S.; Bodlah, I. Diversity and relative abundance of pollinator fauna of canola (Brassica napus L. var Chakwal Sarsoon) with managed Apis mellifera L. in Pothwar region, Gujar Khan, Pakistan. Pak. J. Zool. 2018, 50, 567–573. [Google Scholar] [CrossRef]
- Ahmad, S.; Zafar, M.; Ahmad, M.; Sultana, S.; Yaseen, G.; Khan, K.; Khan, F. Health benefits of honey and ethnobotanical uses of its bee flora from Lakki Marwat district, Khyber Pakhtunkhwa, Pakistan. Interdiscip. J. Appl. Basics Subj. 2021, 1, 27–35. [Google Scholar]
- Ali, M.; Sajjad, A.; Saeed, S. Yearlong association of Apis dorsata and Apis florea with flowering plants: Planted forest vs. agricultural landscape. Sociobiology 2017, 64, 18–25. [Google Scholar] [CrossRef]
- Akram, W.; Sajjad, A.; Ali, M.; Khan, H.A.A.; Maqsood, S.; Farooq, S.U. Toxicity of Commonly Used Insecticides against Apis dorsata (Hymenoptera: Apidae) in South Punjab, Pakistan. Pak. J. Zool. 2024, 1–16. [Google Scholar] [CrossRef]
- Ashkani, H.; Badinij, K.; Bulfati, A.; Chutani, U.; Dareshak, T.; Darzada, D. Assessment of physico-chemical and antimicrobial of honey of Apis dorsata from different locations of Pakistan. Glob. Sci. Res. J. 2014, 2, 186–191. [Google Scholar]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Peterson, A.; Soberón, J.; Pearson, R.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M. Evaluating model performance and significance. Ecol. Niches Geogr. Distrib. 2011, 150–181. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A jackknife approach for species with small sample sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Sánchez-Mercado, A.; Ferrer-Paris, J.; Franklin, J. Mapping species distributions: Spatial inference and prediction. Oryx 2010, 44, 615. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. J. R. Meteorol. Soc. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Sbrocco, E.J.; Barber, P.H. MARSPEC: Ocean climate layers for marine spatial ecology: Ecological Archives E094-086. Ecology 2013, 94, 979. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Hijmans, R.; Barbosa, M.; Ghosh, A.; Mandel, A. Geodata: Download Geographic Data, R Package Version 0.5-8; R Core Team: Cary, CA, USA, 2023. [Google Scholar]
- Kaeslin, E.; Redmond, I.; Dudley, N. Wildlife in a Changing Climate; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012. [Google Scholar]
- Yang, X.-Q.; Kushwaha, S.; Saran, S.; Xu, J.; Roy, P. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Molloy, S.W.; Davis, R.A.; Van Etten, E.J. Species distribution modelling using bioclimatic variables to determine the impacts of a changing climate on the western ringtail possum (Pseudocheirus occidentals; Pseudocheiridae). Environ. Conserv. 2014, 41, 176–186. [Google Scholar] [CrossRef]
- Yi, Y.-j.; Zhou, Y.; Cai, Y.-p.; Yang, W.; Li, Z.-w.; Zhao, X. The influence of climate change on an endangered riparian plant species: The root of riparian Homonoia. Ecol. Indic. 2018, 92, 40–50. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Ma, J.; Jiao, Z.; Xiao, J.; Hayat, M.A.; Wang, H. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in Mainland China. Sci. Total Environ. 2019, 664, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, L.H.; Lindsey, P.; Somers, M.J.; Van Hoven, W.; Dalerum, F. Extent and fragmentation of suitable leopard habitat in South Africa. Anim. Conserv. 2013, 16, 41–50. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Márcia Barbosa, A.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef]
- Phillips, A.J.; Vidafar, P.; Burns, A.C.; McGlashan, E.M.; Anderson, C.; Rajaratnam, S.M.; Lockley, S.W.; Cain, S.W. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. USA 2019, 116, 12019–12024. [Google Scholar] [CrossRef]
- Phillips, J.J.; Phillips, P.P. Handbook of Training Evaluation and Measurement Methods; Routledge: London, UK, 2016. [Google Scholar]
- Zhao, X.; Ren, B.; Li, D.; Garber, P.A.; Zhu, P.; Xiang, Z.; Grueter, C.C.; Liu, Z.; Li, M. Climate change, grazing, and collecting accelerate habitat contraction in an endangered primate. Biol. Conserv. 2019, 231, 88–97. [Google Scholar] [CrossRef]
- Bosso, L.; Rebelo, H.; Garonna, A.P.; Russo, D. Modelling geographic distribution and detecting conservation gaps in Italy for the threatened beetle Rosalia alpina. J. Nat. Conserv. 2013, 21, 72–80. [Google Scholar] [CrossRef]
- Fois, M.; Fenu, G.; Lombrana, A.C.; Cogoni, D.; Bacchetta, G. A practical method to speed up the discovery of unknown populations using Species Distribution Models. J. Nat. Conserv. 2015, 24, 42–48. [Google Scholar] [CrossRef]
- Vasconcelos, T.S.; Rodríguez, M.Á.; Hawkins, B.A. Species distribution modelling as a macroecological tool: A case study using New World amphibians. Ecography 2012, 35, 539–548. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Kumar, S.; Stohlgren, T.J. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ. 2009, 1, 94–98. [Google Scholar]
- Harte, J.; Zillio, T.; Conlisk, E.; Smith, A.B. Maximum entropy and the state-variable approach to macroecology. Ecology 2008, 89, 2700–2711. [Google Scholar] [CrossRef]
- Zhang, F.; Xiang, X.; Dong, Y.; Yan, S.; Song, Y.; Zhou, L. Significant differences in the gut bacterial communities of Hooded Crane (Grus monacha) in different seasons at a stopover site on the flyway. Animals 2020, 10, 701. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Bohl, C.L.; Kass, J.M.; Anderson, R.P. A new null model approach to quantify performance and significance for ecological niche models of species distributions. J. Biogeogr. 2019, 46, 1101–1111. [Google Scholar] [CrossRef]
- Yi, Y.-j.; Cheng, X.; Yang, Z.-F.; Zhang, S.-H. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized maxent model predictions of climate change impacts on the suitable distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J.; Hijmans, M.R.J. Package ‘dismo’. Circles 2017, 9, 1–68. [Google Scholar]
- Ahmad, S.; Khattak, R.H.; Teng, L.; Kaneez, K.; Liu, Z. Factors Affecting Habitat Selection of Endangered Steppe Eagle (Aquila nipalensis) in Pakistan: Implications for Raptors Conservation. Diversity 2022, 14, 1135. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Hijmans, R.J.; Elith, J. Species Distribution Modeling with R; R Cran Project: Vienna, Austria, 2013. [Google Scholar]
- El-Gabbas, A.; Dormann, C.F. Improved species-occurrence predictions in data-poor regions: Using large-scale data and bias correction with down-weighted Poisson regression and Maxent. Ecography 2018, 41, 1161–1172. [Google Scholar] [CrossRef]
- Kevan, P.G. Forest application of the insecticide Fenitrothion and its effect on wild bee pollinators (Hymenoptera: Apoidea) of lowbush blueberries (Vaccinium spp.) in Southern New Brunswick, Canada. Biol. Conserv. 1975, 7, 301–309. [Google Scholar] [CrossRef]
- Heinrich, B. The Hot-Blooded Insects: Strategies and Mechanisms of Thermoregulation; Harvard University Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Seeley, T.D. The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies; Harvard University Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Stabentheiner, A.; Kovac, H.; Brodschneider, R. Honeybee colony thermoregulation–regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE 2010, 5, e8967. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Roubik, D.W.; Roubik, D.W. Ecology and Natural History of Tropical Bees; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Usha, V.; Devi, M.S. Effect of environmental factors on the foraging activities of major bee pollinators. J. Entomol. Zool. Stud. 2020, 8, 450–454. [Google Scholar]
- Singh, P.; Gargi, B.; Semwal, P. Global trends, knowledge mapping and visualization of current research on climate change and their impact on plant-pollinators interaction. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Shi, Y.; Ren, Z.; Zhao, Y.; Wang, H. Effect of climate change on the distribution and phenology of plants, insect pollinators, and their interactions. Biodivers. Sci. 2021, 29, 495. [Google Scholar] [CrossRef]
- Javed, K. Climate Change Perspective in Pakistan. J. Politics Soc. 2023, 1, 10–17. [Google Scholar] [CrossRef]
- Fahad, S.; Wang, J. Climate change, vulnerability, and its impacts in rural Pakistan: A review. Environ. Sci. Pollut. Res. 2020, 27, 1334–1338. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M. Climate change 2021: The physical science basis. Contrib. Work. Group I Sixth Assess. Rep. Intergov. Panel Clim. Change 2021, 2, 2391. [Google Scholar]
- Gérard, M.; Cariou, B.; Henrion, M.; Descamps, C.; Baird, E. Exposure to elevated temperature during development affects bumblebee foraging behavior. Behav. Ecol. 2022, 33, 816–824. [Google Scholar] [CrossRef]
- Karbassioon, A.; Yearlsey, J.; Dirilgen, T.; Hodge, S.; Stout, J.C.; Stanley, D.A. Responses in honeybee and bumblebee activity to changes in weather conditions. Oecologia 2023, 201, 689–701. [Google Scholar] [CrossRef]
- Jaboor, S.K.; da Silva, C.R.B.; Kellermann, V. The effect of environmental temperature on bee activity at strawberry farms. Austral Ecol. 2022, 47, 1470–1479. [Google Scholar] [CrossRef]
- Beltran-Peña, A.; Rosa, L.; D’Odorico, P. Global food self-sufficiency in the 21st century under sustainable intensification of agriculture. Environ. Res. Lett. 2020, 15, 095004. [Google Scholar] [CrossRef]
- Kattel, G.R. Climate warming in the Himalayas threatens biodiversity, ecosystem functioning and ecosystem services in the 21st century: Is there a better solution? Biodivers. Conserv. 2022, 31, 2017–2044. [Google Scholar] [CrossRef]
- Thapa, K.; Subba, S.A.; Thapa, G.J.; Dewan, K.; Acharya, B.P.; Bohara, D.; Subedi, S.; Karki, M.T.; Gotame, B.; Paudel, G. Wildlife in climate refugia: Mammalian diversity, occupancy, and tiger distribution in the Western Himalayas, Nepal. Ecol. Evol. 2022, 12, e9600. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.L.; Selden, R.L.; Kitchel, Z.J. Climate-driven shifts in marine species ranges: Scaling from organisms to communities. Annu. Rev. Mar. Sci. 2020, 12, 153–179. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Schindler, D.E. Getting ahead of climate change for ecological adaptation and resilience. Science 2022, 376, 1421–1426. [Google Scholar] [CrossRef]
- Eckstein, D.; Künzel, V.; Schäfer, L. The Global Climate Risk Index 2021; Germanwatch: Bonn, Germany, 2021. [Google Scholar]
- Lynn, J.; Peeva, N. Communications in the IPCC’s Sixth Assessment Report cycle. Clim. Change 2021, 169, 18. [Google Scholar] [CrossRef]
- Gélvez-Zúñiga, I.; Beirão, M.; Novais, S.; Santiago, J.; Fernandes, G. Floral resource availability declines and florivory increases along an elevation gradient in a highly biodiverse community. Ann. Bot. 2024, 135, 199–210. [Google Scholar] [CrossRef]
- Pontarp, M.; Runemark, A.; Friberg, M.; Opedal, Ø.H.; Persson, A.S.; Wang, L.; Smith, H.G. Evolutionary plant–pollinator responses to anthropogenic land-use change: Impacts on ecosystem services. Biol. Rev. 2024, 99, 372–389. [Google Scholar] [CrossRef]
- Imran, M.; Sheikh, U.A.A.; Rahim, J.; Nasir, M. A Threat to Pollinators: Understanding the Impacts and Solutions: A Review. Planta Anim. 2023, 2, 79–87. [Google Scholar]
- Mehmood, Y.; Arshad, M.; Mahmood, N.; Kächele, H.; Kong, R. Occupational hazards, health costs, and pesticide handling practices among vegetable growers in Pakistan. Environ. Res. 2021, 200, 111340. [Google Scholar] [CrossRef]
- Rashid, S.; Rashid, W.; Tulcan, R.X.S.; Huang, H. Use, exposure, and environmental impacts of pesticides in Pakistan: A critical review. Environ. Sci. Pollut. Res. 2022, 29, 43675–43689. [Google Scholar] [CrossRef]
- Mei, T. Estimating the Causal Effects of Neonicotinoid Use on Wild Bee Abundance in the Mid-Atlantic United States. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2023. [Google Scholar]
- Douglas, M.R.; Sponsler, D.B.; Lonsdorf, E.V.; Grozinger, C.M. County-level analysis reveals a rapidly shifting landscape of insecticide hazard to honey bees (Apis mellifera) on US farmland. Sci. Rep. 2020, 10, 797. [Google Scholar] [CrossRef] [PubMed]

| Future Projection | Scenario | Habitat Categories | Total | ||||
|---|---|---|---|---|---|---|---|
| Model | Year | Unsuitable (km2) | Less Suitable (km2) | Moderately Suitable (km2) | Highly Suitable (km2) | ||
| Current | 629,817 | 51,312 | 150,975 | 49,792 | 881,896 | ||
| CNRM-CM6-1 | 2050 | SSP245 | 708,119 | 31,569 | 103,135 | 39,073 | 881,896 |
| SSP585 | 725,579 | 27,196 | 93,156 | 35,965 | 881,896 | ||
| 2070 | SSP245 | 741,449 | 18,162 | 86,274 | 36,011 | 881,896 | |
| SSP585 | 792,177 | 14,285 | 45,691 | 29,743 | 881,896 | ||
| EPI-ESM1-2-HR-1 | 2050 | SSP245 | 713,598 | 43,872 | 96,561 | 27,865 | 881,896 |
| SSP585 | 737,895 | 34,916 | 86,756 | 22,329 | 881,896 | ||
| 2070 | SSP245 | 741,035 | 29,593 | 86,721 | 24,547 | 881,896 | |
| SSP585 | 802,169 | 14,325 | 54,756 | 10,646 | 881,896 | ||
| Future Projection | Period | Scenario | Habitat Categories | |||
|---|---|---|---|---|---|---|
| Model | US | LS | MS | HS | ||
| Current | 629,817 | 51,312 | 150,975 | 49,792 | ||
| CNRM-CM6-1 | 2050 | SSP245% Change | 12 | −38 | −32 | −22 |
| SSP585% Change | 15 | −47 | −38 | −28 | ||
| 2070 | SSP245% Change | 18 | −65 | −43 | −28 | |
| SSP585% Change | 26 | −72 | −70 | −40 | ||
| EPI-ESM1-2-HR-1 | 2050 | SSP245% Change | 13 | −14 | −36 | −44 |
| SSP585% Change | 17 | −32 | −43 | −55 | ||
| 2070 | SSP245% Change | 18 | −42 | −43 | −51 | |
| SSP585% Change | 27 | −72 | −64 | −79 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.U.; Luan, X.; Nabi, G.; Raza, M.F.; Iqbal, A.; Khan, S.N.; Hu, H. Forecasting the Impact of Climate Change on Apis dorsata (Fabricius, 1793) Habitat and Distribution in Pakistan. Insects 2025, 16, 289. https://doi.org/10.3390/insects16030289
Khan TU, Luan X, Nabi G, Raza MF, Iqbal A, Khan SN, Hu H. Forecasting the Impact of Climate Change on Apis dorsata (Fabricius, 1793) Habitat and Distribution in Pakistan. Insects. 2025; 16(3):289. https://doi.org/10.3390/insects16030289
Chicago/Turabian StyleKhan, Tauheed Ullah, Xiaofeng Luan, Ghulam Nabi, Muhammad Fahad Raza, Arshad Iqbal, Shahid Niaz Khan, and Huijian Hu. 2025. "Forecasting the Impact of Climate Change on Apis dorsata (Fabricius, 1793) Habitat and Distribution in Pakistan" Insects 16, no. 3: 289. https://doi.org/10.3390/insects16030289
APA StyleKhan, T. U., Luan, X., Nabi, G., Raza, M. F., Iqbal, A., Khan, S. N., & Hu, H. (2025). Forecasting the Impact of Climate Change on Apis dorsata (Fabricius, 1793) Habitat and Distribution in Pakistan. Insects, 16(3), 289. https://doi.org/10.3390/insects16030289






