Blood Source and Anesthetics Effects on the Maintenance of Anopheles darlingi in the Lab-Rearing Condition
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
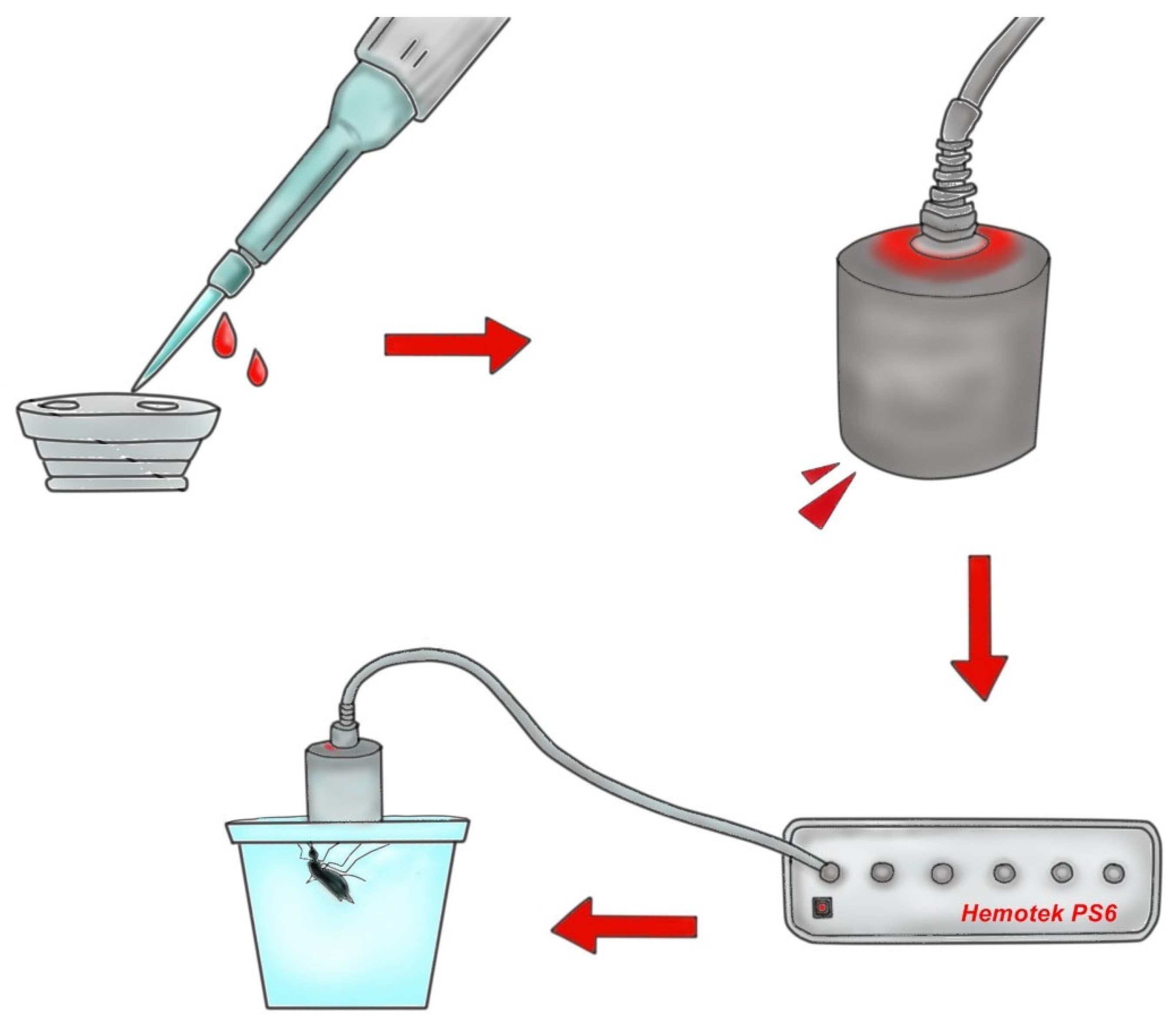
2.2. Blood Source
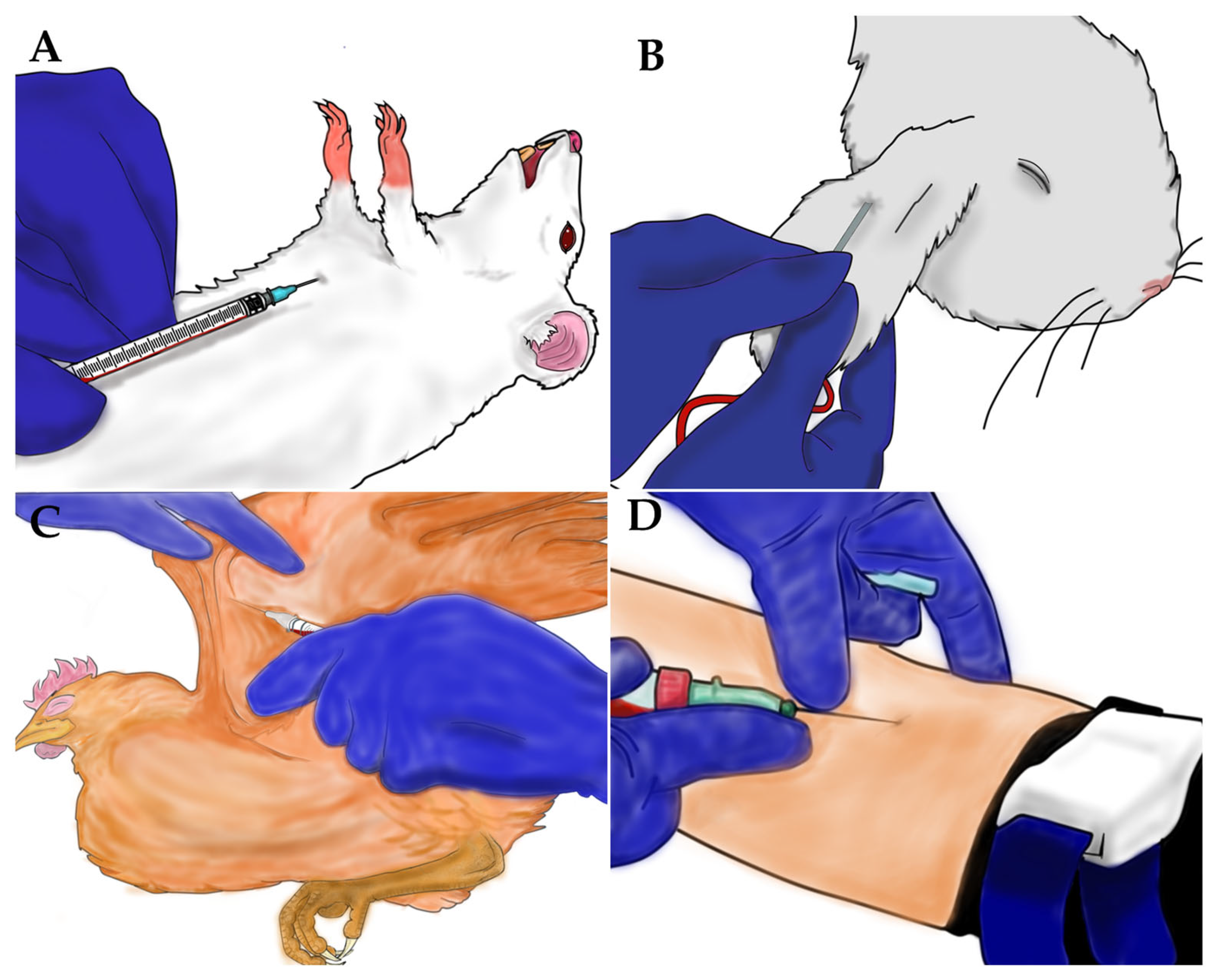
2.3. Anesthesia Procedures and Blood Collection
2.4. Anopheles darlingi Rearing
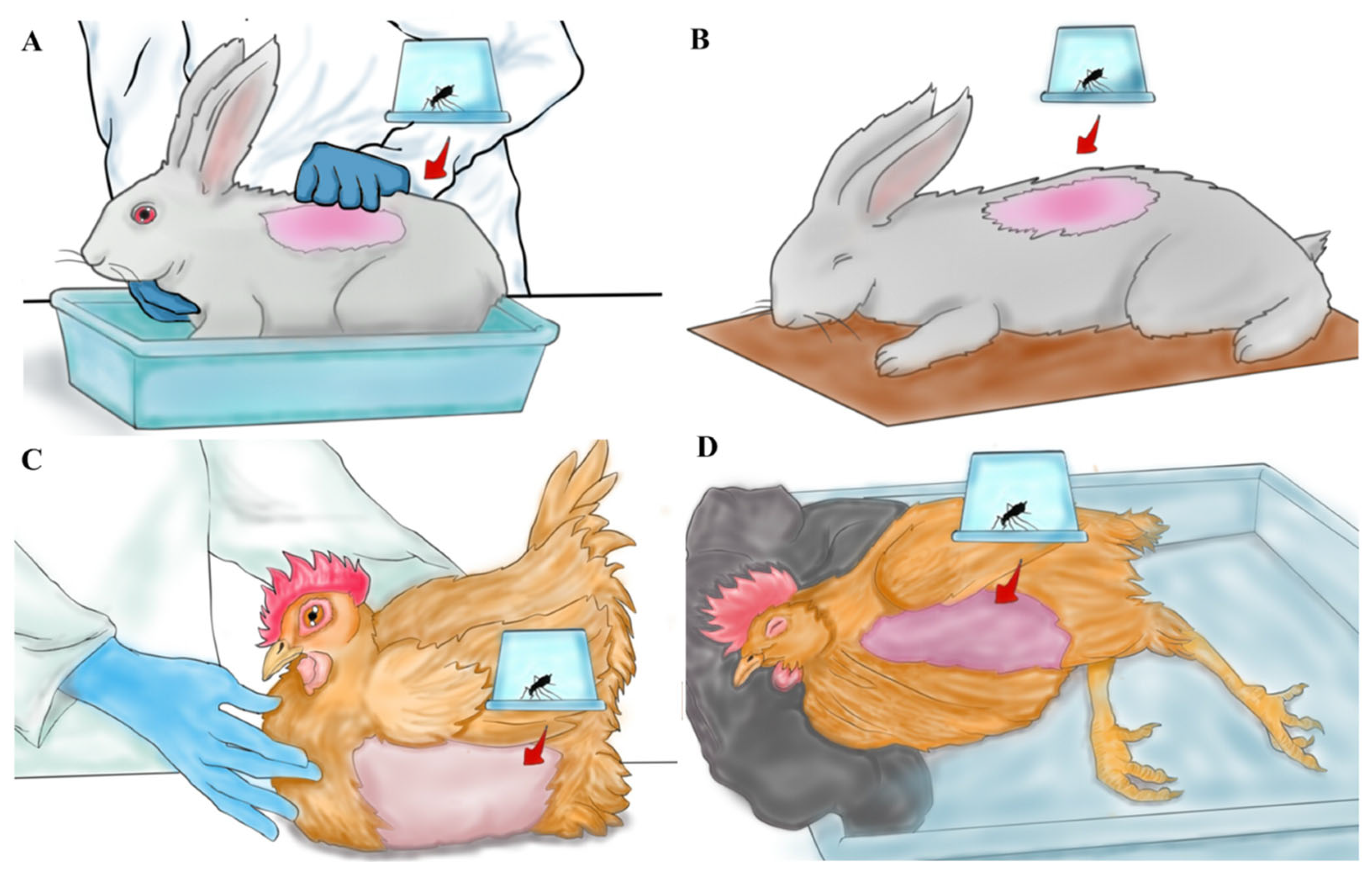
2.5. Blood Feeding
2.6. Biological Parameters Analyzed
2.7. Statistical Analysis
3. Results
3.1. The Effect of Anesthesia on the Reproductive Potential of Anopheles darlingi
3.2. Blood Source Effect by Direct and Indirect Blood Feeding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rougeron, V.; Boundenga, L.; Arnathau, C.; Durand, P.; Renaud, F.; Prugnolle, F. A population genetic perspective on the origin, spread and adaptation of the human malaria agents Plasmodium falciparum and Plasmodium vivax. FEMS Microbiol. Rev. 2022, 46, fuab047. [Google Scholar] [CrossRef]
- World Health Organization. Malaria. 11 December 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 23 January 2025).
- Angrisano, F.; Robinson, L.J. Plasmodium vivax—How hidden reservoirs hinder global malaria elimination. Parasitol. Int. 2022, 87, 102526. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.R.; Gomes, F.L.R.; Ribeiro, C.T.D. Malaria Related Neurocognitive Deficits and Behavioral Alterations. Front. Cell. Infect. Microbiol. 2022, 12, 829413. [Google Scholar] [CrossRef]
- Vitor-Silva, S.; Reyes-Lecca, R.C.; Pinheiro, T.R.; Lacerda, M.V. Malaria is associated with poor school performance in an endemic area of the Brazilian Amazon. Malar. J. 2009, 8, 230–237. [Google Scholar] [CrossRef]
- Tapajós, R.; Castro, D.; Melo, G.; Balogun, S.; James, M.; Pessoa, R. Malaria impact on cognitive function of children in a peri-urban community in the Brazilian Amazon. Malar. J. 2019, 18, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Devine, A.; Pasaribu, A.P.; Teferi, T.; Pham, H.T.; Awab, G.R.; Contantia, F.; Nguyen, T.N.; Ngo, V.T.; Tran, T.H.; Hailu, A.; et al. Provider and household costs of Plasmodium vivax malaria episodes: A multicountry comparative analysis of primary trial data. Bull. World Health Organ. 2019, 97, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Gunalan, K.; Rowley, E.H.; Miller, L.H. A Way Forward for Culturing Plasmodium vivax. Trends Parasitol. 2020, 36, 512–519. [Google Scholar] [CrossRef]
- Moreno, M.; Tong, C.; Guzmán, M.; Chuquiyauri, R.; Llanos-Cuentas, A.; Rodriguez, H.; Gamboa, D.; Meister, S.; Winzeler, E.A.; Maguina, P.; et al. Infection of laboratory-colonized Anopheles darlingi mosquitoes by Plasmodium vivax. Am. J. Trop. Med. Hyg. 2014, 90, 612–616. [Google Scholar] [CrossRef]
- Villarreal-Trevino, C.; Vasquez, G.M.; Lopez-Sifuentes, V.M.; Escobedo-Vargas, K.; Huayanay-Repetto, A.; Linton, Y.M.; Flores-Mendoza, C.; Lescano, A.G.; Stell, F.M. Establishment of a free-mating, long-standing and highly productive laboratory colony of Anopheles darling from the Peruvian Amazon. Malar. J. 2015, 14, 227–239. [Google Scholar] [CrossRef]
- Araujo, M.d.S.; Andrade, A.O.; Santos, N.A.C.d.; Pereira, D.B.; Costa, G.d.S.; Paulo, P.F.M.d.; Rios, C.T.; Moreno, M.; Pereira-da-Silva, L.H.; Medeiros, J.F. Brazil’s first free-mating laboratory colony of Nyssorhynchus darlingi. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190159. [Google Scholar] [CrossRef]
- Puchot, N.; Lecoq, M.-T.; Carinci, R.; Duchemin, J.B.; Gendrin, M.; Bourgouin, C. Establishment of a colony of Anopheles darlingi from French Guiana for vector competence studies on malaria transmission. Front. Trop. 2022, 3, 949300. [Google Scholar] [CrossRef]
- Póvoa, M.M.; de Souza, R.T.L.; Lacerda, R.N.d.L.; Rosa, E.S.; Galiza, D.; de Souza, J.R.; A Wirtz, R.; Schlichting, C.D.; E Conn, J. The importance of Anopheles albitarsis E and An. darlingi in human malaria transmission in Boa Vista, state of Roraima, Brazil. Mem. Do Inst. Oswaldo Cruz 2006, 101, 163–168. [Google Scholar] [CrossRef]
- Andrade, A.O.; dos Santos, N.A.C.; Castro, R.B.; de Araujo, I.S.; Bastos, A.d.S.; Magi, F.N.; Rodrigues, M.M.d.S.; Pereira, D.B.; Medeiros, J.F.; Araújo, M.d.S. Description of malaria vectors (Diptera: Culicidae) in two agricultural settlements in the Western Brazilian Amazon. Rev. Inst. Med. Trop. São Paulo 2021, 63, e60. [Google Scholar] [CrossRef] [PubMed]
- Vittor, A.Y.; Pan, W.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Sánchez-Lozano, W.; Pinedo, V.V.; Salas-Cobos, E.; Flores, S.; et al. Linking deforestation to malaria in the Amazon: Characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am. J. Trop. Med. Hyg. 2009, 81, 5–12. [Google Scholar] [PubMed]
- Bastos, A.S.; dos Santos, N.A.C.; Andrade, A.O.; Pontual, J.D.C.; Araújo, J.E.; Medeiros, J.F.; Araujo, M.S. Evaluation of insemination, blood feeding, and Plasmodium vivax infection effects on locomotor activity patterns of the malaria vector Anopheles darlingi (Diptera: Culicidae). Parasitol. Res. 2024, 123, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.S.; Guo, F.; Rosbash, M. Video Recording Can Conveniently Assay Mosquito Locomotor Activity. Sci. Rep. 2020, 10, 4994–5003. [Google Scholar] [CrossRef]
- Santos, N.A.C.; Martins, M.M.; Andrade, A.O.; Bastos, A.S.; Pontual, J.D.C.; Araújo, J.E.; Rocha, M.L.; Medeiros, J.F.; Araujo, M.S. Effects of Carbohydrate Intake on Anopheles darlingi and Anopheles deaneorum Fitness Under Lab-Reared Conditions. Insects 2024, 15, 240. [Google Scholar] [CrossRef]
- Acford-Palmer, H.; Andrade, A.O.; Phelan, J.E.; Santana, R.A.; Lopes, S.C.P.; Medeiros, J.F.; Clark, T.G.; Araujo, M.S.; Campino, S. Application of a targeted amplicon sequencing panel to screen for insecticide resistance mutations in Anopheles darlingi populations from Brazil. Sci. Rep. 2025, 15, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Penna-Coutinho, J.; Araujo, M.S.; Aguiar, A.C.C.; Sá, P.M.; Rios, C.T.; Medeiros, J.F.; Pereira, D.B.; Boechat, N.; Krettli, A.U. MEFAS, a hybrid of artesunate-mefloquine active against asexual stages of Plasmodium vivax in field isolates, inhibits malaria transmission. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 150–155. [Google Scholar] [CrossRef]
- Calit, J.; Araújo, J.E.; Deng, B.; Miura, K.; Gaitán, X.A.; Araujo, M.S.; Medeiros, J.F.; Long, C.A.; Simeonov, A.; Eastman, R.T.; et al. Novel Transmission-Blocking Antimalarials Identified by High-Throughput Screening of Plasmodium berghei Ookluc. Antimicrob. Agents Chemother. 2023, 67, e0146522. [Google Scholar] [CrossRef]
- Bansal, G.P.; Araujo, M.S.; Cao, Y.; Shaffer, E.; Araujo, J.E.; Medeiros, J.F.; Hayashi, C.; Vinetz, J.; Kumar, N. Transmission-reducing and -enhancing monoclonal antibodies against Plasmodium vivax gamete surface protein Pvs48/45. Infect. Immun. 2024, 12, e0037423. [Google Scholar] [CrossRef]
- Khan, J.; Gholizadeh, S.; Zhang, D.; Wang, G.; Guo, Y.; Zheng, X.; Wu, Z.; Wu, Y. Identification of a biological form in the Anopheles stephensi laboratory colony using the odorant-binding protein 1 intron I sequence. PLoS ONE 2022, 17, e0263836. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.C.; Andrade, A.O.; Santos, T.C.; Martinez, L.N.; Ferreira, A.S.; Bastos, A.S.; Martins, M.M.; Pontual, J.D.C.; Teles, C.B.G.; Medeiros, J.F.; et al. Evaluation of sustainable susceptibility to Plasmodium vivax infection among colonized Anopheles darlingi and Anopheles deaneorum. Malar. J. 2022, 21, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.C.; Magi, F.N.; Andrade, A.O.; Bastos, A.S.; Pereira, S.S.; Medeiros, J.F.; Araujo, M.S. Assessment of antibiotic treatment on Anopheles darlingi survival and susceptibility to Plasmodium vivax. Front. Microbiol. 2022, 13, 971083. [Google Scholar] [CrossRef]
- Benedict, M.Q.; Knols, B.G.; Bossin, H.C.; I Howell, P.; Mialhe, E.; Caceres, C.; Robinson, A.S. Colonization and mass rearing: Learning from others. Malar. J. 2009, 8, S4. [Google Scholar] [CrossRef]
- Williams, J.; Flood, L.; Praulins, G.; Ingham, V.A.; Morgan, J.; Less, R.S.; Hanson, H. Characterization of Anopheles strains used for laboratory screening of new vector control products. Parasites Vectors 2009, 12, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Facchinelli, L.; Valerio, L.; Lees, R.S.; Oliva, C.F.; Persampieri, T.; Collins, C.M.; Crisanti, A.; Spaccapelo, R.; Benedict, M.Q. Stimulating Anopheles gambiae swarms in the laboratory: Application for behavioral and fitness studies. Malar. J. 2015, 14, 271–280. [Google Scholar] [CrossRef]
- Villarreal, C.; Arredondo-Jiménez, J.I.; Rodriguez, M.H.; Ulloa, A. Colonization of Anopheles pseudopunctipennis from Mexico. J. Am. Mosq. Control Assoc. 1998, 14, 369–372. [Google Scholar]
- Baker, R.H. Mating problems as related to the establishment and maintenance of laboratory colonies of mosquitos. Bul. World Health Org. 1964, 31, 467–468. [Google Scholar]
- Phasomkusolsil, S.; Tawong, J.; Monkanna, N.; Pantuwatana, K.; Damdangdee, N.; Khongtak, W.; Kertmanee, Y.; Evans, B.P.; Schuster, A.L. Maintenance of mosquito vectors: Effects of blood source on feeding, survival, fecundity, and egg hatching rates. J. Vector Ecol. 2013, 38, 38–45. [Google Scholar] [CrossRef]
- Dias, L.d.S.; da Bauzer, L.G.S.R.; Lima, J.B.P. Artificial blood feeding for Culicidae colony maintenance in laboratories: Does the blood source condition matter? Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e45. [Google Scholar] [CrossRef]
- Harrington, L.C.; Edman, J.D.; Scott, T.W. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 2001, 38, 411–422. [Google Scholar] [CrossRef]
- Turell, M.J. Reduced Rift Valley fever virus infection rates in mosquitoes associated with pledget feedings. Am. J. Trop. Med. Hyg. 1988, 39, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Bailey, D.L.; Dame, D.A. Maintenance of Anopheles albimanus on frozen blood. J. Am. Mosq. Control. Assoc. 1985, 1, 538–540. [Google Scholar] [PubMed]
- Benedict, M.Q. Methods in Anopheles Research, 2nd ed.; CDC: Atlanta, GA, USA, 2015; Available online: https://www.beiresources.org/Publications/MethodsinAnophelesResearch.aspx (accessed on 15 January 2025).
- Buchta, J.N.; Zarndt, B.S.; Garver, L.S.; Rowland, T.; Shi, M.; Davidson, S.A.; Rowton, E.D. Blood-Feeding Behaviors of Anopheles stephensi but not Phlebotomus papatasi are influenced by Actively Warming Guinea Pigs (Cavia porcellus) Under General Anesthesia. J. Am. Mosq. Control. Assoc. 2015, 31, 149–154. [Google Scholar] [CrossRef]
- Luo, Y.P. A novel multiple membrane blood-feeding system for investigating and maintaining Aedes aegypti and Aedes albopictus mosquitoes. J. Vector Ecol. 2014, 39, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, N.; Ranathunge, T.; Udayanga, L.; Abeyewickreme, W. Efficacy of Blood Sources and Artificial Blood Feeding Methods in Rearing of Aedes aegypti (Diptera: Culicidae) for Sterile Insect Technique and Incompatible Insect Technique Approaches in Sri Lanka. Biomed Res. Int. 2017, 2017, 3196924. [Google Scholar] [CrossRef]
- Moreno, M.; Saavedra, M.P.; Bickersmith, S.A.; Prussing, C.; Michalski, A. Intensive trapping of blood-fed Anopheles darlingi in Amazonian Peru reveals unexpectedly high proportions of avian blood-meals. PLoS Negl. Trop Dis. 2017, 11, e0005337. [Google Scholar] [CrossRef]
- Oliveira-Ferreira, J.; Lourenço-de-Oliveira, R.; Deane, L.M.; Daniel-Ribeiro, C.T. Feeding preference of Anopheles darlingi in malaria endemic areas of Rondônia state, northwestern Brazil. Mem. Inst. Oswaldo Cruz 1992, 87, 601–602. [Google Scholar] [CrossRef]
- Nagaki, S.S.; Chaves, L.S.M.; López, R.V.M.; Bergo, E.S.; Laporta, G.Z.; Conn, J.E.; Sallum, M.A.M. Host feeding patterns of Nyssorhynchus darlingi (Diptera: Culicidae) in the Brazilian Amazon. Acta Trop. 2021, 213, 105751. [Google Scholar] [CrossRef]
- Deane, L.; Vernin, C.S.; Damasceno, R.G. Avaliação das preferências alimentares das fêmeas de Anopheles darlingi e Anopheles aquasalis em Belém, Pará, por meio de Provas de precipitina. Rev. Serv. Esp. Saúde Públ. 1949, 2, 793–808. [Google Scholar]
- Bennett, G.F. The influence of the blood meal type on the fecundity of Aedes aegypti L. (Stegomyia). (Diptera: Culicidae). Can. J. Zool. 1970, 48, 539–543. [Google Scholar] [CrossRef]
- Harrison, R.E.; Brown, M.R.; Strand, M.R. Whole blood and blood components from vertebrates differentially affect egg formation in three species of anautogenous mosquitoes. Parasites Vectors 2021, 14, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Altamiranda-Saavedra, M.; Conn, J.E.; Correa, M.M. Genetic structure and phenotypic variation of Anopheles darlingi in northwest Colombia. Infect. Genet. Evol. 2017, 56, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.H.; Galardo, A.K.R.; Lounibos, L.P.; Arruda, M.; Wirtz, R. Bloodmeal Hosts of Anopheles Species (Diptera: Culicidae) in a Malaria-Endemic Area of the Brazilian Amazon. J. Med. Entomol. 2006, 43, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita, S.; Álvarez, N.; Naranjo-Díaz, N.; Bickersmith, S.; Conn, J.E.; Correa, M.M. Anopheles blood meal sources and entomological indicators related to Plasmodium transmission in malaria endemic areas of Colombia. Acta Trop. 2022, 233, 106567. [Google Scholar] [CrossRef]
- Mirabello, L.; Vineis, J.H.; Yanoviak, S.P.; Scarpassa, V.M.; Póvoa, M.M.; Padilla, N.; Achee, N.L.; E Conn, J. Microsatellite data suggest significant population structure and differentiation within the malaria vector Anopheles darlingi in Central and South America. BMC Ecol. 2008, 8, 3–18. [Google Scholar] [CrossRef]
- Emerson, K.J.; Conn, J.E.; Bergo, E.S.; Randel, M.A.; Sallum, M.A.M. Brazilian Anopheles darlingi Root (Diptera: Culicidae) Clusters by Major Biogeographical Region. PLoS ONE. 2015, 10, e0130773. [Google Scholar] [CrossRef]
- Conn, J.E.; Ribolla, P.E. Ecology of Anopheles darlingi, the Primary Malaria Vector in the Americas and Current Nongenetic Methods of Vector Control. In Genetic Control of Malaria and Dengu; Adelman, Z.N., Ed.; Academic Press: Oxford, UK, 2016; pp. 81–102. [Google Scholar] [CrossRef]
| Parameters | Initial n | Biological Replicates | Rabbit | Chicken | ||
|---|---|---|---|---|---|---|
| Anesthetized (±SEM) | Non-Anesthetized (±SEM) | Anesthetized (±SEM) | Non-Anesthetized (±SEM) | |||
| Feeding rate (%) | 100 females | 2 | 80.8 (2.8) | 76.4 (2.5) | 72.0 (8.0) | 80.3 (7.8) |
| Survival rate (%) | 2 | 92.4 (2.9) | 94.8 (2.0) | 87.5 (4.8) | 92.0 (2.8) | |
| Fecundity (mean of eggs/female) | 2 | 36.3 (6.2) | 35.7 (6) | 15.4 (5.7) | 19.6 (5.9) | |
| Hatching rate (%) | 2 | 70.8 (7.0) | 79.7 (4.2) | 64.4 (5.1) | 64.1 (5.0) | |
| Pupation rate (%) | 200 larvae | 2 | 75.4 (8.2) | 80.6 (6.9) | 54.8 (4.5) | 66.3 (10.4) |
| Adult emergence rate (%) | 2 | 93.6 (1.8) | 91.6 (1.9) | - | - | |
| Parameters | Initial n | Biological Replicates | Blood Type | p-Value (ANOVA) | ||
|---|---|---|---|---|---|---|
| Human (±SEM) | Chicken (±SEM) | Rabbit (±SEM) | ||||
| Feeding rate (%) | 100 females | 2 | 92.6 (4.0) | 88.8 (6.2) | 77.2 (6.8) | 0.200 |
| Survival rate (%) | - | 2 | 94.1 (1.8) | 86.8 (5.7) | 91.2 (2.3) | 0.411 |
| Fecundity (mean of eggs/female) | - | 2 | 11.5 (3.0) | 12.3 (2.1) | 12.9 (3.3) | 0.943 |
| Hatching rate (%) | - | 2 | 54.4 (9.0) | 49.8 (10.5) | 50.4 (12.2) | 0.947 |
| Pupation rate (%) | 200 larvae | 2 | 78.7 (2.9) | 72.5 (2.5) | 83.5 (4.9) | 0.150 |
| Adult emergence rate (%) | - | 2 | 97.4 (1.4) | 96.5 (1.5) | 97.1 (1.5) | 0.908 |
| Parameters | Initial n | Blood Type | p-Value (ANOVA) | ||||
|---|---|---|---|---|---|---|---|
| Mice (±SEM) | Rabbit (±SEM) | Chicken (±SEM) | Bovine (±SEM) | Human (±SEM) | |||
| Feeding rate (%) | 100 females | 72.4 (17.0) | 75.0 (3.5) | 75.4 (0.4) | 80.5 (6.6) | 74.1 (7.4) | 0.920 K |
| Survival rate (%) | - | 90.8 (3.4) | 98.2 (0.3) | 97.9 (0.4) | 83.7 (13.4) | 96.5 (1.0) | 0.448 |
| Fecundity (mean of eggs/female) | - | 100.2 (2.5) | 74.6 (9.6) | 67.0 (13.7) | 88.3 (18.4) | 73.9 (4.8) | 0.270 |
| Hatching rate (%) | - | 76.4 (10.2) | 78.2 (4.7) | 74.1 (5.1) | 64.1 (3.6) | 73.9 (4.8) | 0.557 |
| Pupation rate (%) | 400 larvae | 88.1 (3.7) | 85.6 (6.0) | 91.5 (3.6) | 78.9 (10.0) | 88.4 (5.3) | 0.675 |
| Adult emergence rate (%) | - | 98.3 (0.10) | 98.2 (0.6) | 98.6 (0.2) | 98.6 (0.5) | 99.0 (0.3) | 0.667 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontual, J.D.C.; Coelho, N.V.; Santos, N.A.C.d.; Bastos, A.d.S.; Araújo, J.E.; Andrade, A.O.; Medeiros, J.F.; Araujo, M.d.S. Blood Source and Anesthetics Effects on the Maintenance of Anopheles darlingi in the Lab-Rearing Condition. Insects 2025, 16, 281. https://doi.org/10.3390/insects16030281
Pontual JDC, Coelho NV, Santos NACd, Bastos AdS, Araújo JE, Andrade AO, Medeiros JF, Araujo MdS. Blood Source and Anesthetics Effects on the Maintenance of Anopheles darlingi in the Lab-Rearing Condition. Insects. 2025; 16(3):281. https://doi.org/10.3390/insects16030281
Chicago/Turabian StylePontual, José Daniel Costa, Natália Vitória Coelho, Najara Akira Costa dos Santos, Alessandra da Silva Bastos, Jéssica Evangelista Araújo, Alice Oliveira Andrade, Jansen Fernandes Medeiros, and Maisa da Silva Araujo. 2025. "Blood Source and Anesthetics Effects on the Maintenance of Anopheles darlingi in the Lab-Rearing Condition" Insects 16, no. 3: 281. https://doi.org/10.3390/insects16030281
APA StylePontual, J. D. C., Coelho, N. V., Santos, N. A. C. d., Bastos, A. d. S., Araújo, J. E., Andrade, A. O., Medeiros, J. F., & Araujo, M. d. S. (2025). Blood Source and Anesthetics Effects on the Maintenance of Anopheles darlingi in the Lab-Rearing Condition. Insects, 16(3), 281. https://doi.org/10.3390/insects16030281






