Gone with Water or Mountain: The Population Genetic Diversity of Rhopalopsole sinensis Yang and Yang, 1993 in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
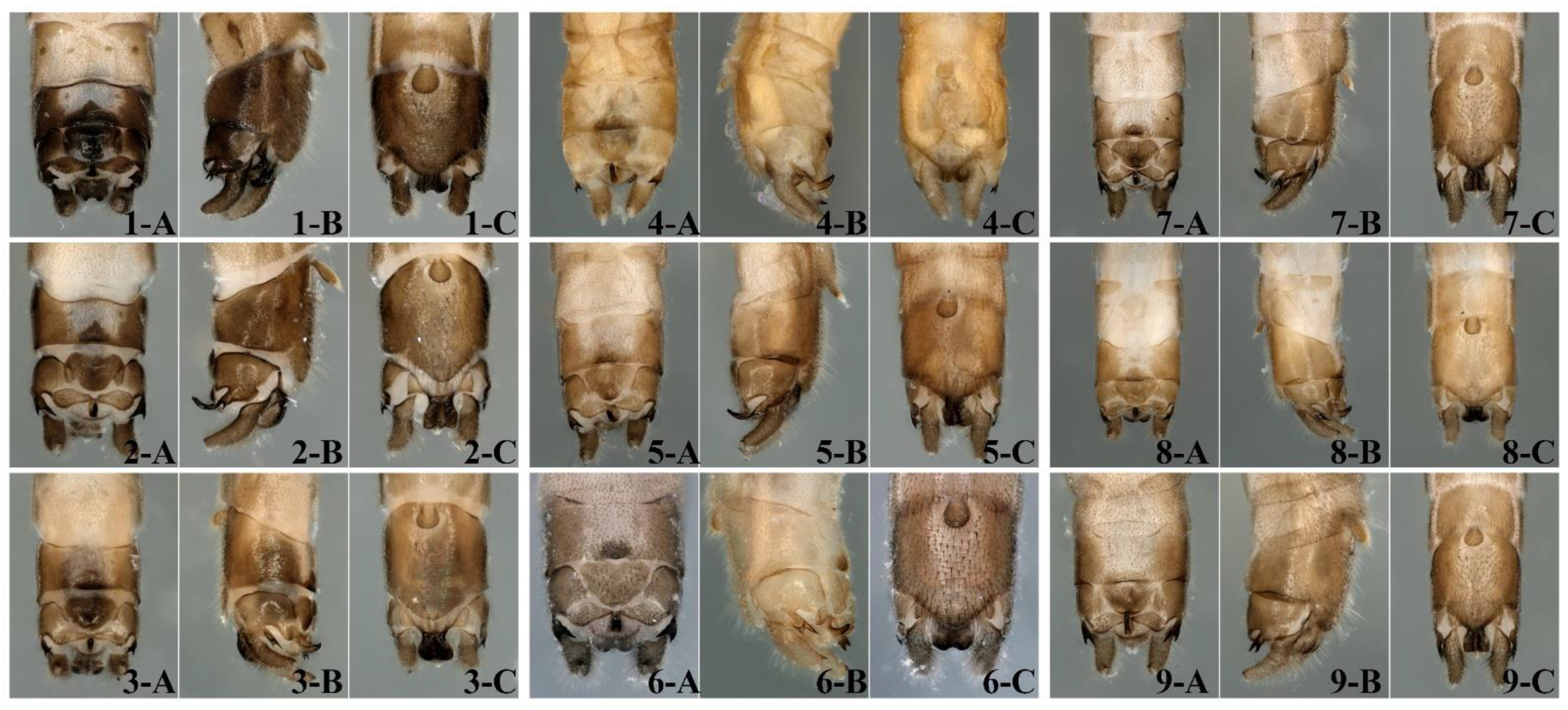
2.2. Observation and Description
2.3. DNA Extraction, Amplification and Sequencing
2.4. Sequential Analysis
2.5. Population Relationship Analyses
2.6. Population Genetic Structure Analyses
2.7. Demographic History Analyses
2.8. Niche Model Analysis
3. Results
3.1. High Level of Genetic Diversity
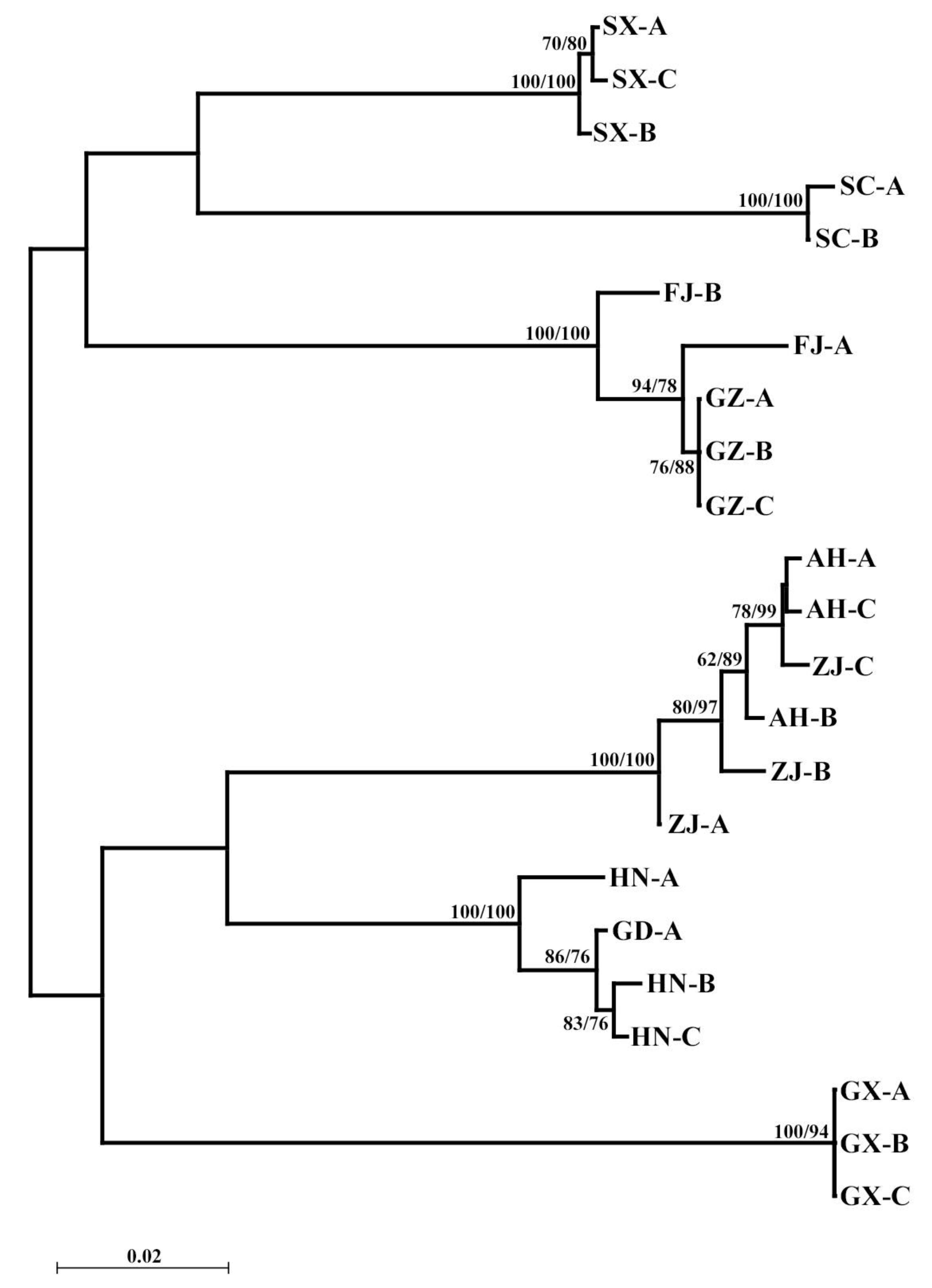
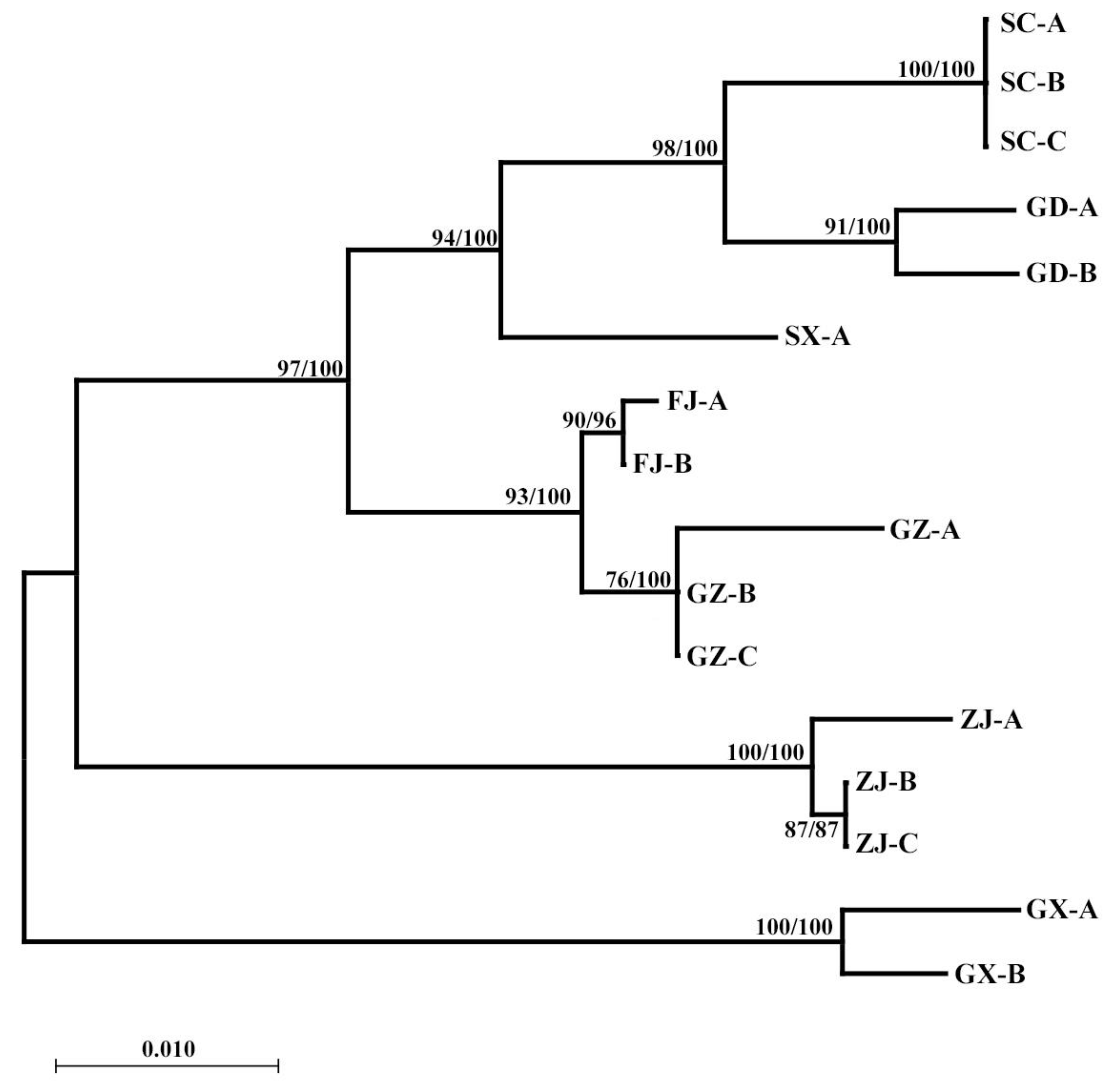
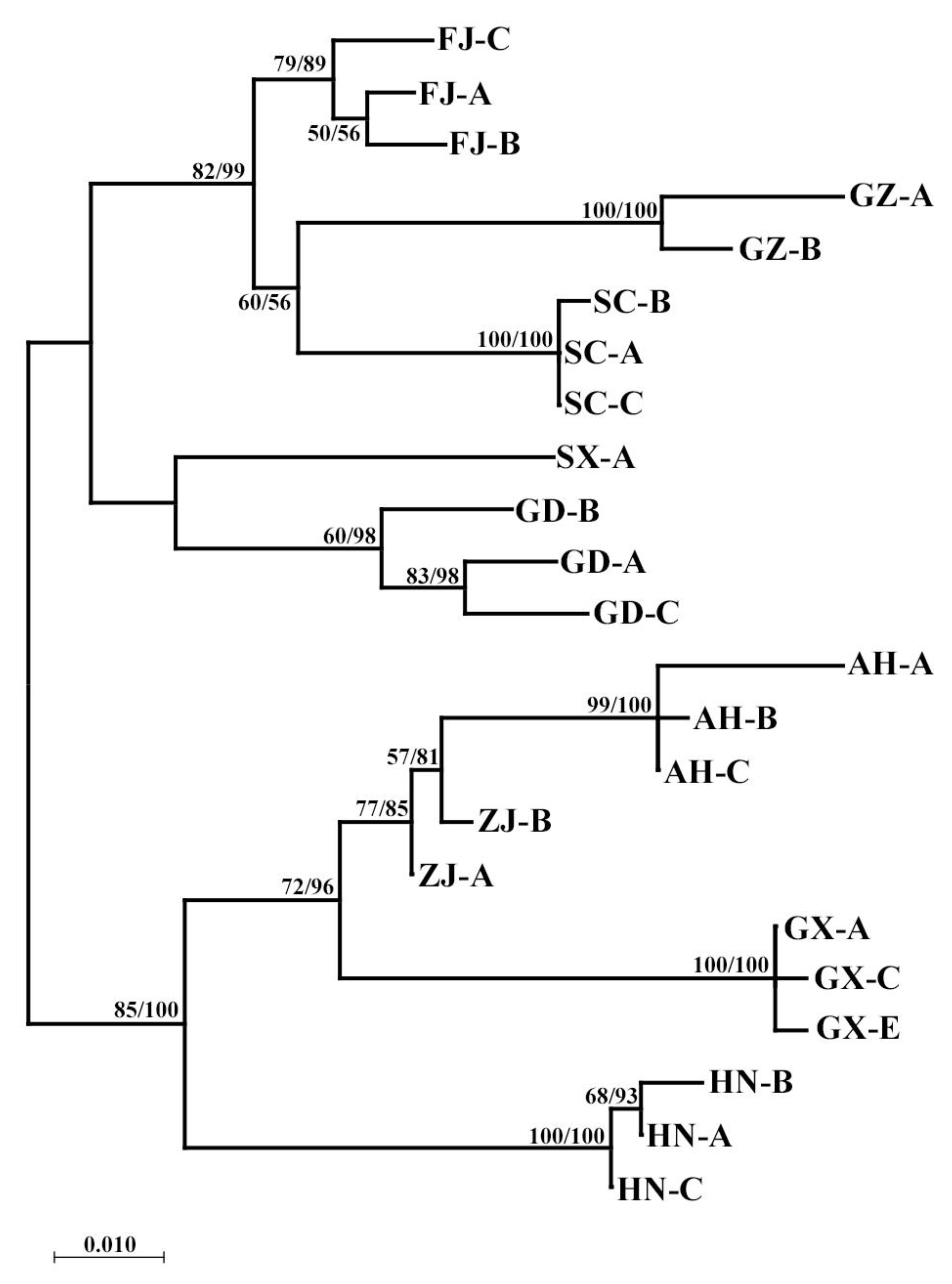
3.2. COI Gene
3.3. 18S Gene
3.4. ITS2 Gene
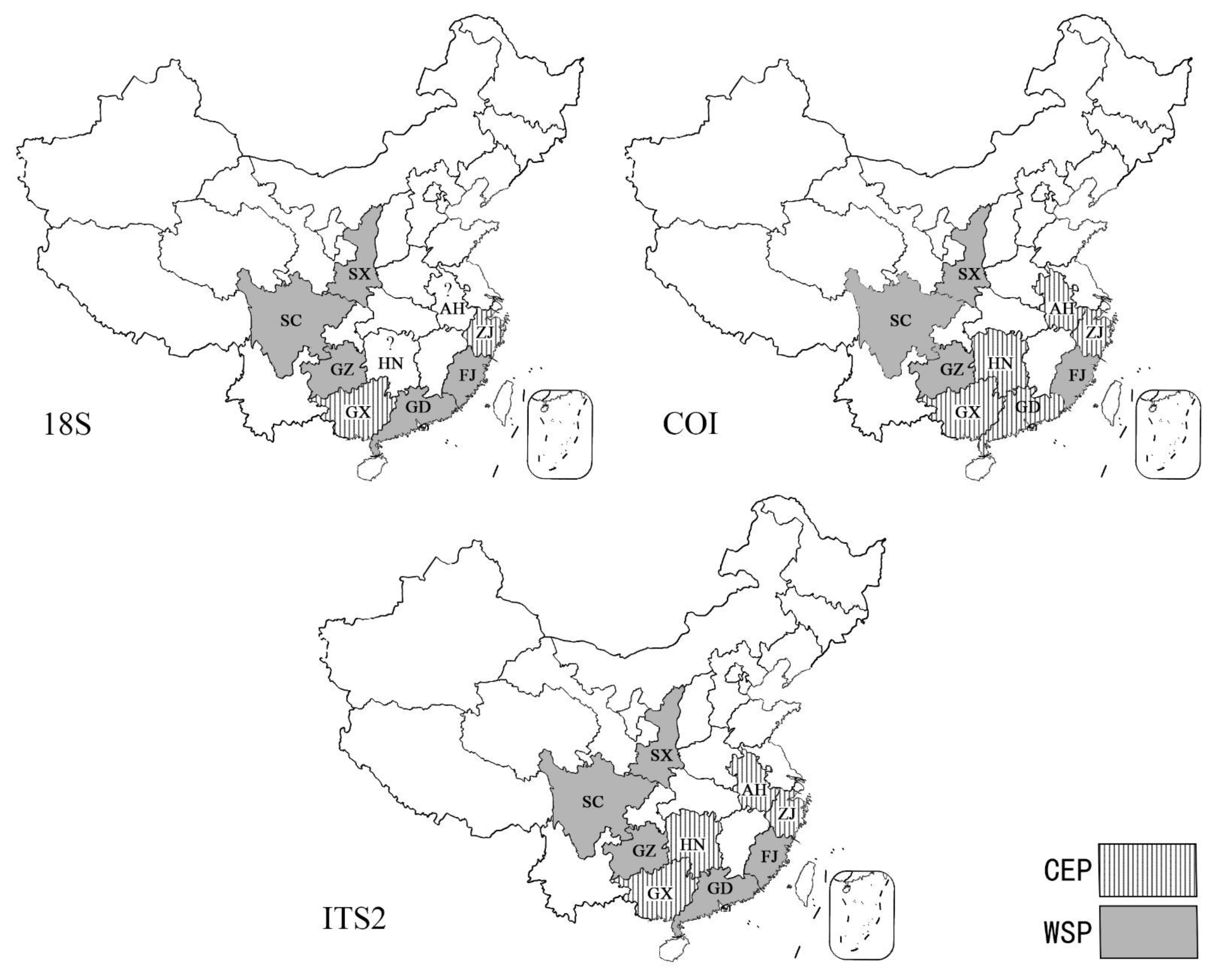
3.5. Population Genetic Structure
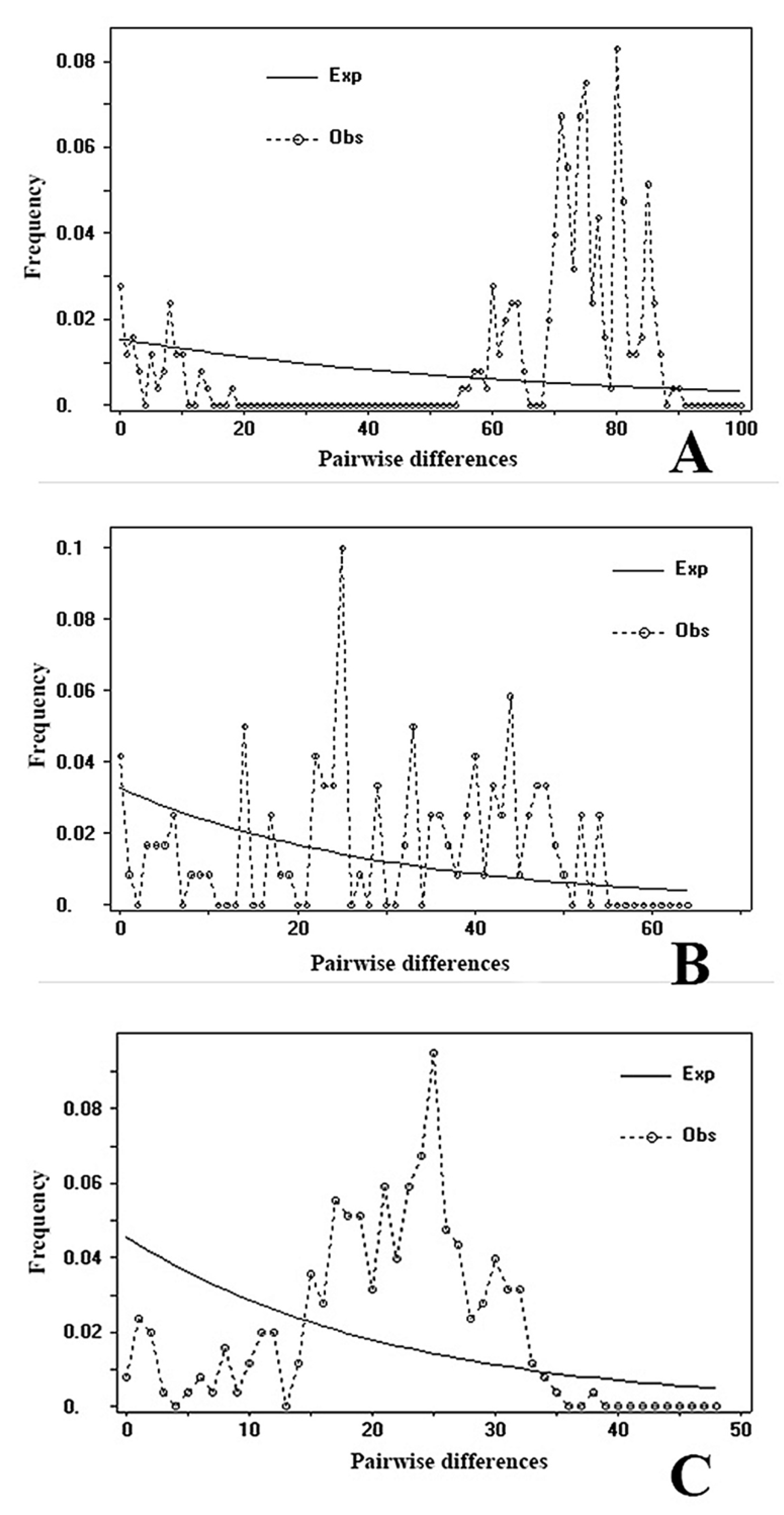
3.6. Demographic History
4. Conclusions
5. Discussion
5.1. Molecular Evolution
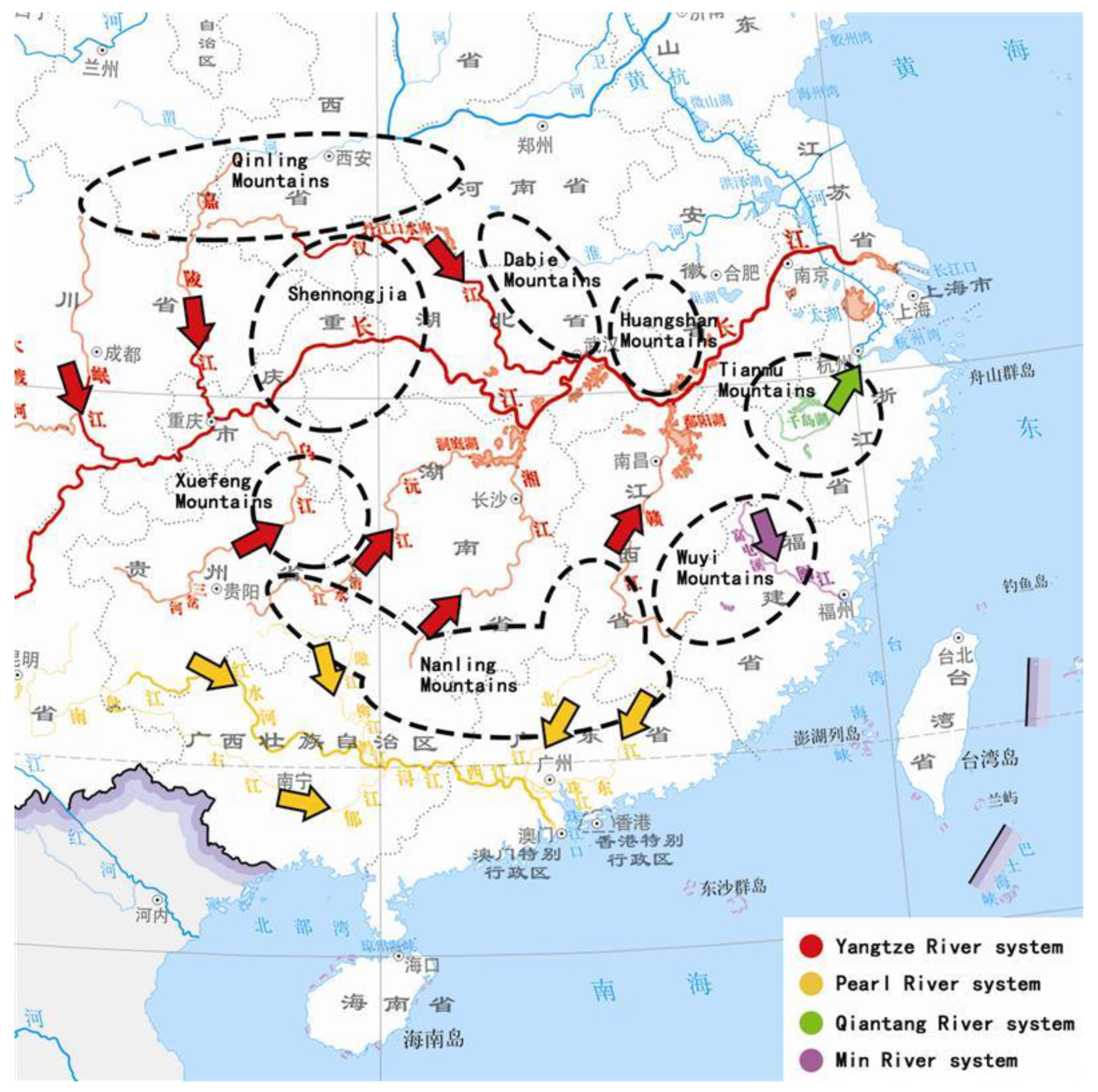
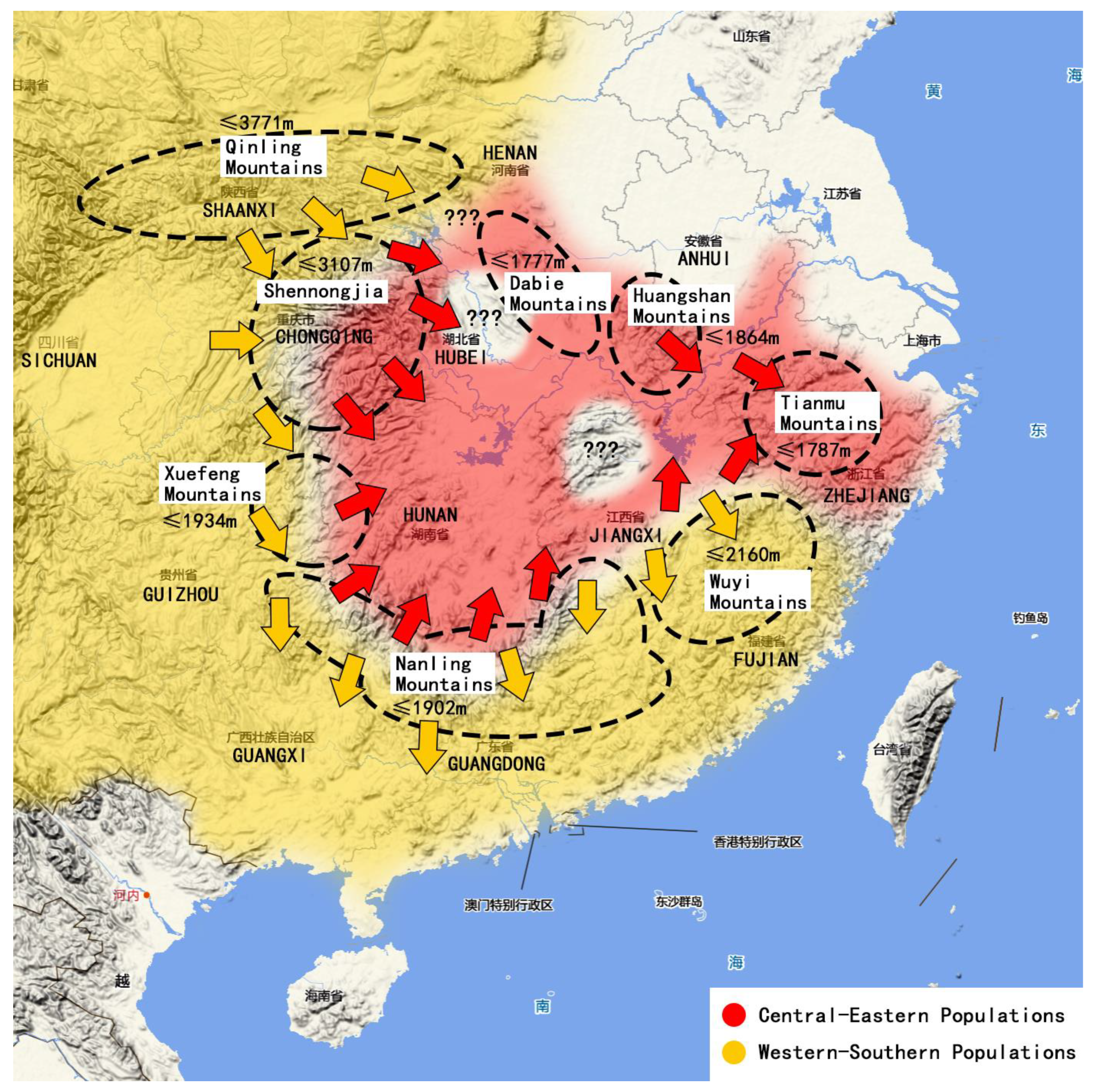
5.2. The Origin and Immigration
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeWalt, R.E.; Ower, G.D. Ecosystem Services, Global Diversity, and Rate of Stonefly Species Descriptions (Insecta: Plecoptera). Insects 2019, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- South, E.J.; Skinner, R.K.; DeWalt, R.E.; Davis, M.A.; Johnson, K.P.; Teslenko, V.A.; Lee, J.J.; Malison, R.L.; Hwang, J.M.; Bae, Y.J.; et al. A New Family of Stoneflies (Insecta: Plecoptera), Kathroperlidae, fam. n., with a Phylogenomic Analysis of the Paraperlinae (Plecoptera: Chloroperlidae). Insect Syst. Divers. 2021, 5, 1–27. [Google Scholar] [CrossRef]
- DeWalt, R.E.; Hopkins, H.; Neu-Becker, U.; Stueber, G. Plecoptera Species File. Available online: https://plecoptera.speciesfile.org (accessed on 10 February 2025).
- Béthoux, O.; Cui, Y.; Kondratieff, B.; Stark, B.; Ren, D. At last, a Pennsylvanian stem-stonefly (Plecoptera) discovered. BMC Ecol. Evol. 2011, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Fochetti, R.; Tierno de Figueroa, J.M. Global diversity of stoneflies (Plecoptera: Insecta) in freshwater. Hydrobiologia 2008, 595, 365–377. [Google Scholar] [CrossRef]
- South, E.J.; Skinner, R.K.; DeWalt, R.E.; Kondratieff, B.C.; Johnson, K.P.; Davis, M.A.; Lee, J.J.; Durfee, R.S. Phylogenomics of the North American Plecoptera. Syst. Entomol. 2020, 46, 287–305. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, D.S.; Hwang, S.J.; Lee, K.L.; Park, Y.S. Distribution patterns and vulnerability of stoneflies (Plecoptera: Insecta) in South Korean streams with conservation perspectives. Glob. Ecol. Conserv. 2022, 34, e02030. [Google Scholar] [CrossRef]
- Murányi, D. Balkanian species of the genus Isoperla Banks, 1906 (Plecoptera: Perlodidae). Zootaxa 2011, 3049, 1–46. [Google Scholar] [CrossRef]
- Graf, W.; Popijač, A.; Previšić, A.; Gamboa, M.; Kučinić, M. Contribution to the knowledge of Siphonoperla in Europe (Plecoptera: Chloroperlidae): Siphonoperla korab sp. n. Zootaxa 2012, 3164, 41–48. [Google Scholar] [CrossRef]
- Pessino, M.; Chabot, E.T.; Giordano, R.; DeWalt, R.E. Refugia and postglacial expansion of Acroneuria frisoni Stark & Brown (Plecoptera: Perlidae) in North America. Freshw. Sci. 2014, 33, 232–249. [Google Scholar]
- Stevens, D.M.; Bishop, J.; Picker, M.D. Phylogenetic analysis reveals high local endemism and clear biogeographic breaks in southern African stoneflies (Notonemouridae, Plecoptera). Zootaxa 2018, 4483, 428–454. [Google Scholar] [CrossRef]
- Gamboa, M.; Murányi, D.; Kanmori, S.; Watanabe, K. Molecular phylogeny and diversification timing of the Nemouridae family (Insecta, Plecoptera) in the Japanese Archipelago. PLoS ONE 2019, 14, e0210269. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, W.H. Species Catalogue of China. Vols. 2 Animals, Insecta (III), Plecoptera; Science Press: Beijing, China, 2018; pp. 1–71. [Google Scholar]
- Yang, Y.-B.; Du, Y.-Z. Three new synonyms of Rhopalopsole sinensis Yang & Yang, 1993 (Plecoptera: Leuctridae). Zootaxa 2021, 5040, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Elwess, N.L.; Latourelle, S.M.; Myers, L. DNA barcoding of stoneflies (Plecoptera) in a general genetics course. J. Biol. Educ. 2017, 52, 406–414. [Google Scholar] [CrossRef]
- Duarte, T.; Calor, A.R.; Bispo, P.d.C. Systematic revision and phylogeny of Paragripopteryx Enderlein, 1909 (Plecoptera: Gripopterygidae). PLoS ONE 2022, 17, e0264264. [Google Scholar] [CrossRef]
- Teslenko, V.A.; Palatov, D.M.; Semenchenko, A.A. Overview of the Caucasian Perla Geoffroy, 1762 (Plecoptera: Perlidae) based on morphological and molecular data with description of two new species. Zootaxa 2024, 5507, 1–56. [Google Scholar] [CrossRef]
- Hlebec, D.; Sivec, I.; Podnar, M.; Kučinić, M. DNA barcoding for biodiversity assessment: Croatian stoneflies (Insecta: Plecoptera). Peer J 2022, 10, e13213. [Google Scholar] [CrossRef]
- Vuataz, L.; Reding, J.P.; Reding, A.; Roesti, C.; Stoffel, C.; Vinçon, G.; Gattolliat, J.L. A comprehensive DNA barcoding reference database for Plecoptera of Switzerland. Sci. Rep. 2024, 14, 6322. [Google Scholar] [CrossRef]
- Kermek, D.; Pischiutta, N.; Hlebec, D.; Sivec, I.; Kučinić, M. Utilising public sequence databases to investigate genetic diversity of stoneflies in Medvednica Nature Park. Biodivers. Data J. 2024, 12, e121398. [Google Scholar] [CrossRef]
- Ellstrand, N.C. Is gene flow the most important evolutionary force in plants? Am. J. Bot. 2014, 101, 737–753. [Google Scholar] [CrossRef]
- Garrido, J.L.; Fenu, G.; Mattana, E.; Bacchetta, G. Spatial genetic structure of aquilegia taxa endemic to the Island of Sardinia. Ann. Bot. 2012, 109, 953–964. [Google Scholar] [CrossRef]
- Saeb, A.; Al-Naqeb, D. The impact of evolutionary driving forces on human complex diseases: A population genetics approach. Scientifica 2016, 2016, 2079704. [Google Scholar] [CrossRef] [PubMed]
- Sork, V.L. Gene flow and natural selection shape spatial patterns of genes in tree populations: Implications for evolutionary processes and applications. Evol. Appl. 2016, 9, 291–310. [Google Scholar] [CrossRef]
- Mynott, J.H. Mitochondrial DNA allows the association of life stages to facilitate species recognition and delimitation in Australian stoneflies (Plecoptera: Gripopterygidae: Newmanoperla). Invertebr. Syst. 2015, 29, 223–238. [Google Scholar] [CrossRef]
- Boumans, L.; Tierno de Figueroa, J.M. Introgression and species demarcation in western European Leuctra fusca (Linnaeus, 1758) and L. digitata Kempny, 1899 (Plecoptera: Leuctridae). Aquat. Insects 2016, 37, 115–126. [Google Scholar] [CrossRef]
- Tang, X.T.; Lu, M.X.; Du, Y.Z. Molecular phylogeography and evolutionary history of the pink rice borer (Lepidoptera: Noctuidae): Implications for refugia identification and pest management. Syst. Entomol. 2021, 47, 371–383. [Google Scholar] [CrossRef]
- Hackett, B.J.; Gimnig, J.; Guelbeogo, W.; Costantini, C.; Koekemoer, L.L.; Coetzee, M.; Collins, F.H.; Besansky, N.J. Ribosomal DNA internal transcribed spacer (ITS2) sequences differentiate Anopheles funestus and An. rivulorum, and uncover a cryptic taxon. Insect Mol. Biol. 2000, 4, 369–374. [Google Scholar] [CrossRef]
- Vahtera, V.; Muona, J. The molecular phylogeny of the Miarus campamnulae (Coleoptera: Curculionidae) species group inferred from COI and ITS2 sequences. Cladistics 2006, 22, 222–229. [Google Scholar] [CrossRef]
- Wang, L.P.; Du, Y.Z.; He, Y.T.; Lu, Y.J.; Lu, Z.Q. Sequence analysis and comparison of ITS1 in different geographical populations of American leaf miner. Acta Entomol. Sin. 2007, 50, 597–603. (In Chinese) [Google Scholar]
- Pairot, P.; Piyamas, N. Phylogenetic analysis based on multiple gene sequencesrevealing cryptic biodiversity in Simulium mulistriatum group (Diptera: Simulidae) in Thailand. Entomol. Sci. 2012, 15, 202–213. [Google Scholar]
- Yang, Y.B.; Du, Y.Z. Two new synonyms of Paraleuctra orientalis (Chu, 1928) (Plecoptera: Leuctridae) based on morphological and molecular data, with notes on Paraleuctra cervicornis Du & Qian, 2012. Insects 2022, 13, 468. [Google Scholar] [CrossRef]
- Grubbs, S.A.; DeWalt, R.E.; Hart, L.V.; Layer, M.R. Systematics and updated range alter the conservation status of the Louisiana Needlefly, Leuctra szczytkoi Stark & Stewart, 1981 (Plecoptera: Leuctridae). Zoosymposium 2023, 24, 137–148. [Google Scholar] [CrossRef]
- Verdone, C.J.; South, E.J.; Kondratieff, B.C. Acroneuria kirchneri Stark & Kondratieff, 2004 is a synonym of Acroneuria kosztarabi Kondratieff & Kirchner, 1993 (Plecoptera: Perlidae). Zootaxa 2022, 5094, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Verdone, C.J.; Beaty, S.R.; Holland, V.B.; Williams, B.W. Isoperla riverae, a new stonefly species from the southeast Nearctic, with notes on sympatric species including the larval description of Isoperla lenati Szczytko & Kondratieff, 2015 (Plecoptera: Perlodidae). Zootaxa 2023, 5270, 437–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.T.; Jiang, C.; Li, Y.S. DNA barcodes of Plecoptera from China: The preliminary dataset and its performance in species delimitation. Zootaxa 2020, 4751, 345–356. [Google Scholar] [CrossRef]
- Yang, Y.-B.; Du, Y.-Z. A new synonym species with description of a new species of Rhopalopsole from China (Plecoptera: Leuctridae). Eur. Zool. J. 2022, 89, 190–196. [Google Scholar] [CrossRef]
- Chenna, R.; Sugawara, H.; Koike, T.; Lopez, R.; Gibson, T.J.; Higgins, D.G.; Thompson, J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003, 31, 3497–3500. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Chen, Y.K.; Wang, Y.; Li, J.L.; Wang, W.T.; Feng, D.Y.; Mao, K.S. Principles, error sources and application suggestions of prevailing molecular dating methods. Biodivers. Sci. 2021, 29, 629–646. (In Chinese) [Google Scholar] [CrossRef]
- Lin, X.; Stur, E.; Ekrem, T. Exploring genetic divergence in a species-rich insect genus using 2790 DNA barcodes. PLoS ONE 2015, 10, e0138993. [Google Scholar] [CrossRef]
- Lin, C.P.; Danforth, B.N. How do insect nuclear and mitochondrial gene substitution patterns differ? Insights from Bayesian analyses of combined datasets. Mol. Phylogenet. Evol. 2004, 30, 686–702. [Google Scholar] [CrossRef]
- Linnen, C.R.; Farrell, B.D. Mitonuclear discordance is caused by rampant mitochondrial introgression in Neodiprion (Hymenoptera: Diprionidae) sawflies. Evolution 2007, 61, 1417–1438. [Google Scholar] [CrossRef]
- Du, Y.Z. A Taxonomic Study on Plecoptera from China; Postdoctoral Research Report; Zhejiang University: Hangzhou, China, 1999; pp. 1–324. [Google Scholar]
- Yang, D.; Li, W.H.; Zhu, F. Fauna Sinica, Insecta. Plecoptera: Nemouroidea; Science Press: Beijing, China, 2015; Volume 58, 518p. [Google Scholar]
- Du, Y.Z. Advances in Plecoptera systematics in China. Chin. J. Appl. Entomol. 2020, 57, 284–297. [Google Scholar]
- Zheng, H.; Clift, P.D.; Wang, P.; Tada, R.; Jia, J.; He, M.; Jourdan, F. Pre-Miocene birth of the Yangtze River. Proc. Natl. Acad. Sci. USA 2013, 110, 7556–7561. [Google Scholar] [CrossRef]
- Zheng, H.B.; Clift, P.D.; He, M.Y.; Bian, Z.X.; Liu, G.Z.; Liu, X.C.; Xia, L.; Yang, Q.; Jourdan, F. Formation of the First Bend in the late Eocene gave birth to the modern Yangtze River, China. Geology 2020, 49, 35–39. [Google Scholar] [CrossRef]
- Dong, Y.P.; Hui, B.; Sun, S.S.; Yang, Z.; Zhang, F.F.; He, D.F.; Sun, J.P.; Shi, X.H. Multiple orogeny and geodynamics from Proto-Tethys to Paleo-Tethys of the Central China Orogenic Belt. Acta Geol. Sin. 2022, 96, 3426–3448. [Google Scholar]
- Zwick, P. Phylogenetic System and Zoogeography of the Plecoptera. Annu. Rev. Entomol. 2000, 45, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Heiman, D.R.; Knight, A.W. Upper-lethal-temperature relations of the nymphs of the stonefly, Paragnetina media. Hydrobiologia 1972, 39, 479–493. [Google Scholar] [CrossRef]
- DeWalt, R.E.; Favret, C.; Webb, D.W. Just how imperiled are aquatic insects? A case study of stoneflies (Plecoptera) in Illinois. Ann. Entomol. Soc. Am. 2005, 98, 941–950. [Google Scholar] [CrossRef]

| Primer | Sequence |
|---|---|
| COⅠ-LCO1490 | GGTCAACAAATCATAAAGATATTGG |
| COⅠ-HCO2198 | TAAACTTCAGGGTGACCAAAAAATCA |
| 18S-a0.7 | ATTAAAGTTGTTGCGGTT |
| 18S-b2.9 | TATCTGATCGCCTTCGAACCTCT |
| ITS2-Forward | GCATCGATGAAG AACGCAGC |
| ITS2-Reverse | TCCTCCGCTTATTGATATGC |
| Gene Name | Sample Size | Number of Polymorphic Sites | Average number of Nucleotide Differences (K) | Haplotype Number | Haplotype Diversity (Hd) | Mean Nucleotide Diversity (π) |
|---|---|---|---|---|---|---|
| COI | 23 | 169 | 63.972 | 18 | 0.972 | 0.09678 |
| 18S | 16 | 98 | 29.683 | 12 | 0.958 | 0.04645 |
| ITS2 | 23 | 75 | 20.984 | 21 | 0.992 | 0.06082 |
| Gene Name | Tajima’s D Test | Fu’s Fs Test |
|---|---|---|
| COⅠ | 0.70655 (p > 0.10) | 2.361 (p > 0.10) |
| 18S | −0.10697 (p > 0.10) | 1.639 (p > 0.10) |
| ITS2 | −0.51755 (p > 0.10) | −4.771 (p > 0.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Q.-B.; Yang, Y.-B.; Eichert, A.; Du, Y.-Z. Gone with Water or Mountain: The Population Genetic Diversity of Rhopalopsole sinensis Yang and Yang, 1993 in China. Insects 2025, 16, 244. https://doi.org/10.3390/insects16030244
Huo Q-B, Yang Y-B, Eichert A, Du Y-Z. Gone with Water or Mountain: The Population Genetic Diversity of Rhopalopsole sinensis Yang and Yang, 1993 in China. Insects. 2025; 16(3):244. https://doi.org/10.3390/insects16030244
Chicago/Turabian StyleHuo, Qing-Bo, Yu-Ben Yang, Anna Eichert, and Yu-Zhou Du. 2025. "Gone with Water or Mountain: The Population Genetic Diversity of Rhopalopsole sinensis Yang and Yang, 1993 in China" Insects 16, no. 3: 244. https://doi.org/10.3390/insects16030244
APA StyleHuo, Q.-B., Yang, Y.-B., Eichert, A., & Du, Y.-Z. (2025). Gone with Water or Mountain: The Population Genetic Diversity of Rhopalopsole sinensis Yang and Yang, 1993 in China. Insects, 16(3), 244. https://doi.org/10.3390/insects16030244







