Biological Control Potential of the Reduviid Predator Rhynocoris fuscipes (Fabricius) in Managing Noctuid Pests: Insights Into Predation and Prey Preference
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Colonies
2.2. Experimental Conditions
2.3. Functional Response
2.4. Intraspecific Interference Competition
2.5. Prey Preference
2.6. Statistical Analyses
3. Results
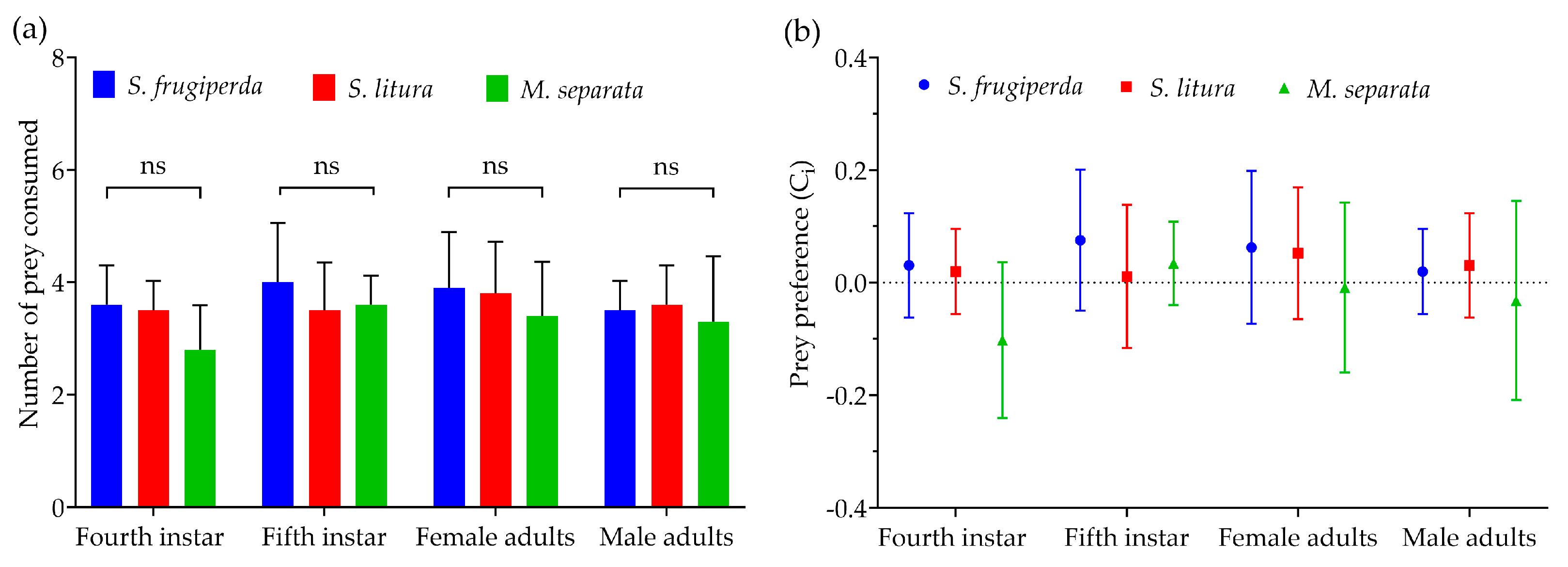
3.1. Prey Consumption
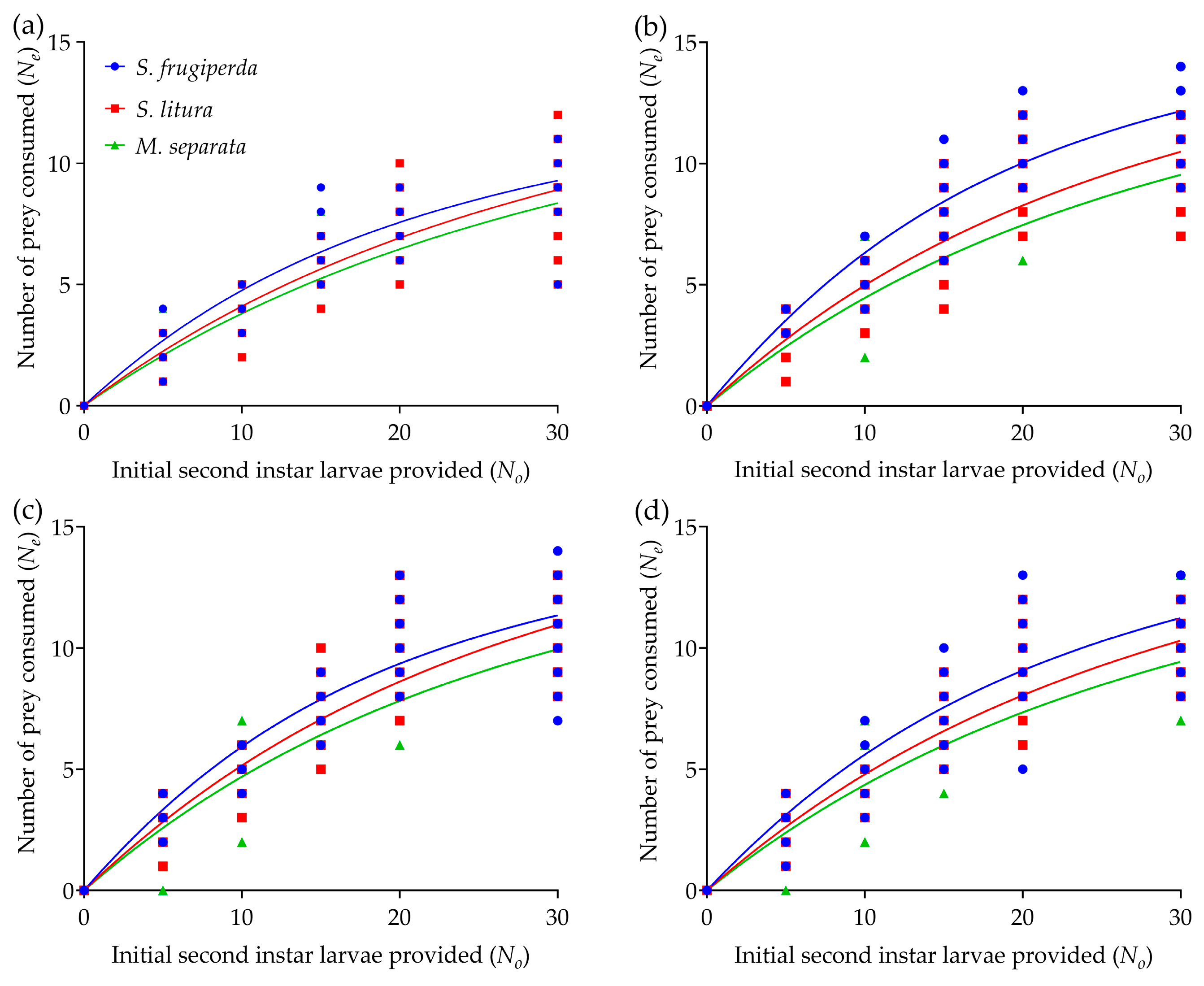
3.2. Functional Response
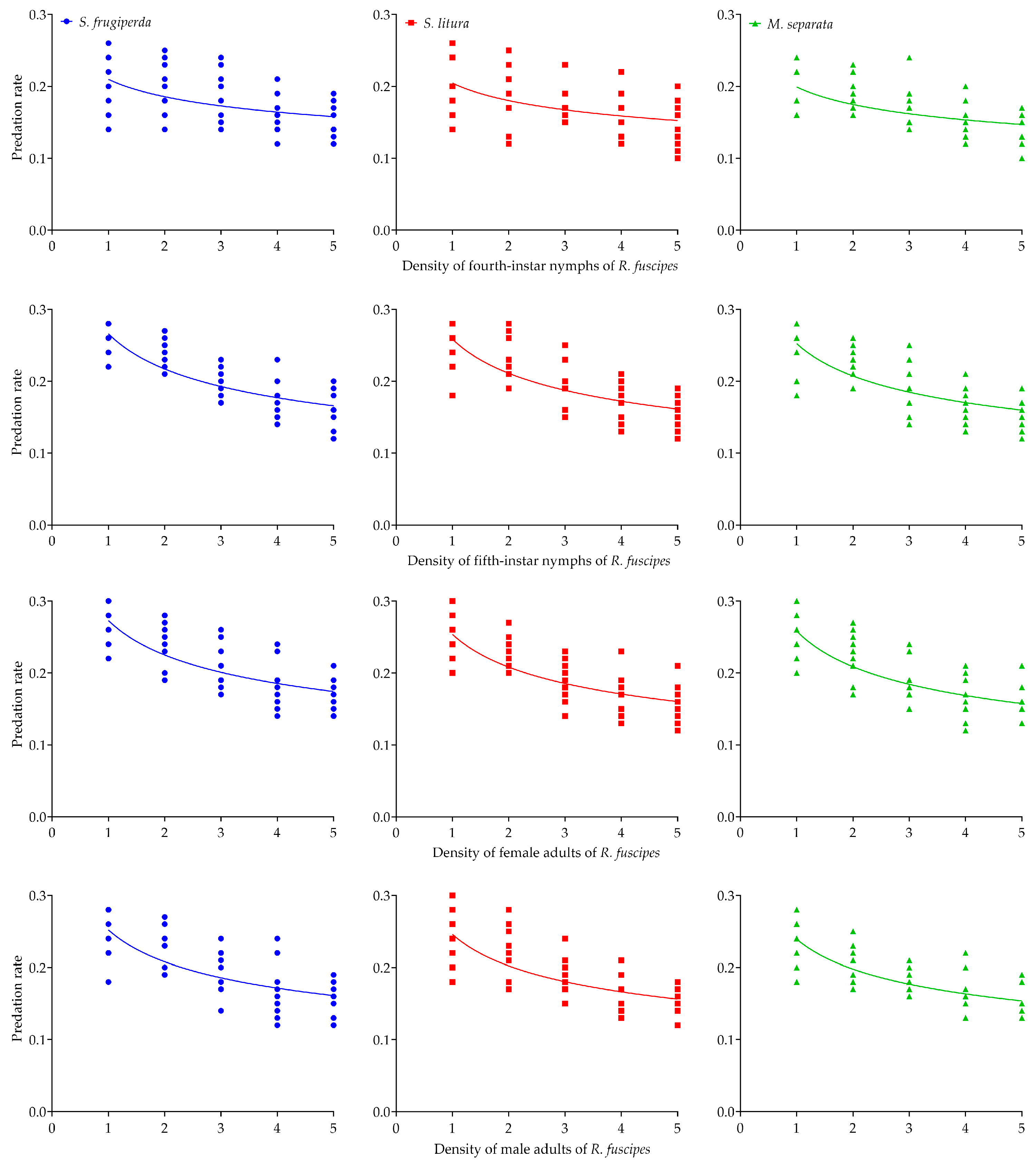
3.3. Intraspecific Interference Competition
3.4. Prey Preference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chimweta, M.; Nyakudya, I.W.; Jimu, L.; Mashingaidze, A.B. Fall armyworm [Spodoptera frugiperda (J.E. Smith)] damage in maize: Management options for flood-recession cropping smallholder farmers. Int. J. Pest Manag. 2020, 66, 142–154. [Google Scholar] [CrossRef]
- Li, Y.Y.; Qu, C.; Zhang, Q.H.; Zhang, L.P.; Luo, C.; Wang, R. Baseline Susceptibility, Cross-Resistance, and Sublethal Effects of Broflanilide, a Novel Meta-Diamide Pesticide, in Spodoptera litura. Int. J. Mol. Sci. 2023, 24, 5351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Liu, H.W.; Liu, Y.P.; Wang, C.; Ma, B.W.; Zhang, M.J.; Zhang, Y.; Liu, Y.; Yang, B.; Wang, S.; et al. Chromosome-level genomes of two armyworms, Mythimna separata and Mythimna loreyi, provide insights into the biosynthesis and reception of sex pheromones. Mol. Ecol. Resour. 2023, 23, 1423–1441. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.P.; Guo, J.F.; Jiang, Y.Y.; Zhao, J.Z.; Sethi, A.; He, K.L.; Wang, Z.Y. Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in cornfields using molecular techniques. Insect Sci. 2020, 27, 780–790. [Google Scholar] [CrossRef]
- Lu, Y.H.; Tian, J.; Ullah, F.; Desneux, N.; Guo, J.W.; Wang, S.S.; Xu, H.X. Sublethal and transgenerational effects of lufenuron on biological characteristics and expression of reproductive related genes in the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 2023, 196, 105593. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Jin, P.; Li, J.; Wang, J.; Shu, Y. Effects of Cd accumulation on cutworm Spodoptera Litura larvae via Cd treated Chinese flowering cabbage Brassica campestris and artificial diets. Chemosphere 2018, 200, 151–163. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.H.; Wang, J.; Liu, J.; Tang, Q.B.; Xiang-Rui, L.; Cheng, D.F.; Zhu, X. Analysis on the migration of first-generation Mythimna separata (Walker) in China in 2013. J. Integr. Agric. 2018, 17, 1527–1537. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Hoesain, M.; Suharto; Prastowo, S.; Pradana, A.P.; Alfarisy, F.K.; Adiwena, M. Investigating the plant metabolite potential as botanical insecticides against Spodoptera litura with different application methods. Cogent Food Agric. 2023, 9, 2229580. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, Q.W.; Ye, Z.H.; Luo, L.Z. The role of nectar plants in severe outbreaks of armyworm Mythimna separata (Lepidoptera: Noctuidae) in China. Bull. Entomol. Res. 2006, 96, 445–455. [Google Scholar] [CrossRef]
- Global Action for Fall Armyworm Control. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/fall-armyworm/background/zh/ (accessed on 5 April 2024).
- Zhou, L.; Meng, J.Y.; Ruan, H.Y.; Yang, C.L.; Zhang, C.Y. Expression stability of candidate RT-qPCR housekeeping genes in Spodoptera frugiperda (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 2021, 108, e21831. [Google Scholar] [CrossRef] [PubMed]
- Mallapur, C.P.; Naik, A.K.; Hagari, S.; Prabhu, S.T.; Patil, P.K. Status of alien pest fall armyworm, Spodoptera frugiperda (J.E. Smith) on maize in Northern Karnataka. J. Entomol. Zool. Stud. 2018, 6, 432–436. [Google Scholar]
- Zhou, Y.; Wu, Q.L.; Zhang, H.W.; Wu, K.M. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Gong, J.; Cheng, T.C.; Wu, Y.Q.; Yang, X.; Feng, Q.L.; Mita, K. Genome-wide patterns of copy number variations in Spodoptera litura. Genomics 2019, 111, 1231–1238. [Google Scholar] [CrossRef]
- Takatsuka, J.; Okuno, S.; Nakai, M.; Kunimi, Y. Genetic and phenotypic comparisons of viral genotypes from two nucleopolyhedroviruses interacting with a common host species, Spodoptera litura (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 2016, 139, 42–49. [Google Scholar] [CrossRef]
- Ming, L.; Du, Y.W.; Yuan, G.G.; Su, Q.; Shi, X.B.; Yu, H.; Chen, G. Spodoptera litura larvae are attracted by HvAV-3h-infected S. litura larvae-damaged pepper leaves. Pest Manag. Sci. 2023, 79, 2713–2724. [Google Scholar] [CrossRef]
- Xu, C.; Ji, J.C.; Zhu, X.Z.; Huangfu, N.; Xue, H.; Wang, L.; Zhang, K.X.; Li, D.Y.; Niu, L.; Chen, R.; et al. Chromosome level genome assembly of oriental armyworm Mythimna separata. Sci. Data 2023, 10, 597–608. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, Y.N.; Zhang, H.Z.; Zhang, M.S.; Wang, M.Q.; Mao, J.J.; Zhang, L.S. The green lacewing Chrysopa formosa as a potential biocontrol agent for managing Spodoptera frugiperda and Spodoptera litura. Bull. Entomol. Res. 2023, 113, 49–62. [Google Scholar] [CrossRef]
- Tian, T.A.; Yu, L.C.; Sun, G.J.; Xiao, F.Y.; Li, L.T.; Wu, C.X.; Chen, Y.C.; Yang, M.F.; Liu, J.F. Biological control efficiency of an ectoparasitic mite Pyemotes zhonghuajia on oriental armyworm Mythimna separata. Syst. Appl. Acarol. 2020, 25, 1683–1692. [Google Scholar]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Phillips, T.W.; Throne, J.E. Biorational approaches to managing stored-product insects. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Di, X.Y.; Yan, B.; Ren, P.; Wu, H.Z.; Yang, M.F. Parasitism success of Microplitis manilae (Hymenoptera: Braconidae) on different diet-fed Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. J. Appl. Entomol. 2023, 148, 199–204. [Google Scholar] [CrossRef]
- Sarkhandia, S.; Sharma, G.; Mahajan, R.; Koundal, S.; Kumar, M.; Chadha, P.; Saini, H.S.; Kaur, S. Synergistic and additive interactions of Shewanella sp., Pseudomonas sp. and Thauera sp. with chlorantraniliprole and emamectin benzoate for controlling Spodoptera litura (Fabricius). Sci. Rep. 2023, 13, 14648. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Freed, S.; Sabir, H.; Rafique, S.; Naeem, A.; Ahmed, R. Effect of sub-lethal and lethal concentrations of the entomopathogenic fungus Metarhizium anisopliae Sorokin on detoxification enzymes and demographic parameters of Mythimna separata (Walker). Crop Prot. 2023, 172, 106323. [Google Scholar] [CrossRef]
- Lenteren, J.C.V.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Tang, Y.T.; Li, Y.Y.; Liu, C.X.; Mao, J.J.; Chen, H.Y.; Zhang, L.S.; Wang, M.Q. Predation and behavior of Arma chinensis to Spodoptera frugiperda. Plant Prot. 2019, 45, 65–68. [Google Scholar]
- Tang, Y.T.; Wang, M.Q.; Li, Y.Y.; Liu, C.X.; Mao, J.J.; Chen, H.Y.; Zhang, L.S. Predation of Arma chinensis on Spodoptera litura larvae. Chin. Tob. Sci. 2020, 41, 62–66. [Google Scholar]
- Pan, M.Z.; Zhang, H.P.; Zhang, L.S.; Chen, H.Y. Effects of starvation and prey availability on predation and dispersal of an omnivorous predator Arma chinensis Fallou. J. Insect Behav. 2019, 32, 134–144. [Google Scholar] [CrossRef]
- Tang, Y.T.; Wang, M.Q.; Chen, H.Y.; Wang, Y.; Zhang, H.M.; Chen, F.S.; Zhao, X.Q.; Zhang, L.S. Predatory capacity and behavior of Picromerus lewisi Scott against Spodoptera frugiperda higher instar larve. Chin. J. Biol. Control 2019, 35, 698–703. [Google Scholar]
- Yang, Q.Q.; Chen, L.; Fang, L.; Wu, Z.Y.; Ding, Y.S.; Hu, X.D.; Li, W.B.; Kong, C.X. Evaluation of predation ability of Picromerus lewisi to Spodoptera litura larvae. J. Anhui Agric. Sci. 2022, 50, 140–142. [Google Scholar]
- Tang, Y.T.; Guo, Y.; He, G.W.; Liu, C.X.; Chen, H.Y.; Zhang, L.S.; Wang, M.Q. Functional responses of Picromerus lewisi Scott (Hemiptera: Pentatomidae) attacking Mythimna separata (Walker)(Lepidoptera: Noctuidae). Chin. J. Biol. Control 2018, 34, 825–830. [Google Scholar]
- Pradeep, P.; Deshmukh, S.S.; Kalleshwaraswamy, C.M.; Rajan, S.J. Biology and predation potential of the hemipteran predator, Rhynocoris marginatus (Fab., 1794) on the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2022, 32, 64. [Google Scholar] [CrossRef]
- Zhang, L.S.; Chen, H.Y.; Li, B.P. Mass-Rearing and Utilization of Insect Natural Enemies, 1st ed.; Chinese Agriculture Press: Beijing, China, 2014; pp. 119–122. [Google Scholar]
- Zhao, P.; Yuan, J.L. The insect list and faunal analysis of Harpactorinae in Guizhou Province. Guizhou Agric. Sci. 2011, 39, 99–102. [Google Scholar]
- Tomson, M. Functional response, host stage preference and development of Rhynocoris fuscipes (Fab.) (Heteroptera: Reduviidae) for two cotton pests. J. Biopestic. 2021, 14, 12–21. [Google Scholar] [CrossRef]
- Sunil, V.; Sampathkumar, M.; Lydia, C.; Chiranjeevi, K.; Shanker, C. Biology, predatory potential and functional response of Rhynocoris fuscipes (Fabricius) (Hemiptera: Reduviidae) on rice brown planthopper, Nilaparvata lugens (Stal) (Homoptera: Delphacidae). J. Exp. Zool. India 2018, 21, 259–263. [Google Scholar]
- Tomson, M.; Sahayaraj, K.; Kumar, V.; Avery, P.B.; McKenzie, C.L.; Osborne, L.S. Mass rearing and augmentative biological control evaluation of Rhynocoris fuscipes (Hemiptera: Reduviidae) against multiple pests of cotton. Pest Manag. Sci. 2017, 73, 1743–1752. [Google Scholar] [CrossRef]
- Deng, H.B.; Wang, Z.; Chen, Y.M.; Wu, W.B.; Peng, W.S. Predation of Harpactor fuscipes on Helicoverpa assulta and Spodoptera litura. Guangdong Agric. Sci. 2012, 39, 107–109. [Google Scholar]
- Solomon, M.E. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Poncio, S.; Montoya, P.; Cancino, J.; Nava, D.E. Determining the Functional Response and Mutual Interference of Utetes anastrephae (Hymenoptera: Braconidae) on Anastrepha obliqua (Diptera: Tephritidae) Larvae for Mass Rearing Purposes. Ann. Entomol. Soc. Am. 2016, 109, 518–525. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Dick, J.T.A.; Callaghan, A.; Dickey, J.W.E. Biological control agent selection under environmental change using functional responses, abundances and fecundities; the relative control potential (RCP) metric. Biol. Control 2018, 121, 50–57. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Messelink, G.J.; Bodino, N.; Lliadou, A.; Driss, L.; Woelke, J.B.; Leman, A.; Tavella, L. Functional response of the mirid predators Dicyphus bolivari and Dicyphus errans and their efficacy as biological control agents of Tuta absoluta on tomato. J. Pest Sci. 2019, 92, 1457–1466. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Juliano, S.A. Nonlinear Curve Fitting: Predation and Functional Response Curves, 2nd ed.; Chapman and Hall: London, UK, 2001; pp. 178–196. [Google Scholar]
- Uiterwaal, S.F.; Lagerstrom, I.T.; Lyon, S.R.; Delong, J.P. Data paper: FoRAGE (Functional Responses from Around the Globe in all Ecosystems) database: A compilation of functional responses for consumers and parasitoids. BioRxiv 2018. [Google Scholar] [CrossRef]
- Messina, F.J.; Hanks, J.B. Host plant alters the shape of the functional response of an aphid predator (Coleoptera: Coccinellidae). Environ. Entomol. 1998, 27, 1196–1202. [Google Scholar] [CrossRef]
- Hassanzadeh-Avval, M.; Sadeghi-Namaghi, H.; Fekrat, L. Factors influencing functional response, handling time and searching efficiency of Anthocoris minki Dohrn (Hem.: Anthocoridae) as predator of Psyllopsis repens Loginova (Hem.: Psyllidae). Phytoparasitica 2019, 47, 341–350. [Google Scholar] [CrossRef]
- Hassanpour, M.; Mohaghegh, J.; Iranipour, S.; Nouri-Ganbalani, G.; Enkegaard, A. Functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) to Helicoverpa armigera (Lepidoptera: Noctuidae): Effect of prey and predator stages. Insect Sci. 2011, 18, 217–224. [Google Scholar] [CrossRef]
- Ziaei Madbouni, M.A.; Samih, M.A.; Namvar, P.; Biondi, A. Temperature-dependent functional response of Nesidiocoris tenuis (Hemiptera: Miridae) to different densities of pupae of cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Eur. J. Entomol. 2017, 114, 325–331. [Google Scholar] [CrossRef]
- Pakyari, H.; Kasirloo, F.; Arbab, A. Effect of sublethal doses of Abamectin and fenpropathrin on functional response of Cryptolaemus Montrouzieri (Coleoptera: Coccinellidae) predator of Planococcus citri (Hemiptera: Pseudococcidae). J. Entomol. Zool. Stud. 2016, 4, 469–473. [Google Scholar]
- Hassell, M.P. A population model for the interaction between Cyzenisal bicans (Fall.) (Techinidae) and Opero phterabrumata (L.) (Geometridae) at Wytham, Berkshire. J. Anim. Ecol. 1969, 38, 567–576. [Google Scholar] [CrossRef]
- Hassanzadeh-Avval, M.; Sadeghi-Namaghi, H.; Fekrat, L. Prey preference and prey switching in Anthocoris minki Dohrn (Hemiptera: Anthocoridae). J. Asia-Pac. Entomol. 2018, 21, 1116–1121. [Google Scholar] [CrossRef]
- Greene, G.L.; Leppla, N.C.; Dickerson, W.A. Velvetbean caterpillar: A rearing procedure and artificial medium. J. Econ. Entomol. 1976, 69, 487–488. [Google Scholar] [CrossRef]
- Chen, W.B.; Li, Y.Y.; Wang, M.Q.; Mao, J.J.; Zhang, L.S. Evaluating the potential of using Spodoptera litura eggs for mass-rearing Telenomus remus, a promising egg parasitoid of Spodoptera frugiperda. Insects 2021, 12, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D. Random search and insect population models. J. Anim. Ecol. 1972, 41, 369–383. [Google Scholar] [CrossRef]
- Hassell, M.P.; Varley, G.C. New inductive population model for insect parasites and its bearing on biological control. Nature 1969, 223, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Lechowicz, M.J. The sampling characteristics of electivity indices. Oecologia 1982, 52, 22–30. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Dickey, J.W.E.; Coughlan, N.E.; Joyce, P.W.S.; Dick, J.T.A. The functional response ratio (FRR): Advancing comparative metrics for predicting the ecological impacts of invasive alien species. Biol. Invasions 2019, 21, 2543–2547. [Google Scholar] [CrossRef]
- George, P.J.E.; Seenivasagan, R. Predatory Efficiency of Rhynocoris marginatus (Fabricius)(Heteroptera: Reduviidae) on Helicoverpa armigera (Hiibner) and Spodoptera litura (Fabricius). J. Biol. Control 1998, 12, 25–29. [Google Scholar]
- Feng, Y.; Zhou, Z.X.; An, M.R.; Yu, X.L.; Liu, T.X. The effects of prey distribution and digestion on functional response of Harmonia axyridis (Coleoptera: Coccinellidae). Biol. Control 2018, 124, 74–81. [Google Scholar] [CrossRef]
- Varshney, R.; Budhlakoti, N.; Ballal, C.R. Functional response of Geocoris ochropterus Fieber (Hemiptera: Geocoridae) to different egg densities of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Phytoparasitica 2018, 46, 451–458. [Google Scholar] [CrossRef]
- Liu, P.; Jia, W.; Zheng, X.; Zhang, L.; Sangbaramou, R.; Tan, S.Q.; Liu, Y.Q.; Shi, W.P. Predation functional response and life table parameters of Orius sauteri (Hemiptera: Anthocoridae) feeding on Megalurothrips usitatus (Thysanoptera: Thripidae). Fla. Entomol. 2018, 101, 254–259. [Google Scholar] [CrossRef]
- Nagarajan, K.; Rajan, K.; Ambrose, D.P. Functional response of assassin bug, Rhynocoris fuscipes (Fabricius) (Hemiptera: Reduviidae) to cucumber leaf folder, Diaphania indicus Saunders (Lepidoptera: Pyraustidae). Entomon 2010, 35, 1–7. [Google Scholar]
- Ambrose, D.P.; Nagarajan, K. Functional response of Rhynocoris fuscipes (Fabricius) (Hemiptera: Reduviidae) to teak skeletonizer Eutectona machaeralis Walker (Lepidoptera: Pyralidae). J. Biol. Control 2010, 24, 175–178. [Google Scholar]
- Stasek, D.J.; Radl, J.N.; Crist, T.O. The functional response and prey preference of generalist Nabis (Hemiptera: Nabidae) predators to leafhopper prey (Hemiptera: Cicadellidae). Can. Entomol. 2018, 150, 190–200. [Google Scholar] [CrossRef]
- Sajjad, S.; Sultan, A.; Khan, M.F.; Keerio, I.D.; Channa, M.S.; Akbar, M.F. Biology, life table parameters, and functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) on different stages of invasive Paracoccus marginatus (Hemiptera: Pseudococcidae). J. Asia-Pac. Biodivers. 2021, 14, 174–182. [Google Scholar] [CrossRef]
- Mohaghegh, J.; Clercq, P.D.; Tirry, L. Functional response of the predators Podisus maculiventris (Say) and Podisus nigrispinus (Dallas) (Het., Pentatomidae) to the beet armyworm, Spodoptera exigua (Hübner) (Lep., Noctuidae): Ef fect of temperature. J. Appl. Entomol. 2001, 125, 131–134. [Google Scholar] [CrossRef]
- Hassanpour, M.; Maghami, R.; Rafiee-Dastjerdi, H.; Golizadeh, A.; Yazdanian, M.; Enkegaard, A. Predation activity of Chrysoperla carnea (Neuroptera: Chrysopidae) upon Aphis fabae (Hemiptera: Aphididae): Effect of different hunger levels. J. Asia-Pac. Entomol. 2015, 18, 297–302. [Google Scholar] [CrossRef]
- Schenk, D.; Bacher, S. Functional response of a generalist insect predator to one of its prey species in the field. J. Anim. Ecol. 2002, 71, 524–531. [Google Scholar] [CrossRef]
- Khan, M.A.Z.; Liang, Q.; Liu, T.X. Effect of cage size on functional response of the parasitoid Aphidius Gifuensis (Ashmead) (Hymenoptera: Braconidae) against Myzus Persicae (Sulzer) (Hemiptera: Aphididae). Egypt. J. Biol. Pest Control 2016, 26, 373. [Google Scholar]
- Kalinkat, G.; Rall, B.C.; Uiterwaal, S.F.; Uszko, W. Empirical evidence of type III functional responses and why it remains rare. Front. Ecol. Evol. 2023, 11, 1033818. [Google Scholar] [CrossRef]
- Viteri Jumbo, L.O.; Teodoro, A.V.; Rego, A.S.; Haddi, K.; Galvao, A.S.; de Oliveira, E.E. The lacewing Ceraeochrysa caligata as a potential biological agent for controlling the red palm mite Raoiella indica. PeerJ 2019, 7, e7123. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Kalidas, S.; Tomson, M. Stage preference and functional response of Rhynocoris longifrons (Stål) (Hemiptera: Reduviidae) on three hemipteran cotton pests. Braz. Arch. Biol. Technol. 2012, 55, 733–740. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Kumar, V.; Avery, P.B. Functional response of Rhynocoris kumarii (Hemiptera: Reduviidae) to different population densities of Phenacoccus solenopsis (Hemiptera: Pseudococcidae) recorded in the laboratory. Eur. J. Entomol. 2015, 112, 69–74. [Google Scholar] [CrossRef]
- Atrchian, H.; Mahdian, K.; Shahidi Noghabi, S. Effect of temperature on predation of Chilocorus bipustulatus L. (Col.: Coccinellidae) feeding on Agonoscena pistaciae (Hem.: Psyllidae). Phytoparasitica 2023, 51, 215–226. [Google Scholar]
- Mondal, R.P.; Chandra, G.; Bandyopadhyay, S.; Ghosh, A. Effect of temperature and search area on the functional response of Anisops sardea (Hemiptera: Notonectidae) against Anopheles stephensi in laboratory bioassay. Acta Trop. 2017, 166, 262–267. [Google Scholar] [CrossRef]
- Gholami, N.; Aleosfoor, M.; Hosseini, M.; Fekrat, L. Predation functional response and demographic parameters of Orius albidipennis (Hemiptera: Anthocoridae) on Schizaphis graminum (Hemiptera: Aphididae): Effect of host plant morphological attributes. Biocontrol Sci. Technol. 2022, 32, 362–380. [Google Scholar] [CrossRef]
- Maselou, D.; Perdikis, D.; Fantinou, A. Effect of hunger level on prey consumption and functional response of the predator Macrolophus pygmaeus. Bull. Insectology 2015, 68, 211–218. [Google Scholar]
- Ambrose, D.P.; Rajan, S.J.; Raja, J.M. Impacts of Synergy-505 on the functional response and behavior of the reduviid bug, Rhynocoris marginatus. J. Insect Sci. 2010, 10, 187. [Google Scholar] [CrossRef]
- Zhang, D.J.; Maiga, H.; Li, Y.J.; Bakhoum, M.T.; Wang, G.; Sun, Y.; Damiens, D.; Mamai, W.; Somda, N.S.B.; Wallner, T.; et al. Mating harassment may boost the effectiveness of the sterile insect technique for Aedes mosquitoes. Nat. Commun. 2024, 15, 1980. [Google Scholar] [CrossRef]
- Wu, P.X.; He, J.; Dong, H.; Zhang, R.Z. Functional response and intraspecific competition of three ladybird species feeding on aphids on goji berry plants in laboratory and semi-field conditions. Insects 2023, 14, 853. [Google Scholar] [CrossRef]
- Wu, P.X.; Zhang, J.; Haseeb, M.; Yan, S.; Kanga, L.; Zhang, R.Z. Functional responses and intraspecific competition in the ladybird Harmonia axyridis (Coleoptera: Coccinellidae) provided with Melanaphis sacchari (Homoptera: Aphididae) as prey. Eur. J. Entomol. 2018, 115, 232–241. [Google Scholar] [CrossRef]
- Nachappa, P.; Braman, S.K.; Guillebeau, L.P.; All, J.N. Functional response of the tiger beetle Megacephala carolina carolina (Coleoptera: Carabidae) on twolined spittlebug (Hemiptera: Cercopidae) and fall armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 2006, 99, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Messelink, G.J.; Vijverberg, R.; Leman, A.; Janssen, A. Biological control of mealybugs with lacewing larvae is affected by the presence and type of supplemental prey. BioControl 2016, 61, 555–565. [Google Scholar] [CrossRef]
- Nagarajan, K.; Ambrose, D.P. Chemically mediated prey-approaching behaviour of the reduviid predator Rhynocoris fuscipes (Fabricius) (Insecta: Heteroptera: Reduviidae) by Y-arm olfactometer. Pak. J. Biol. Sci. 2013, 16, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.X.; Enkegaard, A. Predation capacity and prey preference of Chrysoperla carnea on Pieris brassicae. BioControl 2010, 55, 379–385. [Google Scholar] [CrossRef]
- Daniel, S.; Kumar, S.P. Impact of prey types on the predatory behaviour of Rhynocoris fuscipes (Insecta: Heteroptera). J. Ecobiol. 2003, 15, 121–126. [Google Scholar]
- Sujatha, S.; Vidya, L.S.; Sumi, G.S. Prey-predator interaction and info-chemical behavior of Rhynocoris fuscipes (Fab.) on three agricultural pests (Heteroptera: Reduviidae). J. Entomol. 2012, 9, 130–136. [Google Scholar] [CrossRef]
- Kumar, R.M.; Gadratagi, B.G.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Thakur, N.; Sharma, A.; Kaur, S.; Ahluwalia, K.K.; Sidhu, A.K.; Kumar, S.; Rustagi, S.; Singh, S.; Rai, A.K.; Sheikh, S. Insect pest Spodoptera litura (Fabricius) and its resistance against the chemical insecticides: A review. Plant Sci. Today 2024, 11, 192–203. [Google Scholar] [CrossRef]
- Xu, Y.C.; Yang, C.H.; Li, Y.H.; Shi, C.J.; He, Y.G.; Yang, M.H.; Zhang, Y.; Tang, G.W. Larvae head morphology and ultrastructure of sensilla in tyrannophagous Mythimna separata and Spodoptera litura. Plant Prot. 2024, 50, 196–204. [Google Scholar]
| R. fuscipes | S. frugiperda | S. litura | M. separata | F, p |
|---|---|---|---|---|
| Fourth-instar nymphs | 9.05 ± 0.29 Ab | 8.65 ± 0.37 Ab | 8.15 ± 0.31 Ab | F(2, 57) = 1.839, p = 0.168 |
| Fifth-instar nymphs | 11.75 ± 0.37 Aa | 10.05 ± 0.32 Ba | 9.15 ± 0.33 Ba | F(2, 57) = 14.272, p < 0.0001 |
| Female adults | 10.90 ± 0.40 Aa | 10.45 ± 0.36 ABa | 9.50 ± 0.24 Ba | F(2, 57) = 4.228, p < 0.05 |
| Male adults | 10.85 ± 0.32 Aa | 9.85 ± 0.32 Ba | 9.05 ± 0.30 Ba | F(2, 57) = 7.854, p < 0.01 |
| F, p | F(3, 76) = 10.268, p < 0.0001 | F(3, 76) = 4.877, p < 0.05 | F(3, 76) = 3.513, p = 0.019 |
| R. fuscipes | Prey Species | Attack Rate ± SE (a) | Handling Time ± SE (Th) | Maximum Consumption (T/Th) | FRR (a/Th) | R2 |
|---|---|---|---|---|---|---|
| Fourth-instar nympha | S. frugiperda | 0.9390 ± 0.0364 a | 0.0650 ± 0.0030 a | 15.3752 | 14.3804 | 0.8875 |
| S. litura | 0.6820 ± 0.0503 b | 0.0533 ± 0.0038 a | 18.5117 | 12.5546 | 0.8750 | |
| M. separata | 0.6125 ± 0.0389 b | 0.0547 ± 0.0052 a | 18.1422 | 10.9851 | 0.8786 | |
| F, p | F(2, 57) = 12.394, p < 0.05 | F(2, 57) = 1.836, p = 0.214 | ||||
| Fifth-instar nympha | S. frugiperda | 1.5205 ± 0.0544 a | 0.0540 ± 0.0023 a | 18.5048 | 28.0718 | 0.9217 |
| S. litura | 0.9272 ± 0.0458 b | 0.0507 ± 0.0025 a | 19.6580 | 18.1168 | 0.8860 | |
| M. separata | 0.7748 ± 0.0263 b | 0.0531 ± 0.0028 a | 18.9036 | 14.5898 | 0.8749 | |
| F, p | F(2, 57) = 60.829, p < 0.0001 | F(2, 57) = 0.337, p = 0.723 | ||||
| Female adult | S. frugiperda | 1.3913 ± 0.1010 a | 0.0575 ± 0.0064 a | 17.4338 | 23.7099 | 0.9087 |
| S. litura | 0.9780 ± 0.0446 b | 0.0488 ± 0.0052 a | 20.7512 | 19.9875 | 0.8866 | |
| M. separata | 0.8489 ± 0.0486 b | 0.0522 ± 0.0038 a | 19.1534 | 16.0563 | 0.8756 | |
| F, p | F(2, 57) = 12.422, p < 0.05 | F(2, 57) = 0.533, p = 0.604 | ||||
| Male adult | S. frugiperda | 1.1778 ± 0.0619 a | 0.0534 ± 0.0021 a | 18.7406 | 21.9453 | 0.8969 |
| S. litura | 0.8578 ± 0.0254 b | 0.0499 ± 0.0049 a | 20.3666 | 17.3381 | 0.8929 | |
| M. separata | 0.7555 ± 0.0521 b | 0.0524 ± 0.0078 a | 19.2864 | 14.2469 | 0.8760 | |
| F, p | F(2, 57) = 15.185, p = 0.001 | F(2, 57) = 0.085, p = 0.919 |
| R. fuscipes | Prey Species | Prey-Predator Ratio | ||||
|---|---|---|---|---|---|---|
| 50:1 | 100:2 | 150:3 | 200:4 | 250:5 | ||
| Fourth-instar nymphs | S. frugiperda | 10.10 ± 0.61 a | 9.75 ± 0.56 a | 9.27 ± 0.54 ab | 8.00 ± 0.39 bc | 7.38 ± 0.39 c |
| S. litura | 9.90 ± 0.57 a | 9.45 ± 0.63 a | 8.77 ± 0.44 ab | 7.70 ± 0.48 b | 7.26 ± 0.51 b | |
| M. separata | 9.60 ± 0.45 a | 9.30 ± 0.35 a | 8.57 ± 0.43 a | 7.20 ± 0.39 b | 7.10 ± 0.35 b | |
| Fifth-instar nymphs | S. frugiperda | 12.70 ± 0.35 a | 12.07 ± 0.32 a | 9.82 ± 0.38 b | 8.24 ± 0.47 c | 7.85 ± 0.40 c |
| S. litura | 12.40 ± 0.47 a | 11.60 ± 0.43 a | 9.67 ± 0.45 b | 8.08 ± 0.44 c | 7.60 ± 0.36 c | |
| M. separata | 12.10 ± 0.46 a | 11.40 ± 0.30 a | 9.50 ± 0.51 b | 7.95 ± 0.38 c | 7.60 ± 0.31 c | |
| Female adults | S. frugiperda | 13.10 ± 0.48 a | 12.35 ± 0.49 a | 10.33 ± 0.49 b | 8.90 ± 0.50 c | 8.08 ± 0.35 c |
| S. litura | 12.20 ± 0.53 a | 11.45 ± 0.32 a | 9.40 ± 0.40 b | 8.08 ± 0.45 c | 7.58 ± 0.41 c | |
| M. separata | 12.60 ± 0.45 a | 11.20 ± 0.49 b | 9.17 ± 0.44 c | 7.88 ± 0.47 c | 7.78 ± 0.39 c | |
| Male adults | S. frugiperda | 12.10 ± 0.50 a | 11.40 ± 0.41 a | 9.57 ± 0.45 b | 8.20 ± 0.55 bc | 7.56 ± 0.38 c |
| S. litura | 11.90 ± 0.64 a | 10.95 ± 0.57 a | 9.17 ± 0.41 b | 7.90 ± 0.48 bc | 7.52 ± 0.31 c | |
| M. separata | 11.80 ± 0.46 a | 10.20 ±0.39 b | 9.00 ± 0.26 c | 7.85 ± 0.44 cd | 7.50 ± 0.39 d | |
| R. fuscipes | Prey Species | Maximum Predation (Q) | 95% Confidence Interval (CI) | Interference Coefficient (m) | 95% Confidence Interval (CI) | R2 |
|---|---|---|---|---|---|---|
| Fourth-instar nympha | S. frugiperda | 0.2097 | 0.1906–0.2292 | 0.1765 | 0.08671–0.2650 | 0.2410 |
| S. litura | 0.2045 | 0.1846–0.2247 | 0.1828 | 0.08666–0.2776 | 0.2290 | |
| M. separata | 0.1992 | 0.1837–0.2149 | 0.1887 | 0.1118–0.2647 | 0.3298 | |
| Fifth-instar nympha | S. frugiperda | 0.2661 | 0.2497–0.2826 | 0.2939 | 0.2302–0.3575 | 0.6305 |
| S. litura | 0.2586 | 0.2410–0.2763 | 0.2930 | 0.2228–0.3630 | 0.5831 | |
| M. separata | 0.2523 | 0.2357–0.2697 | 0.2837 | 0.2158–0.3513 | 0.5836 | |
| Female adults | S. frugiperda | 0.2730 | 0.2543–0.2919 | 0.2790 | 0.2084–0.3492 | 0.5570 |
| S. litura | 0.2538 | 0.2364–0.2713 | 0.2854 | 0.2147–0.3559 | 0.5669 | |
| M. separata | 0.2583 | 0.2408–0.2760 | 0.3075 | 0.2364–0.3784 | 0.5999 | |
| Male adults | S. frugiperda | 0.2521 | 0.2335–0.2709 | 0.2779 | 0.2019–0.3536 | 0.5183 |
| S. litura | 0.2459 | 0.2267–0.2653 | 0.2815 | 0.2008–0.3619 | 0.4946 | |
| M. separata | 0..2392 | 0.2243–0.2542 | 0.2744 | 0.2101–0.3385 | 0.5940 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, C.; Mao, J.; Xu, B.; Zhou, L.; Zhou, H.; Mao, J.; Shen, Z.; Zhang, L.; Wang, M.; Li, Y. Biological Control Potential of the Reduviid Predator Rhynocoris fuscipes (Fabricius) in Managing Noctuid Pests: Insights Into Predation and Prey Preference. Insects 2025, 16, 224. https://doi.org/10.3390/insects16020224
Xue C, Mao J, Xu B, Zhou L, Zhou H, Mao J, Shen Z, Zhang L, Wang M, Li Y. Biological Control Potential of the Reduviid Predator Rhynocoris fuscipes (Fabricius) in Managing Noctuid Pests: Insights Into Predation and Prey Preference. Insects. 2025; 16(2):224. https://doi.org/10.3390/insects16020224
Chicago/Turabian StyleXue, Chuanzhen, Jiaying Mao, Bowen Xu, Lei Zhou, Haihang Zhou, Jianjun Mao, Zhongjian Shen, Lisheng Zhang, Mengqing Wang, and Yuyan Li. 2025. "Biological Control Potential of the Reduviid Predator Rhynocoris fuscipes (Fabricius) in Managing Noctuid Pests: Insights Into Predation and Prey Preference" Insects 16, no. 2: 224. https://doi.org/10.3390/insects16020224
APA StyleXue, C., Mao, J., Xu, B., Zhou, L., Zhou, H., Mao, J., Shen, Z., Zhang, L., Wang, M., & Li, Y. (2025). Biological Control Potential of the Reduviid Predator Rhynocoris fuscipes (Fabricius) in Managing Noctuid Pests: Insights Into Predation and Prey Preference. Insects, 16(2), 224. https://doi.org/10.3390/insects16020224







