Daily Prey Consumption and Functional Response of Orius insidiosus: Implications for Biological Control of Scirtothrips dorsalis in Strawberries
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Daily Consumption Rate of O. insidiosus on S. dorsalis
3.2. Functional Response of O. insidiosus on S. dorsalis (Second-Instar Larvae)
3.3. Functional Response of O. insidiosus on S. dorsalis (Adults)
4. Discussion
4.1. Daily Prey Consumption
4.2. Functional Response of O. insidiosus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA/NASS 2023 State Agriculture Overview for Florida. Available online: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=FLORIDA (accessed on 8 January 2025).
- Liburd, O.; Rhodes, E. Management of Strawberry Insect and Mite Pests in Greenhouse and Field Crops. In Strawberry—Pre- and Post-Harvest Management Techniques for Higher Fruit Quality; IntechOpen: London UK, 2019. [Google Scholar] [CrossRef]
- Panthi, B.; Renkema, J. Managing Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in Florida Strawberry with Flupyradifurone. Int. J. Fruit Sci. 2020, 20 (Suppl. S1), 967–977. [Google Scholar] [CrossRef]
- Reddy, G.P.V.; Prasad, V.D.; Rao, R.S. Relative resistance in chilli thrips Scirtothrips dorsalis Hood population in Andhra Pradesh to some conventional insecticides. Indian J. Plant Prot. 1992, 20, 218–222. [Google Scholar]
- Dale, A.G.; Borden, M.A. Evaluation of Reduced-Risk Insecticides to Control Chilli Thrips (Thysanoptera: Thripidae) and Conserve Natural Enemies on Ornamental Plants. Fla. Entomol. 2018, 101, 237–243. [Google Scholar] [CrossRef]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for unifying the terminology in biological control. BioControl 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Isenhour, D.J.; Marston, N.L. Seasonal cycles of Orius insidiosus (Hemiptera: Anthocoridae) in Missouri soybeans. J. Kans Entomol. Soc 1981, 54, 129–142. [Google Scholar]
- Bosco, L.; Giacometto, E.; Tavella, L. Colonization and predation of thrips (Thysanoptera: Thripidae) by Orius spp. (Heteroptera: Anthocoridae) in sweet pepper greenhouses in Northwest Italy. Biol. Control 2008, 44, 331–340. [Google Scholar] [CrossRef]
- Kawai, A. Control of Thrips palmi Karny (Thysanoptera: Thripidae) by Orius spp. (Heteroptera: Anthocoridae) on Greenhouse Eggplant. Appl. Entomol. Zool. 1995, 30, 1–7. [Google Scholar] [CrossRef]
- Funderburk, J.; Stavisky, J.; Olson, S. Predation of Frankliniella occidentalis (Thysanoptera: Thripidae) in field peppers by Orius insidiosus (Hemiptera: Anthocoridae). Environ. Entomol. 2000, 29, 376–382. [Google Scholar] [CrossRef]
- Frescata, C.; Mexia, A. Biological Control of Thrips (Thysanoptera) by Orius laevigatus (Heteroptera: Anthocoridae) in Organically-Grown Strawberries. Biol. Agric. Hortic. 1996, 13, 141–148. [Google Scholar] [CrossRef]
- Perera, M.T.M.D.R.; Senanayake, N.; Dissanayake, D.M.I.C.B. Evaluation of Amblyseius swirskii (predatory mite) and Orius leavigatus as biological control agents of chilli thrips (Scirtothrips dorsalis). Ceylon J. Sci. 2021, 50, 541–544. [Google Scholar] [CrossRef]
- Doĝramaci, M.; Arthurs, S.P.; Chen, J.; McKenzie, C.; Irrizary, F.; Osborne, L. Management of chilli thrips Scirtothrips dorsalis (Thysanoptera: Thripidae) on peppers by Amblyseius swirskii (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae). Biol. Control 2011, 59, 340–347. [Google Scholar] [CrossRef]
- Lattin, J.D. Bionomics of the anthocoridae. Annu. Rev. Èntomol. 1999, 44, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, S.; Alinç, T.; Achiri, T.D.; Atakan, E. Functional responses of two predatory bugs (Hemiptera: Anthocoridae) to changes in the abundance of Tetranychus urticae (Acari: Tetranychidae) and Bemisia tabaci (Hemiptera: Aleyrodidae). Eur. J. Entomol. 2020, 117, 49–55. [Google Scholar] [CrossRef]
- Solomon, M.E. The Natural Control of Animal Populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Işikber, A.A. Functional response of two coccinellid predators, Scymnus levaillanti and Cycloneda sanguinea, to the cotton aphid, Aphis gossypii. Turk. J. Agric. For. 2005, 29, 347–355. [Google Scholar]
- Fathi, S.A.A.; Nouri-Ganbalani, G. Assessing the potential for biological control of potato field pests in Ardabil, Iran: Functional responses of Orius niger (Wolf.) and O. minutus (L.) (Hemiptera: Anthocoridae). J. Pest Sci. 2010, 83, 47–52. [Google Scholar] [CrossRef]
- Holling, C.S. The Canadian E n t o m o l o g i s t Some Characteristics of Simple Types of Predation and Parasitism1. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Fathipour, Y.; Maleknia, B. Mite Predators. In Ecofriendly Pest Management for Food Security; Academic Press: London, UK, 2016. [Google Scholar] [CrossRef]
- Alvarado, P.; Baltà, O.; Alomar, O. Efficiency of four heteroptera as predators of Aphis gossypii and Macrosiphum euphorbiae (Hom.: Aphididae). Entomophaga 1997, 42, 215–226. [Google Scholar] [CrossRef]
- Montserrat, M.; Albajes, R.; Castane, C. Functional response of four Heteropteran predators preying on greenhouse whitefly (Homoptera: Aleyrodidae) and western flower thrips (Thysanoptera: Thripidae). Environ. Entomol. 2000, 29, 1075–1082. [Google Scholar] [CrossRef]
- Nagai, K.; Yano, E. Predation by Orius sauteri (Poppius) (Heteroptera: Anthocoridae) on Thrips palmi Karny (Thysanoptera: Thripidae): Functional response and selective predation. Appl. Entomol. Zool. 2000, 35, 565–574. [Google Scholar] [CrossRef]
- Whitaker, V.M.; Peres, N.A.; Osorio, L.F.; Fan, Z.; do Nascimento Nunes, M.C.; Plotto, A.; Sims, C.A. ‘Florida Brilliance’ strawberry. HortScience 2019, 54, 2073–2077. [Google Scholar] [CrossRef]
- Juliano, S.A. Nonlinear Curve Fitting: Predation and Functional Response Curves. Des. Anal. Ecol. Exp. 2003, 218, 159–182. [Google Scholar] [CrossRef]
- Dostálková, I.; Kindlmann, P.; Dixon, A.F.G. Are classical predator-prey models relevant to the real world? J. Theor. Biol. 2002, 218, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Mouratidis, A.; de Lima, A.P.; Dicke, M.; Messelink, G.J. Predator-prey interactions and life history of Orius laevigatus and O. majusculus feeding on flower and leaf-inhabiting thrips. Biological. Control. 2022, 172, 104954. [Google Scholar] [CrossRef]
- Isenhour, D.J.; Yeargan, K.V. Predation by Orius insidiosus1 on the Soybean Thrips, Sericothrips variabilis 2: Effect of Prey Stage and Density 3. Environ. Entomol. 1981, 10, 496–500. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Irani-Nejad, K.H.; Iranipour, S.; Vahed, M.M. Daily consumption and functional response of Stethorus gilvifrons (Coleoptera: Coccinellidae) and Orius albidipennis (Hemiptera: Anthocoridae) to Tetranychus urticae (Acari: Tetranychidae). Persian J. Acarol. 2018, 7, 363–380. [Google Scholar] [CrossRef]
- Poley, K.; Bahlai, C.; Grieshop, M. Functional response of generalist predators to Halyomorpha halys (Hemiptera: Pentatomidae) Eggs. Environ. Entomol. 2018, 47, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Hassell, M.P.; Lawton, J.H.; Beddington, J.R. The Components of Arthropod Predation: I. The Prey Death-Rate. J. Anim. Ecol. 1976, 45, 135–164. [Google Scholar] [CrossRef]
- Liu, P.; Jia, W.; Zheng, X.; Zhang, L.; Sangbaramou, R.; Tan, S.; Liu, Y.; Shi, W. Predation Functional Response and Life Table Parameters of Orius sauteri (Hemiptera: Anthocoridae) Feeding on Megalurothrips usitatus (Thysanoptera: Thripidae). Fla. Entomol. 2018, 101, 254–259. [Google Scholar] [CrossRef]
- Gitonga, L.M.; Overholt, W.A.; Löhr, B.; Magambo, J.K.; Mueke, J.M. Functional response of Orius albidipennis (Hemiptera: Anthocoridae) to Megalurothrips sjostedti (Thysanoptera: Thripidae). Biological. Control. 2002, 24, 1–6. [Google Scholar] [CrossRef]
- Pervez, A.; Omkar. Ecology and biological control application of multicoloured Asian ladybird, Harmonia axyridis: A review. Biocontrol Sci. Technol. 2006, 16, 111–128. [Google Scholar] [CrossRef]
- Howard, D.F.; Blum, M.S.; Fales, H.M. Defense in thrips: Forbidding fruitiness of a lactone. Science 1983, 220, 335–336. [Google Scholar] [CrossRef]
- Alves-Silva, E.; Del-Claro, K. Fire triggers the activity of extrafloral nectaries, but ants fail to protect the plant against herbivores in a neotropical savanna. Arthropod Plant Interact. 2014, 8, 233–240. [Google Scholar] [CrossRef]
- O’Neil, R.J. Comparison of Laboratory and Field Measurements of the Functional Response of Podisus maculiventris (Heteroptera: Pentatomidae). J. Kans. Entomol. Soc. 1989, 62, 148–155. [Google Scholar]
- Wiedenmann, R.N.; O’Neil, R.J. Searching behavior and time budgets of the predator Podisus maculiventris. Entomol. Exp. Appl. 1991, 60, 83–93. [Google Scholar] [CrossRef]
- Mohaghegh; Clercq, D.; Tirry. Functional response of the predators Podisus maculiventris (Say) and Podisus nigrispinus (Dallas) (Het., Pentatomidae) to the beet armyworm, Spodoptera exigua (Hübner) (Lep., Noctuidae): Effect of temperature. J. Appl. Entomol. 2001, 125, 131–134. [Google Scholar] [CrossRef]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Pervez, A.; Omkar. Functional responses of coccinellid predators: An illustration of a logistic approach. J. Insect Sci. 2005, 5, 5. [Google Scholar] [CrossRef]
- Bielza, P.; Balanza, V.; Cifuentes, D.; Mendoza, J.E. Challenges facing arthropod biological control: Identifying traits for genetic improvement of predators in protected crops. Pest Manag. Sci. 2020, 76, 3517–3526. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, T.J. Functional response of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) to Aphis gossypii Glover (Homoptera: Aphididae) in the Laboratory. Biol. Control 2004, 31, 306–310. [Google Scholar] [CrossRef]
- O’Neil, R.J. Functional Response and Search Strategy of Podisus maculiventris (Heteroptera: Pentatomidae) Attacking Colorado Potato Beetle (Coleoptera: Chrysomelidae). Environ. Èntomol. 1997, 26, 1183–1190. [Google Scholar] [CrossRef]
- Murdoch, W.W. The functional response of predators. J. Appl. Ecol. 1973, 10, 335–342. [Google Scholar]
- Kareiva, P. The spatial dimension in pest–enemy interaction. In Critical Issues in Biological Control; Mackauer, M., Ehler, L.E., Roland, J., Eds.; Intercept Ltd.: Andover, UK, 1990; pp. 213–227. [Google Scholar]
- Uiterwaal, S.F.; DeLong, J.P. Multiple factors, including arena size, shape the functional responses of ladybird beetles. J. Appl. Ecol. 2018, 55, 2429–2438. [Google Scholar] [CrossRef]
- Skirvin, D.J.; Fenlon, J.S. Plant species modifies the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae): Implications for biological control. Bull. Entomol. Res. 2001, 91, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Riddick, E.W.; Simmons, A.M. Do plant trichomes cause more harm than good to predatory insects? Pest Manag. Sci. 2014, 70, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Guisoni, N.; Rocca, M.; Greco, N. To move or not to move: Dispersal of Orius insidiosus in strawberry plants. Entomol. Exp. Appl. 2024, 172, 883–893. [Google Scholar] [CrossRef]
- Traczyk, E.; Funderburk, J.; Martini, X. Foraging behavior responses of Orius insidiosus to thrips cues. Entomol. Exp. Appl. 2020, 168, 716–722. [Google Scholar] [CrossRef]
- Reitz, S.R.; Funderburk, J.E.; Waring, S.M. Differential predation by the generalist predator Orius insidiosus on congeneric species of thrips that vary in size and behavior. Entomol. Exp. Appl. 2006, 119, 179–188. [Google Scholar] [CrossRef]
- Lehtinen, S.O.; Perälä, T.A.; Uusi-Heikkilä, S.K.; Kuparinen, A.K. Mutually exclusive feeding yields Holling type III functional response. Funct. Ecol. 2024, 38, 403–416. [Google Scholar] [CrossRef]
- Nault, B.A.; Shelton, A.M. Impact of insecticide efficacy on developing action thresholds for pest management: A case study of onion thrips (Thysanoptera: Thripidae) on onion. J. Econ. Entomol. 2010, 103, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.K.; Garg, H.; Gill, A.K.; Gillett-Kaufman, J.L.; Nault, B.A. Onion thrips (Thysanoptera: Thripidae) biology, ecology, and management in onion production systems. J. Integr. Pest Manag. 2015, 66, 6. [Google Scholar] [CrossRef]
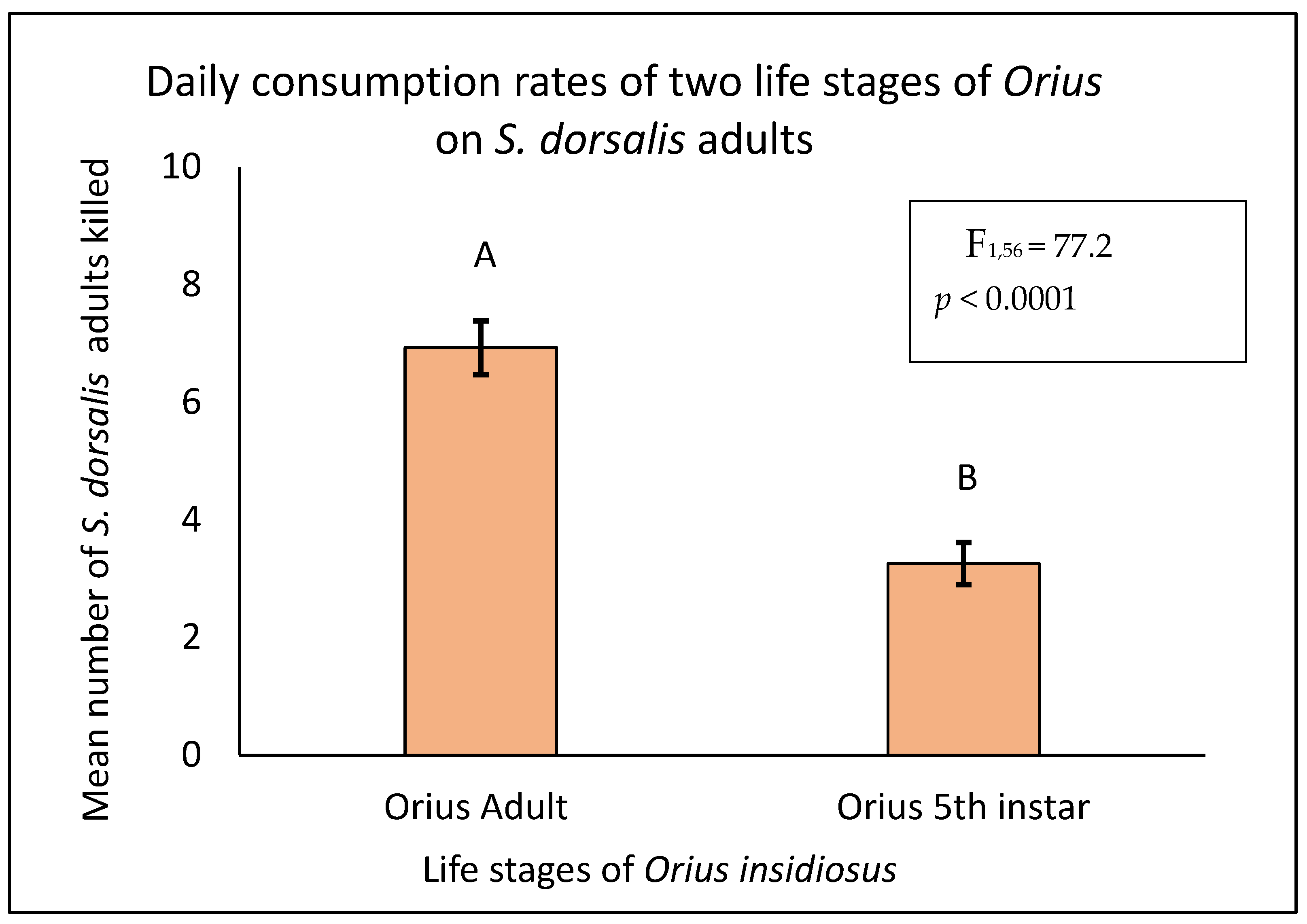
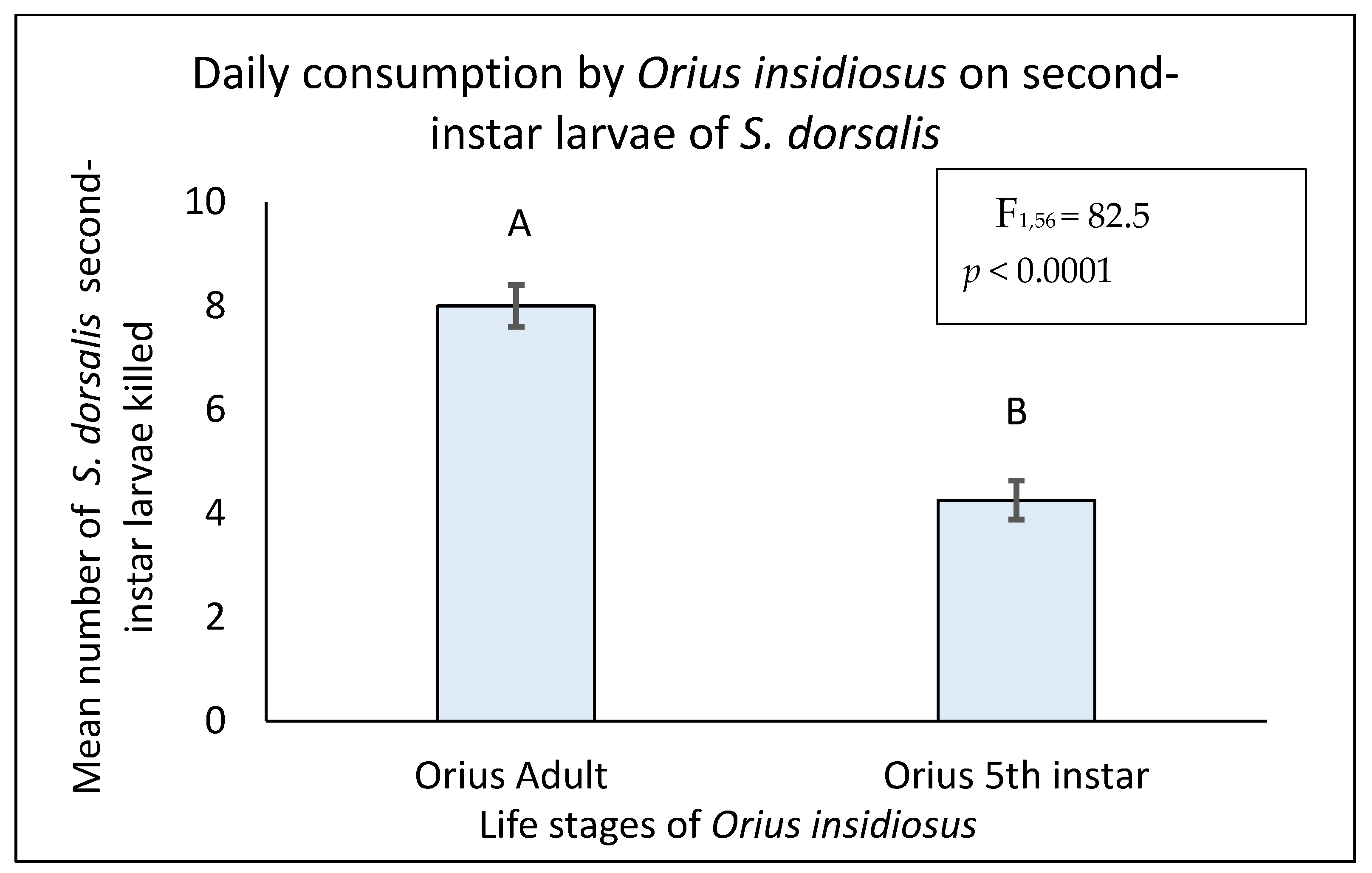
| Parameter | Co-Efficient | Function Number | Estimate | STD Err | Chi-Square | Pr > Chi-Square | |
|---|---|---|---|---|---|---|---|
| Intercept | P0 Intercept | 1 | −1.2203 | 0.1357 | 80.87 | <0.0001 | |
| P0 Intercept | 2 | 0.1951 | 0.0918 | 4.52 | 0.033 | ||
| Initial_prey_cat | High | P1 Linear | 1 | −0.2338 | 0.1601 | 2.13 | 0.1441 |
| High | P1 Linear | 2 | −0.2158 | 0.1057 | 4.17 | 0.0412 | |
| Low | P2 Quadratic | 1 | 0.6607 | 0.2267 | 8.53 | 0.0035 | |
| Low | P2 Quadratic | 2 | 0.7211 | 0.1584 | 20.73 | <0.0001 |
| Parameter | Function Number | Estimate | STD Err | Chi-Square | Pr > ChiSq | ||
|---|---|---|---|---|---|---|---|
| Intercept | P0 Intercept | 1 | 0.8500 | 0.1597 | 28.34 | <0.0001 | |
| P0 Intercept | 2 | 0.3973 | 0.1701 | 5.46 | 0.0195 | ||
| Initial_prey_cat | High | P1 Linear | 1 | −0.4989 | 0.1732 | 8.30 | 0.004 |
| High | P1 Linear | 2 | −2.3432 | 0.2238 | 109.60 | <0.0001 | |
| Low | P2 Quadratic | 1 | 0.6698 | 0.2890 | 5.37 | 0.0205 | |
| Low | P2 Quadratic | 2 | 1.9053 | 0.2852 | 44.64 | <0.0001 |
| Prey Stage | Parameters | Estimate ± SE | Asymptomatic 95% CL | R2 | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Second-instar larvae | a | 0.0342 ± 0.0018 | 0.0306 | 0.0379 | 0.735 |
| Th | 0.01 ± 0.00 | 0.011 | 0.01 | ||
| S. dorsalis adult | a | 0.0335 ± 0.004 | 0.0248 | 0.0423 | 0.789 |
| Th | 0.8135 ± 0.2419 | 0.3328 | 1.2941 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikary, L.; Smith, H.A.; Lahiri, S. Daily Prey Consumption and Functional Response of Orius insidiosus: Implications for Biological Control of Scirtothrips dorsalis in Strawberries. Insects 2025, 16, 205. https://doi.org/10.3390/insects16020205
Adhikary L, Smith HA, Lahiri S. Daily Prey Consumption and Functional Response of Orius insidiosus: Implications for Biological Control of Scirtothrips dorsalis in Strawberries. Insects. 2025; 16(2):205. https://doi.org/10.3390/insects16020205
Chicago/Turabian StyleAdhikary, Lovely, Hugh Adam Smith, and Sriyanka Lahiri. 2025. "Daily Prey Consumption and Functional Response of Orius insidiosus: Implications for Biological Control of Scirtothrips dorsalis in Strawberries" Insects 16, no. 2: 205. https://doi.org/10.3390/insects16020205
APA StyleAdhikary, L., Smith, H. A., & Lahiri, S. (2025). Daily Prey Consumption and Functional Response of Orius insidiosus: Implications for Biological Control of Scirtothrips dorsalis in Strawberries. Insects, 16(2), 205. https://doi.org/10.3390/insects16020205






