Assessment of Scalable Fractionation Methodologies to Produce Concentrated Lauric Acid from Black Soldier Fly (Hermetia illucens) Larvae Fat
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Production of Fatty Acids in Free and Ethyl Ester Forms
2.2.1. Chemical Hydrolysis
2.2.2. Chemical Ethanolysis
2.3. Concentration of Lauric Acid
2.3.1. Winterization of FFA
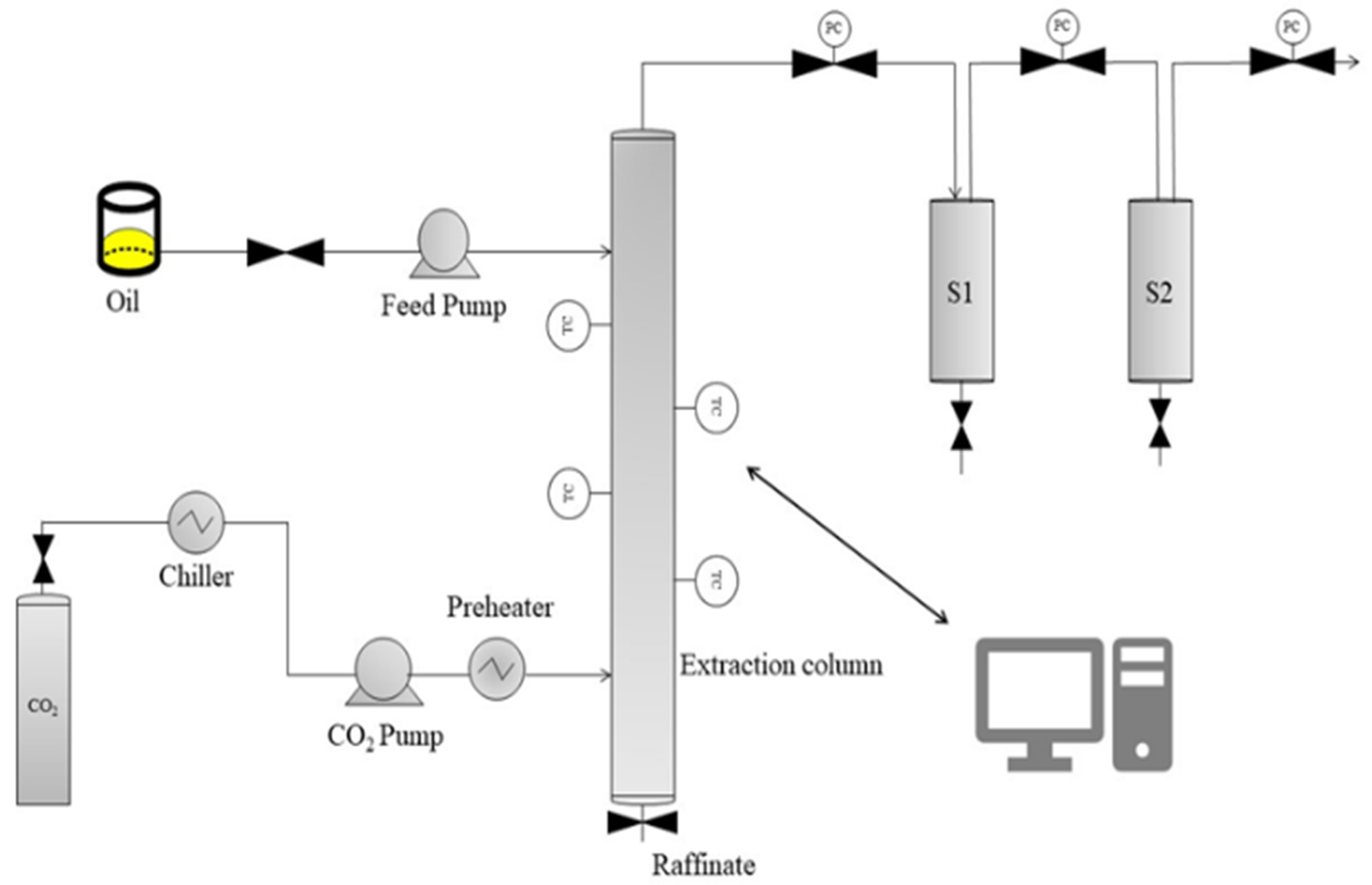
2.3.2. Supercritical Fluid Extraction of FAEE
2.3.3. Simulation of Distillation
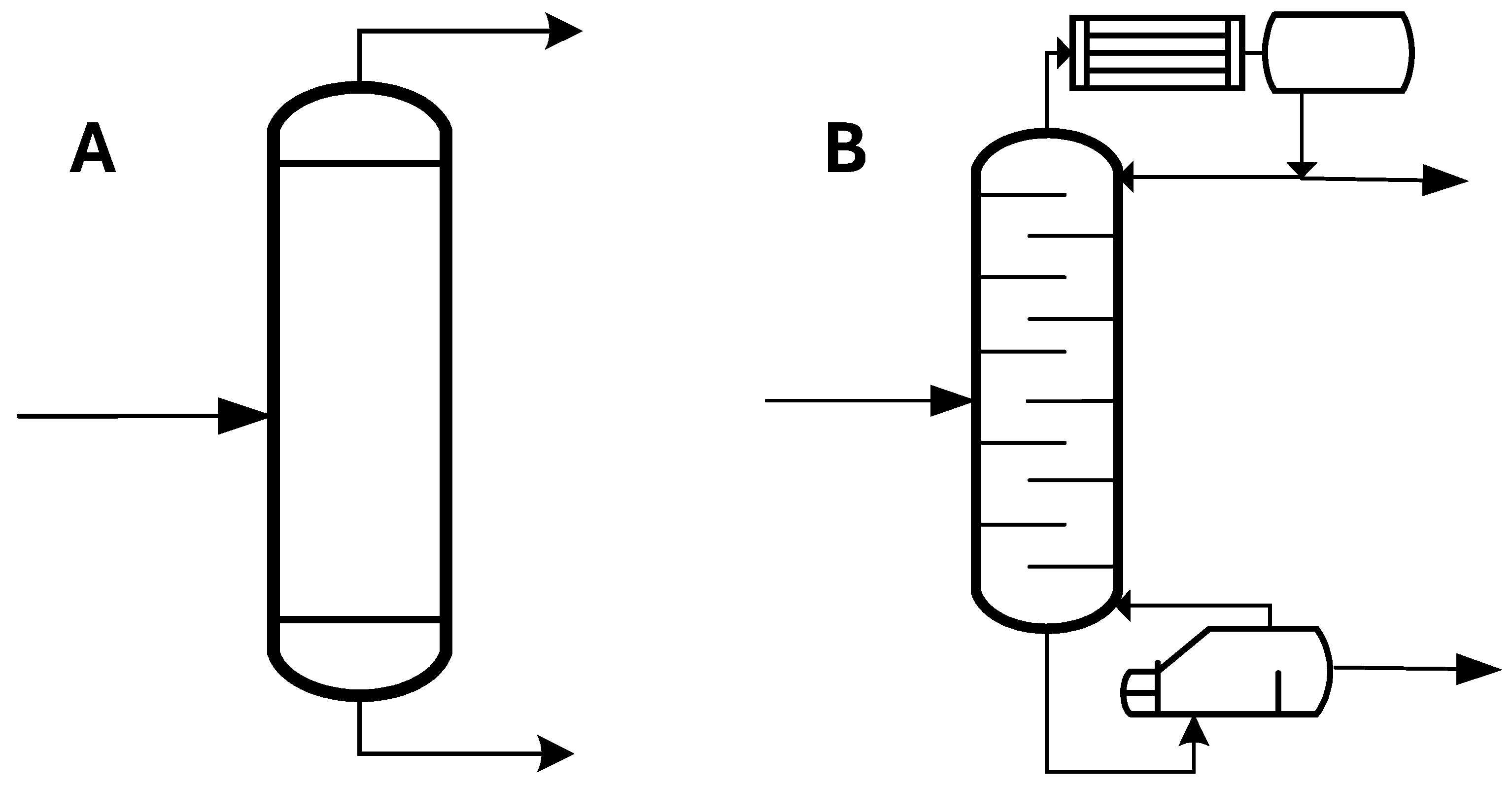
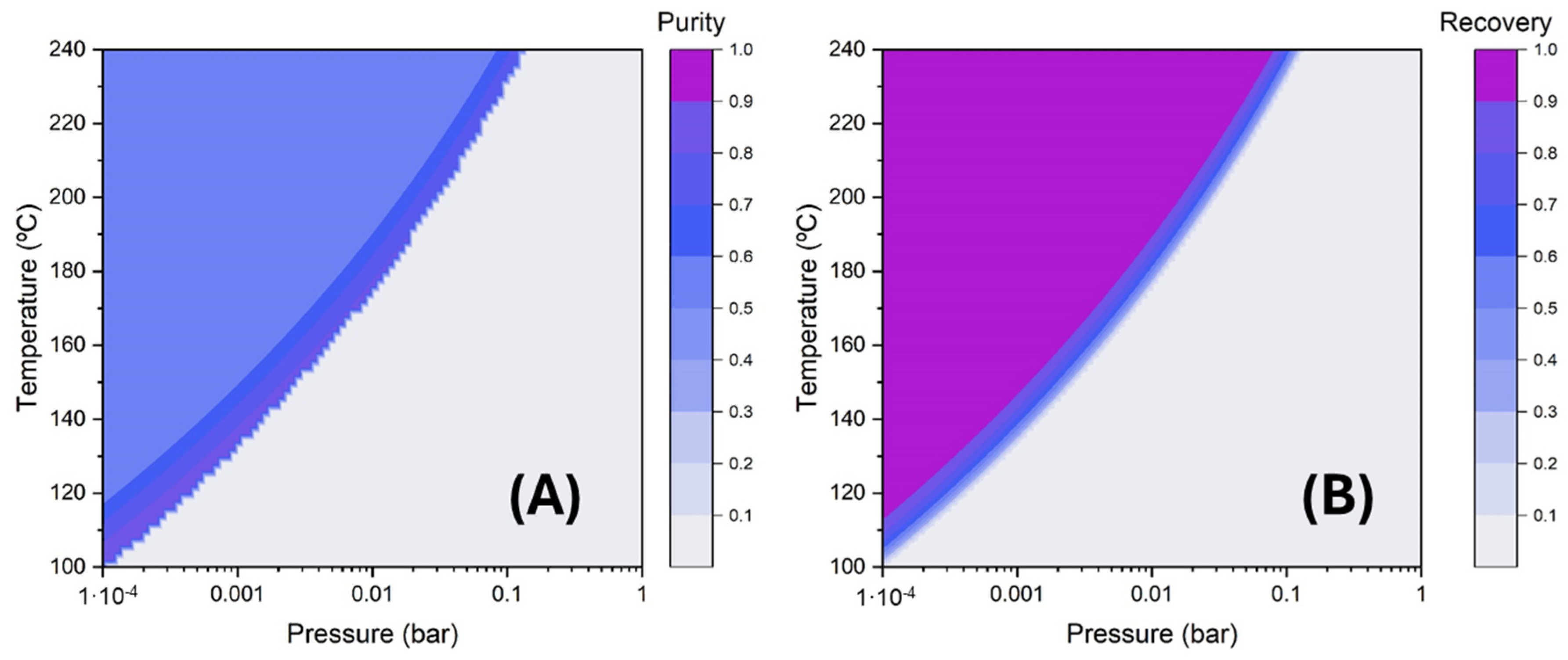
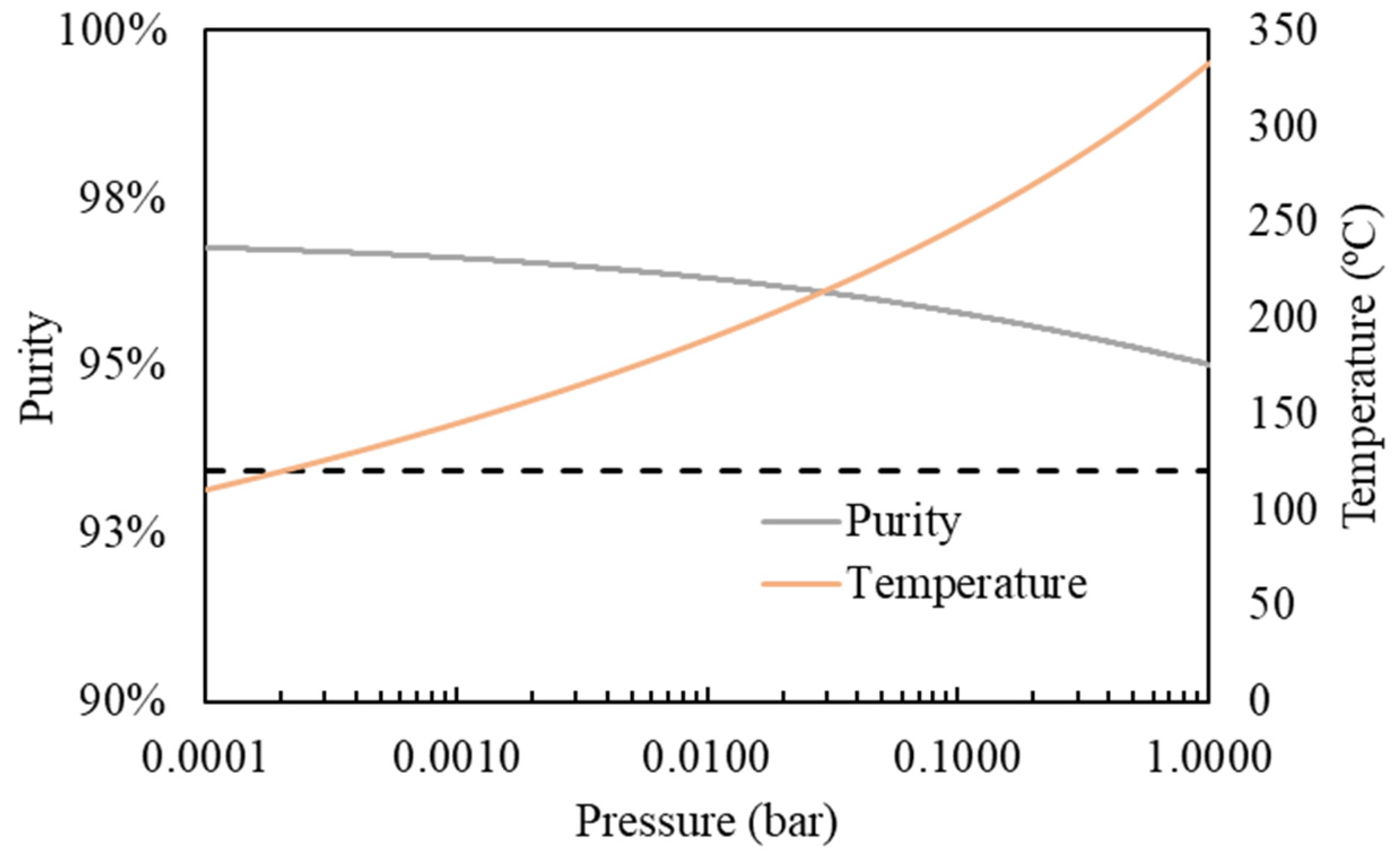
- Simple one-stage flash distillation (Figure 2A). In this case, the FLASH module of the Aspen Plus process simulator was utilized to simulate the separation process. Wide ranges of operating temperature (100–240 °C) and pressure (10-4-1 bar) were studied to analyse separation efficiency, i.e., the purity and recovery of the C12:0 compound in the vapor stream.
- Multistage distillation column (Figure 2B). The RADFRAC module of the Aspen Plus process simulator was employed in equilibrium mode for distillation column modelling, using 10 stages. A total condenser and a kettle reboiler were incorporated with a reflux ratio set to 1. The distillate flow rate from the column was adjusted to ensure an 80% recovery of the C12:0 compound, and the impact of the column’s operating pressure on the reboiler temperature and distillate purity of the C12:0 compound was investigated.
2.4. Analysis by Gas Chromatography
2.5. Peroxide Value Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Lauric Acid Concentration via Winterization
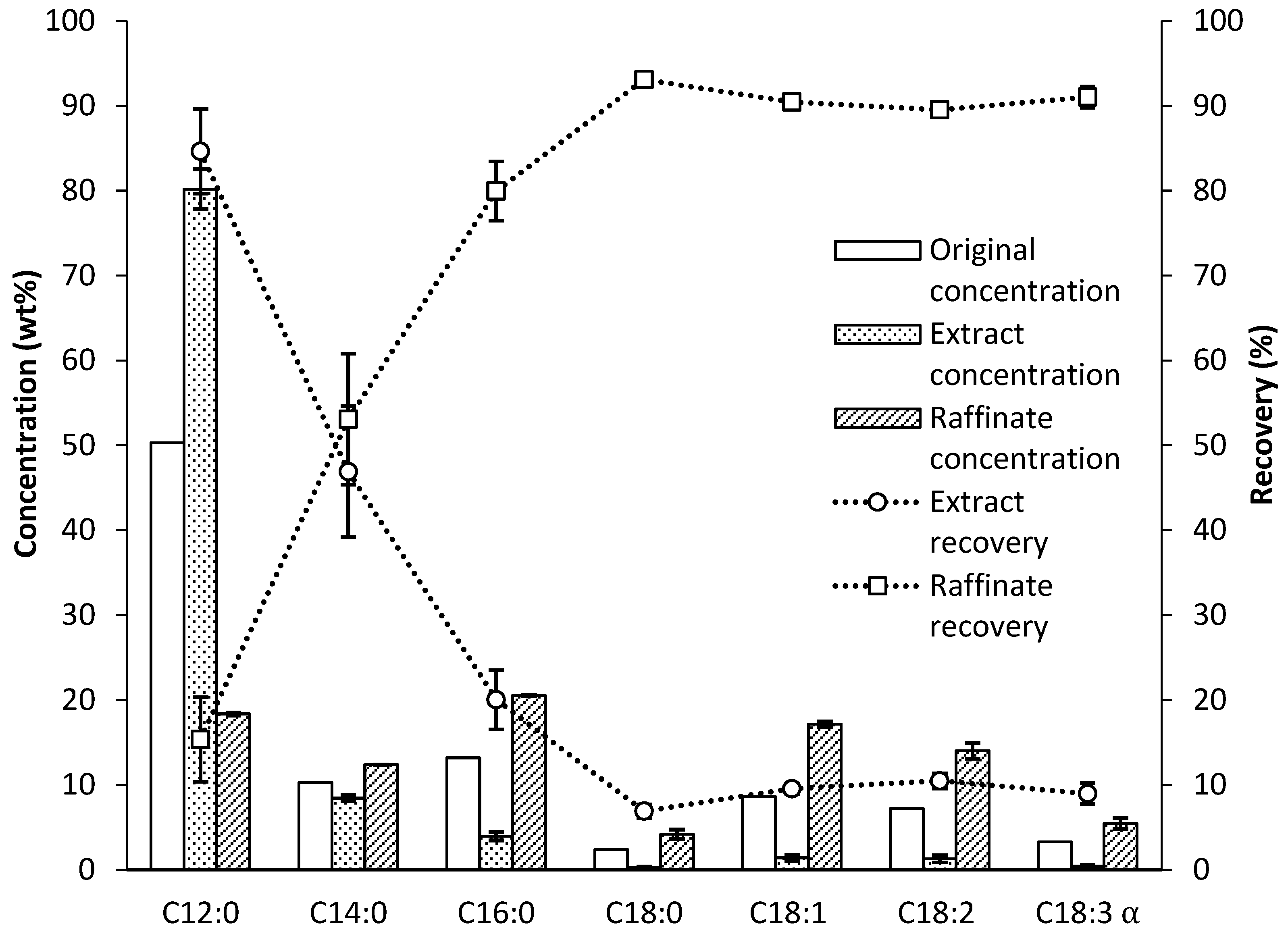
3.2. Lauric Acid Concentration via Supercritical Fluid Extraction
3.3. Lauric Acid Concentration via Simulated Distillation
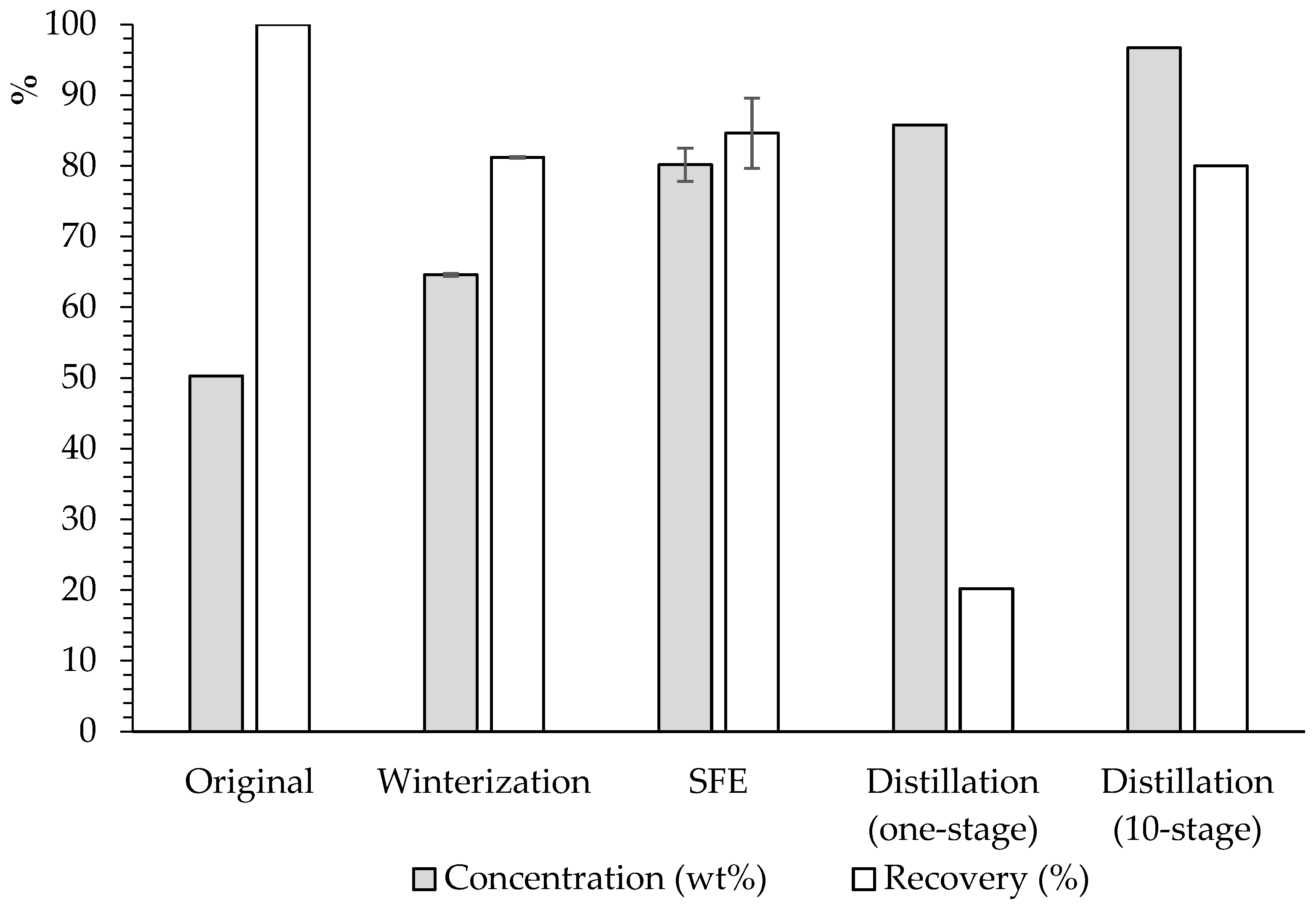
3.4. Comparison of Results from the Three Fractionation Technologies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anneken, D.J.; Both, S.; Christoph, R.; Fieg, G.; Steinberner, U.; Westfechtel, A. Fatty Acids. Ullmann’s Encycl. Ind. Chem. 2006, 14, 73–115. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an Innovative and Sustainable Source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Hurtado-Ribeira, R.; Villanueva-Bermejo, D.; García-Risco, M.R.; Hernández, M.D.; Sánchez-Muros, M.J.; Fornari, T.; Vázquez, L.; Martin, D. Evaluation of the Interrelated Effects of Slaughtering, Drying, and Defatting Methods on the Composition and Properties of Black Soldier Fly (Hermetia illucens) Larvae Fat. Curr. Res. Food Sci. 2023, 7, 100633. [Google Scholar] [CrossRef] [PubMed]
- Salsinha, A.S.; Machado, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Bioactive Lipids: Chemistry, Biochemistry, and Biological Properties. In Bioactive Lipids; Academic Press: Cambridge, MA, USA, 2023; pp. 1–35. ISBN 9780128240434. [Google Scholar]
- Suryati, T.; Julaeha, E.; Farabi, K.; Ambarsari, H.; Hidayat, A.T. Lauric Acid from the Black Soldier Fly (Hermetia illucens) and its Potential Applications. Sustainability 2023, 15, 10383. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Pucciarelli, V.; Borrelli, L.; Addeo, N.F.; Bovera, F.; Laginestra, A.; Schmitt, E.; Falabella, P. Antimicrobial Activity of Lipids Extracted from Hermetia illucens Reared on Different Substrates. Appl. Microbiol. Biotechnol. 2024, 108, 167. [Google Scholar] [CrossRef]
- Franco, A.; Salvia, R.; Scieuzo, C.; Schmitt, E.; Russo, A.; Falabella, P. Lipids from Insects in Cosmetics and for Personal Care Products. Insects 2022, 13, 41. [Google Scholar] [CrossRef]
- Borrelli, L.; Varriale, L.; Dipineto, L.; Pace, A.; Menna, L.F.; Fioretti, A. Insect Derived Lauric Acid as Promising Alternative Strategy to Antibiotics in the Antimicrobial Resistance Scenario. Front. Microbiol. 2021, 12, 620798. [Google Scholar] [CrossRef]
- Hurtado-Ribeira, R.; Silvan, J.M.; Fornari, T.; Vázquez, L.; Martinez-Rodriguez, A.J.; Martin, D. Modulation of the Lipolysis and Subsequent Antibacterial Activity of the Fat from Black Soldier Fly (Hermetia illucens) by the Combined Selection of Slaughtering, Drying and Defatting Methods of the Larvae. Innov. Food Sci. Emerg. Technol. 2023, 90, 103510. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Nitti, F.; Jumina, J.; Detha, A.I.R. Antimicrobial Properties of Lauric Acid and Monolaurin in Virgin Coconut Oil: A Review. ChemBioEng Rev. 2022, 9, 442–461. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Lauric Acid-Rich Medium-Chain Triglycerides Can Substitute for Other Oils in Cooking Applications and May Have Limited Pathogenicity. Open Heart 2016, 3, e000467. [Google Scholar] [CrossRef]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Zhang, R.; Cao, G.; Li, Q.; Zhang, B.; Wang, Y.; Yang, C. Serum Metabolome and Gut Microbiome Alterations in Broiler Chickens Supplemented with Lauric Acid. Poult. Sci. 2021, 100, 101315. [Google Scholar] [CrossRef] [PubMed]
- Hájek, M.; Vávra, A.; de Paz Carmona, H.; Kocík, J. The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review. Catalysts 2021, 11, 1118. [Google Scholar] [CrossRef]
- Mukherjee, K.D. Lipase-Catalyzed Reactions for Modification of Fats and Other Lipids. Biocatal. Biotransform. 1990, 3, 277–293. [Google Scholar] [CrossRef]
- Vázquez, L.; Hurtado-Ribeira, R.; López De Haro, C.S.; Martin, D. Hermetia illucens Larvae Fat vs Coconut Oil to Obtain Free Lauric Acid-Rich Products by Chemical or Enzymatic Hydrolysis. J. Insects Food Feed 2024, 4588, 1583–1593. [Google Scholar] [CrossRef]
- Cermak, S.C.; Evangelista, R.L.; Kenar, J.A. Distillation of Natural Fatty Acids and Their Chemical Derivatives. In Distillation—Advances from Modeling to Applications; IntechOpen: London, UK, 2012; pp. 109–140. [Google Scholar]
- Vázquez, L.; Sánchez-Moyano, M.; de la Iglesia, L.; Reglero, G.; Torres, C.F. A New Urea Adducts Method for PUFA Concentration Using Green Food Grade Solvents and Avoiding Ethyl Carbamate Formation. Food Chem. 2022, 392, 133197. [Google Scholar] [CrossRef]
- Vázquez, L.; Akoh, C.C. Concentration of Stearidonic Acid in Free Fatty Acid and Fatty Acid Ethyl Ester Forms from Modified Soybean Oil by Winterization. J. Am. Oil Chem. Soc. 2011, 88, 1775–1785. [Google Scholar] [CrossRef]
- Torres, C.F.; Torrelo, G.; Señoráns, F.J.; Reglero, G. Supercritical Fluid Fractionation of Fatty Acid Ethyl Esters from Butteroil. J. Dairy Sci. 2009, 92, 1840–1845. [Google Scholar] [CrossRef]
- Nagao, T.; Watanabe, Y.; Kobayashi, T.; Sumida, M.; Kishimoto, N.; Fujita, T.; Shimada, Y. Enzymatic Purification of Dihomo-γ-Linolenic Acid from Mortierella Single-Cell Oil. J. Mol. Catal. B Enzym. 2007, 44, 14–19. [Google Scholar] [CrossRef]
- Han, T.; Yang, G.; Cao, X.; Li, H.; Pei, H.; Zhang, Z. Preparative and Scaled-up Separation of High-Purity α-Linolenic Acid from Perilla Seed Oil by Conventional and PH-Zone Refining Counter Current Chromatography. J. Sep. Sci. 2019, 42, 2360–2370. [Google Scholar] [CrossRef]
- Gervajio, G.C. Fatty Acids and Derivatives from Coconut Oil. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 1–56. [Google Scholar]
- Mahrous, E.A.; Farag, M.A. Trends and Applications of Molecular Distillation in Pharmaceutical and Food Industries. Sep. Purif. Rev. 2022, 51, 300–317. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Liu, Y. Concentration of Omega-3 Polyunsaturated Fatty Acids from Oil of Schizochytrium limacinum by Molecular Distillation: Optimization of Technological Conditions. Ind. Eng. Chem. Res. 2013, 52, 3918–3925. [Google Scholar] [CrossRef]
- Vázquez, L.; Akoh, C.C. Fractionation of Short and Medium Chain Fatty Acid Ethyl Esters from a Blend of Oils via Ethanolysis and Short-Path Distillation. J. Am. Oil Chem. Soc. 2010, 87, 917–928. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Puranik, S.; Radha, S.; Natta, S.; Senapathy, M.; Dhumal, S.; Singh, S.; Kumar, S.; Deshmukh, V.P.; et al. Combination of green extraction techniques and smart solvents for bioactives recovery. TrAC Trends Anal. Chem. 2023, 169, 117286. [Google Scholar] [CrossRef]
- Amiri-Rigi, A.; Abbasi, S.; Scanlon, M.G. Enhanced lycopene extraction from tomato industrial waste using microemulsion technique: Optimization of enzymatic and ultrasound pre-treatments. Innovat. Food Sci. Emerg. Technol. 2016, 35, 160–167. [Google Scholar] [CrossRef]
- Chemat, S.; Tomao, V.; Chemat, F. Limonene as Green Solvent for Extraction of Natural Products. In Green Solvents; Mohammad, I., Inamuddin, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 175–187. [Google Scholar] [CrossRef]
- López-Martínez, J.C.; Campra-Madrid, P.; Guil-Guerrero, J.L. γ-Linolenic Acid Enrichment from Borago officinalis and Echium fastuosum Seed Oils and Fatty Acids by Low Temperature Crystallization. J. Biosci. Bioeng. 2004, 97, 294–298. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils—A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Singh, S.; Verma, D.K.; Thakur, M.; Tripathy, S.; Patel, A.R.; Shah, N.; Utama, G.L.; Srivastav, P.P.; Benavente-Valdés, J.R.; Chávez-González, M.L.; et al. Supercritical Fluid Extraction (SCFE) as Green Extraction Technology for High-Value Metabolites of Algae, Its Potential Trends in Food and Human Health. Food Res. Int. 2021, 150, 110746. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Norulaini, N.A.N.; Omar, A.K.M.; Sato, Y.; Smith, R.L. Separation of Palm Kernel Oil from Palm Kernel with Supercritical Carbon Dioxide Using Pressure Swing Technique. J. Food Eng. 2007, 81, 419–428. [Google Scholar] [CrossRef]
- Markom, M.; Singh, H.; Hasan, M. Supercritical CO2 Fractionation of Crude Palm Oil. J. Supercrit. Fluids 2001, 20, 45–53. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Filho, R.M. Recent Advances in Lipid Extraction Using Green Solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Temelli, F. Perspectives on Supercritical Fluid Processing of Fats and Oils. J. Supercrit. Fluids 2009, 47, 583–590. [Google Scholar] [CrossRef]
- Büyükbeşe, D.; Emre, E.E.; Kaya, A. Supercritical Carbon Dioxide Fractionation of Anhydrous Milk Fat. J. Am. Oil Chem. Soc. 2014, 91, 169–177. [Google Scholar] [CrossRef]
- Md Zaidul, I.S.; Nik Norulaini, N.A.; Mohd Omar, A.K. Separation/Fractionation of Triglycerides in Terms of Fatty Acid Constituents in Palm Kernel Oil Using Supercritical CO2. J. Sci. Food Agric. 2006, 86, 1138–1145. [Google Scholar] [CrossRef]
- Espinosa, S.; Diaz, S.; Brignole, E.A. Thermodynamic Modeling and Process Optimization of Supercritical Fluid Fractionation of Fish Oil Fatty Acid Ethyl Esters. Ind. Eng. Chem. Res. 2002, 41, 1516–1527. [Google Scholar] [CrossRef]
- Palomar, J.; Lemus, J.; Navarro, P.; Moya, C.; Santiago, R.; Hospital-Benito, D.; Hernández, E. Process Simulation and Optimization on Ionic Liquids. Chem. Rev. 2024, 124, 1649–1737. [Google Scholar] [CrossRef]
- Ferro, V.R.; Moya, C.; Moreno, D.; Santiago, R.; De Riva, J.; Pedrosa, G.; Larriba, M.; Diaz, I.; Palomar, J. Enterprise Ionic Liquids Database (ILUAM) for Use in Aspen ONE Programs Suite with COSMO-Based Property Methods. Ind. Eng. Chem. Res. 2018, 57, 980–989. [Google Scholar] [CrossRef]
- Satchithanandam, S.; Fritsche, J.; Rader, J.I. Extension of AOAC Official Method 996.01 to the Analysis of Standard Reference Material (SRM) 1846 and Infant Formulas. J. AOAC Int. 2001, 84, 805–813. [Google Scholar] [CrossRef]
- Vázquez, L.; Prados, I.M.; Reglero, G.; Torres, C.F. Identification and Quantification of Ethyl Carbamate Occurring in Urea Complexation Processes Commonly Utilized for Polyunsaturated Fatty Acid Concentration. Food Chem. 2017, 229, 28–34. [Google Scholar] [CrossRef]
- Martín, D.; Terrón, A.; Fornari, T.; Reglero, G.; Torres, C.F. Oxidative Stabilization of Ultra-High Omega-3 Concentrates as Ethyl Esters or Triacylglycerols. Food Res. Int. 2012, 45, 336–341. [Google Scholar] [CrossRef]
- Vázquez, L.; Akoh, C.C. Enrichment of Stearidonic Acid in Modified Soybean Oil by Low Temperature Crystallisation. Food Chem. 2012, 130, 147–155. [Google Scholar] [CrossRef]
- Liu, P.; Cui, X.; Wang, Y.; Zhang, Z.; Rao, J.; Jiang, S.; Gu, X. Preparation and Characterization of Lauric Acid/Modified Fly Ash/Graphene Composite as Low-Cost and Eco-Friendly Phase Change Materials for Thermal Energy Storage. Energies 2023, 16, 5666. [Google Scholar] [CrossRef]
- Kreulen, H.P. Fractionation and Winterization of Edible Fats and Oils. J. Am. Oil Chem. Soc. 1976, 53, 393–396. [Google Scholar] [CrossRef]
- Nikolai, P.; Rabiyat, B.; Aslan, A.; Ilmutdin, A. Supercritical CO2: Properties and Technological Applications—A Review. J. Therm. Sci. 2019, 28, 394–430. [Google Scholar] [CrossRef]
- Atta, M.S.; Khan, H.; Ali, M.; Tariq, R.; Yasir, A.U.; Iqbal, M.M.; Din, S.U.; Krzywanski, J. Simulation of Vacuum Distillation Unit in Oil Refinery: Operational Strategies for Optimal Yield Efficiency. Energies 2024, 17, 3806. [Google Scholar] [CrossRef]
| Structure | Nomenclature | Mass Fraction (%) | |
|---|---|---|---|
| 1. Free Fatty Acid | 2. Ethyl Ester | ||
| Capric acid | Ethyl Caprate | C10:0 | 0.9 |
| Lauric acid | Ethyl Laurate | C12:0 | 50.3 |
| Myristic acid | Ethyl Myristate | C14:0 | 10.3 |
| Myristoleic acid | Ethyl Myristoleic | C14:1 | 0.4 |
| Palmitic acid | Ethyl Palmitate | C16:0 | 13.2 |
| Palmitoleic acid | Ethyl Palmitoleic | C16:1 | 2.4 |
| Margaric acid | Ethyl Margarate | C17:0 | 0.4 |
| Heptadecenoic acid | Ethyl Heptadecenoic | C17:1 | 0.3 |
| Stearic acid | Ethyl Stearate | C18:0 | 2.4 |
| Oleic acid | Ethyl Oleate | C18:1 n9 | 8.6 |
| Linoleic acid | Ethyl Linoleate | C18:2 | 7.2 |
| γ-linolenic acid | Ethyl Linolenate (γ) | C18:3 n6 | 0.1 |
| α-linolenic acid | Ethyl Linolenate (α) | C18:3 n3 | 3.3 |
| Gadeloic acid | Ethyl Gadeloic | C20:1 | 0.2 |
| FFA | Raw FFA | P1 Hexane 1:4 | P2 Hexane 1:6 | P3 Hexane 1:8 | |||
|---|---|---|---|---|---|---|---|
| SF | LF | SF | LF | SF | LF | ||
| Lauric | 50.3 | 59.4 ± 0.2 b | 28.7 ± 0.6 v | 57.3 ± 0.2 c | 35.6 ± 1.1 x | 59.3 ± 0.2 b | 35.6 ± 0.4 x |
| Myristic | 10.3 | 10.9 ± 0.0 b | 8.4 ± 0.1 x | 10.9 ± 0.0 b | 8.3 ± 0.0 x | 11.1 ± 0.1 a | 8.5 ± 0.1 x |
| Palmitic | 13.2 | 12.3 ± 0.1de | 4.0 ± 0.0 v | 12.0 ± 0.0 e | 5.3 ± 0.4 x | 12.7 ± 0.1 d | 5.0 ± 0.1 xw |
| Stearic | 2.4 | 1.7 ± 0.0 e | 0.9 ± 0.0 x | 1.7 ± 0.0 de | 0.9 ± 0.0 x | 1.8 ± 0.0 cd | 0.9 ± 0.1 x |
| Oleic | 8.6 | 5.6 ± 0.1 b | 21.4 ± 0.1 z | 6.5 ± 0.1 a | 18.3 ± 0.6 y | 5.3 ± 0.1 b | 18.4 ± 0.2 y |
| Linoleic | 7.2 | 4.0 ± 0.1 b | 16.0 ± 0.0 z | 4.6 ± 0.1 a | 13.7 ± 0.4 y | 3.8 ± 0.1 b | 13.7 ± 0.1 y |
| α-linolenic | 3.3 | 1.7 ± 0.0 b | 7.2 ± 0.1 z | 2.0 ± 0.0 a | 6.2 ± 0.1 y | 1.7 ± 0.0 b | 6.2 ± 0.0 y |
| FFA | Raw FFA | P4 Hexane 1:10 | P5 Hexane 1:15 | P6 Hexane 1:20 | |||
| SF | LF | SF | LF | SF | LF | ||
| Lauric | 50.3 | 64.6 ± 0.2 a | 33.0 ± 0.3 w | 64.3 ± 0.0 a | 42.3 ± 0.1 y | 65.0 ± 0.3 a | 45.3 ± 0.2 z |
| Myristic | 10.3 | 10.8 ± 0.0 b | 9.8 ± 0.1 y | 11.2 ± 0.0 a | 9.7 ± 0.1 y | 10.9 ± 0.0 b | 10.1 ± 0.0 z |
| Palmitic | 13.2 | 13.9 ± 0.1 c | 4.6 ± 0.0 wv | 15.2 ± 0.0 b | 6.1 ± 0.1 y | 16.3 ± 0.1 a | 6.8 ± 0.0 z |
| Stearic | 2.4 | 1.9 ± 0.0 c | 1.0 ± 0.0 y | 2.1 ± 0.0 b | 1.0 ± 0.0 y | 2.2 ± 0.0 a | 1.1 ± 0.0 z |
| Oleic | 8.6 | 2.9 ± 0.2 c | 18.9 ± 0.0 y | 2.4 ± 0.0 d | 15.1 ± 0.1 x | 1.8 ± 0.0 e | 13.4 ± 0.0 w |
| Linoleic | 7.2 | 2.0 ± 0.1 c | 14.1 ± 0.0 y | 1.6 ± 0.0 d | 11.2 ± 0.1 x | 1.1 ± 0.0 e | 9.9 ± 0.0 w |
| α-linolenic | 3.3 | 0.9 ± 0.1 c | 6.3 ± 0.0 y | 0.6 ± 0.0 cd | 5.0 ± 0.0 x | 0.5 ± 0.0 d | 4.5 ± 0.0 w |
| FFA | P1 Hexane 1:4 | P2 Hexane 1:6 | P3 Hexane 1:8 | |||
|---|---|---|---|---|---|---|
| SF | LF | SF | LF | SF | LF | |
| Lauric | 91.3 ± 0.5 a | 8.7 ± 0.5 v | 89.9 ± 1.1 a | 10.1 ± 1.1 v | 85.4 ± 0.0 b | 14.6 ± 0.0 w |
| Myristic | 87.0 ± 0.4 a | 13.1 ± 0.4 v | 87.9 ± 1.0 a | 12.2 ± 1.1 v | 82.2 ± 0.0 b | 17.9 ± 0.1 w |
| Palmitic | 94.0 ± 0.3 a | 6.0 ± 0.3 v | 92.6 ± 1.1 a | 7.5 ± 1.1 v | 90.0 ± 0.2 b | 10.0 ± 0.1 w |
| Stearic | 91.0 ± 0.3 a | 9.1 ± 0.2 v | 91.4 ± 1.0 a | 8.6 ± 1.0 v | 88.0 ± 0.2 b | 12.1 ± 0.2 w |
| Oleic | 57.0 ± 1.4 b | 43.1 ± 1.3 v | 66.1 ± 1.8 a | 33.9 ± 1.8 u | 50.5 ± 1.3 c | 49.5 ± 1.3 w |
| Linoleic | 55.8 ± 1.4 b | 44.3 ± 1.5 v | 65.2 ± 1.8 a | 34.8 ± 1.8 u | 49.2 ± 1.3 c | 50.9 ± 1.3 w |
| α-linolenic | 55.2 ± 1.2 b | 44.9 ± 1.2 v | 64.6 ± 2.2 a | 35.5 ± 2.2 u | 48.4 ± 1.3 c | 51.6 ± 1.3 w |
| Total FFA | 85.2 ± 2.6 | 16.8 ± 0.2 | 86.4 ± 2.2 | 15.7 ± 1.1 | 79.6 ± 0.2 | 22.7 ± 0.4 |
| FFA | P4 Hexane 1:10 | P5 Hexane 1:15 | P6 Hexane 1:20 | |||
| SF | LF | SF | LF | SF | LF | |
| Lauric | 81.2 ± 0.1 c | 18.8 ± 0.1 x | 64.8 ± 0.8 d | 35.3 ± 0.8 y | 52.8 ± 0.2 e | 47.2 ± 0.1 z |
| Myristic | 71.0 ± 0.2 c | 29.1 ± 0.2 x | 58.2 ± 1.0 d | 41.8 ± 1.0 y | 45.6 ± 0.3 e | 54.4 ± 0.3 z |
| Palmitic | 87.0 ± 0.1 c | 13.0 ± 0.1 x | 75.3 ± 0.8 d | 24.8 ± 0.8 y | 65.2 ± 0.1 e | 34.9 ± 0.1 z |
| Stearic | 80.6 ± 0.9 c | 19.4 ± 0.8 x | 71.5 ± 0.9 d | 28.6 ± 0.9 y | 60.1 ± 0.3 e | 39.9 ± 0.3 z |
| Oleic | 25.3 ± 1.1 d | 74.7 ± 1.1 x | 16.1 ± 0.4 e | 84.0 ± 0.5 y | 9.4 ± 0.1 f | 90.7 ± 0.1 z |
| Linoleic | 24.3 ± 0.5 d | 75.7 ± 0.4 x | 14.4 ± 0.3 e | 85.7 ± 0.4 y | 8.0 ± 0.1 f | 92.0 ± 0.1 z |
| α-linolenic | 22.9 ± 2.1 d | 77.1 ± 2.1 x | 13.4 ± 0.3 e | 86.6 ± 0.4 y | 7.5 ± 0.0 f | 92.5 ± 0.0 z |
| Total FFA | 70.5 ± 0.8 | 32.0 ± 0.4 | 54.2 ± 0.4 | 44.9 ± 1.1 | 43.2 ± 0.4 | 55.4 ± 0.1 |
| SF | LF | |
|---|---|---|
| P1 | 0.78 ± 0.18 * | 2.01 ± 0.30 b * |
| P2 | 1.11 ± 0.04 * | 3.42 ± 0.07 ab * |
| P3 | 1.06 ± 0.11 * | 3.60 ± 0.48 a * |
| P4 | 1.41 ± 0.47 | 1.99 ± 0.32 b |
| P5 | 1.25 ± 0.20 | 2.60 ± 0.57 ab |
| P6 | 1.84 ± 0.39 | 2.83 ± 0.33 ab |
| CONCENTRATION (wt%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FFA | Raw FFA | Hexane | Ethanol | Acetone | ||||||||
| SF | LF | SF | LF | SF | LF | |||||||
| Lauric | 50.3 | 64.6 ± 0.2 a | 33.0 ± 0.3 | 31.7 ± 1.5 | 59.1 ± 0.4 b | 56.3 ± 0.1 c | 50.8 ± 0.1 | |||||
| Myristic | 10.3 | 10.8 ± 0.0 b | 9.8 ± 0.1 | 13.9 ± 0.6 | 9.8 ± 0.3 c | 12.0 ± 0.0 a | 8.8 ± 0.0 | |||||
| Palmitic | 13.2 | 13.9 ± 0.1 b | 4.6 ± 0.0 | 36.7 ± 1.8 | 5.4 ± 0.5 c | 17.4 ± 0.1 a | 3.7 ± 0.0 | |||||
| Stearic | 2.4 | 1.9 ± 0.0 b | 1.0 ± 0.0 | 5.4 ± 0.1 | 0.8 ± 0.1 c | 2.5 ± 0.0 a | 0.6 ± 0.0 | |||||
| Oleic | 8.6 | 2.9 ± 0.2 c | 18.9 ± 0.0 | 3.8 ± 0.0 | 9.2 ± 0.1 a | 3.9 ± 0.0 b | 12.9 ± 0.1 | |||||
| Linoleic | 7.2 | 2.0 ± 0.1 c | 14.1 ± 0.0 | 2.4 ± 0.0 | 6.5 ± 0.2 a | 2.7 ± 0.0 b | 9.6 ± 0.1 | |||||
| α-linolenic | 3.3 | 0.9 ± 0.1 c | 6.3 ± 0.0 | 1.1 ± 0.0 | 2.6 ± 0.1 a | 1.2 ± 0.0 b | 4.3 ± 0.0 | |||||
| RECOVERY (%) | ||||||||||||
| FFA | Hexane | Ethanol | Acetone | |||||||||
| SF | LF | SF | LF | SF | LF | |||||||
| Lauric | 81.2 ± 0.1 a | 18.8 ± 0.1 | 16.3 ± 2.0 | 83.7 ± 2.0 a | 55.2 ± 0.6 b | 44.8 ± 0.6 | ||||||
| Myristic | 71.0 ± 0.2 a | 29.1 ± 0.2 | 33.9 ± 3.9 | 66.1 ± 3.9 ab | 60.3 ± 0.3 b | 39.7 ± 0.3 | ||||||
| Palmitic | 87.0 ± 0.1 a | 13.0 ± 0.1 | 71.2 ± 3.1 | 28.8 ± 3.1 b | 84.0 ± 0.3 a | 16.0 ± 0.3 | ||||||
| Stearic | 80.6 ± 0.9 a | 19.4 ± 0.8 | 71.6 ± 4.1 | 28.4 ± 4.1 b | 81.4 ± 0.1 a | 18.6 ± 0.1 | ||||||
| Oleic | 25.3 ± 1.1 b | 74.7 ± 1.1 | 13.0 ± 1.0 | 87.0 ± 1.0 a | 25.1 ± 0.5 b | 74.9 ± 0.5 | ||||||
| Linoleic | 24.3 ± 0.5 b | 75.7 ± 0.4 | 11.7 ± 0.8 | 88.3 ± 0.8 a | 24.0 ± 0.0 b | 76.0 ± 0.0 | ||||||
| α-linolenic | 22.9 ± 2.1 b | 77.1 ± 2.1 | 12.8 ± 0.7 | 87.2 ± 0.7 a | 23.7 ± 0.1 b | 76.3 ± 0.1 | ||||||
| Total FFA | 70.5 ± 0.8 | 32.0 ± 0.4 | 28.1 ± 2.5 | 77.6 ± 1.2 | 51.7 ± 0.3 | 46.4 ± 0.6 | ||||||
| Temperature (°C) | Pressure (mbar) | Purity (%) | Recovery (%) | ||
|---|---|---|---|---|---|
| One-stage flash distillation | Case 1 (max purity) | 102 | 0.11 | 85.8 | 20.2 |
| Case 2 (max recovery) | 134 | 0.27 | 53.4 | 98.9 | |
| Multistage distillation (10-stage) | 120 | 0.19 | 96.7 | 80.0 | |
| Temperature (°C) | Pressure (mbar) | Purity (%) | Recovery (%) | ||
|---|---|---|---|---|---|
| One-stage flash distillation | Case 1 (max purity) | 82 | 0.18 | 87.1 | 26.2 |
| Case 2 (max recovery) | 112 | 0.25 | 54.9 | 98.9 | |
| Multistage distillation (10-stage) | 120 | 1.10 | 97.4 | 80.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, L.; Reyero, C.; Hurtado-Ribeira, R.; Villanueva-Bermejo, D.; Belinchón, A.; Palomar, J.; Fornari, T.; Martín, D. Assessment of Scalable Fractionation Methodologies to Produce Concentrated Lauric Acid from Black Soldier Fly (Hermetia illucens) Larvae Fat. Insects 2025, 16, 171. https://doi.org/10.3390/insects16020171
Vázquez L, Reyero C, Hurtado-Ribeira R, Villanueva-Bermejo D, Belinchón A, Palomar J, Fornari T, Martín D. Assessment of Scalable Fractionation Methodologies to Produce Concentrated Lauric Acid from Black Soldier Fly (Hermetia illucens) Larvae Fat. Insects. 2025; 16(2):171. https://doi.org/10.3390/insects16020171
Chicago/Turabian StyleVázquez, Luis, Carlota Reyero, Raúl Hurtado-Ribeira, David Villanueva-Bermejo, Alejandro Belinchón, José Palomar, Tiziana Fornari, and Diana Martín. 2025. "Assessment of Scalable Fractionation Methodologies to Produce Concentrated Lauric Acid from Black Soldier Fly (Hermetia illucens) Larvae Fat" Insects 16, no. 2: 171. https://doi.org/10.3390/insects16020171
APA StyleVázquez, L., Reyero, C., Hurtado-Ribeira, R., Villanueva-Bermejo, D., Belinchón, A., Palomar, J., Fornari, T., & Martín, D. (2025). Assessment of Scalable Fractionation Methodologies to Produce Concentrated Lauric Acid from Black Soldier Fly (Hermetia illucens) Larvae Fat. Insects, 16(2), 171. https://doi.org/10.3390/insects16020171









