A Pre-Exposure to Male-Specific Compound γ-Hexalactone Reduces Oviposition in Bactrocera oleae (Rossi) (Diptera: Tephritidae) Under Laboratory Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Pre-Exposure to γ-Hexalactone
2.3. Oviposition Assays
2.4. Statistical Analysis
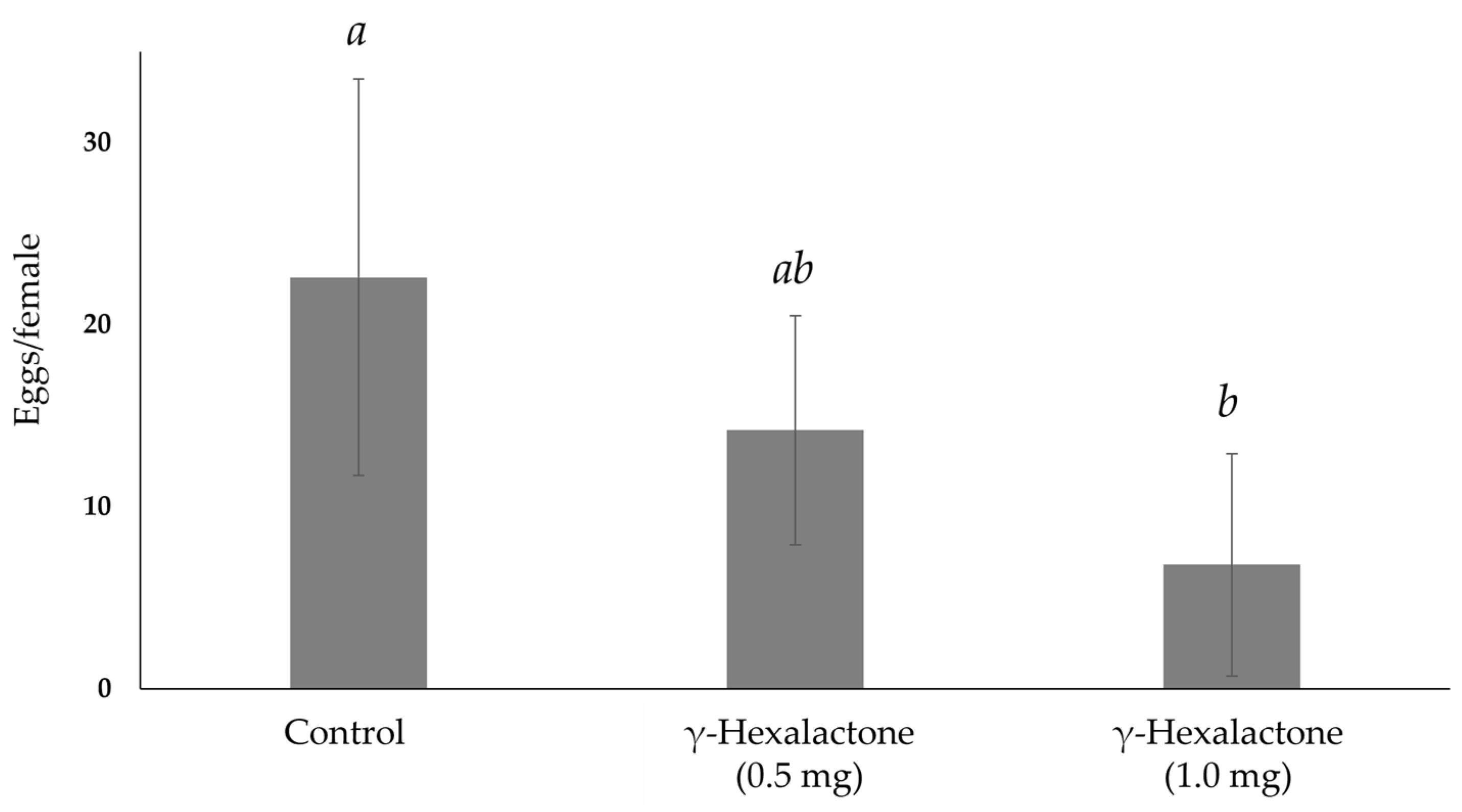
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daane, K.M.; Johnson, M.W. Olive Fruit Fly: Managing an Ancient Pest in Modern Times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.S. The Biology of Dacine Fruit Flies. Annu. Rev. Entomol. 1987, 32, 115–144. [Google Scholar] [CrossRef]
- Medjkouh, L.; Tamendjari, A.; Keciri, S.; Santos, J.; Nunes, M.A.; Oliveira, M. The Effect of the Olive Fruit Fly (Bactrocera oleae) on Quality Parameters, and Antioxidant and Antibacterial Activities of Olive Oil. Food Funct. 2016, 7, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Angerosa, F.; Di Giacinto, L.; Solinas, M. Influence of Dacus oleae Infestation on Flavor of Oils, Extracted from Attacked Olive Fruits, by HPLC and HRGC Analyses of Volatile Compounds. Grasas Y Aceites 1992, 43, 134–142. [Google Scholar] [CrossRef]
- Neuenschwander, P.; Michelakis, S.; Mikros, L.; Mathioudis, M. Compensation for Early Fruit Drop Caused by Dacus oleae (Gmel.) (Diptera, Tephritidae) Due to an Increase in Weight and Oil Content of the Remaining Olives. Zeitschrift Für Angew. Entomol. 1980, 89, 514–525. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Del Carlo, M.; Compagnone, D.; Cichelli, A. Effects of Fly Attack (Bactrocera oleae) on the Phenolic Profile and Selected Chemical Parameters of Olive Oil. J. Agric. Food Chem. 2008, 56, 4577–4583. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A Review of Bactrocera oleae (Rossi) Impact in Olive Products: From the Tree to the Table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Universitat Pompeu Fabra. ENTOMATIC. Bioacustic Identification of the Olive Fruit Fly. Available online: https://www.upf.edu/web/entomatic/concept-objectives (accessed on 11 November 2024).
- Mazomenos, B.E.; Haniotakis, G.E. A Multicomponent Female Sex Pheromone of Dacus oleae Gmelin: Isolation and Bioassay. J. Chem. Ecol. 1981, 7, 437–444. [Google Scholar] [CrossRef]
- Baker, R.; Herbert, R.; Howse, P.E.; Jones, O.T.; Francke, W.; Reith, W. Identification and Synthesis of the Major Sex Pheromone of the Olive Fly (Dacus oleae). J. Chem. Soc. Chem. Commun. 1106, 52–53. [Google Scholar] [CrossRef]
- Mavraganis, V.G.; Papadopoulos, N.T.; Kouloussis, N.A. Extract of Olive Fruit Fly Males (Diptera: Tephritidae) Attract Virgin Females. Entomol. Hell. 2010, 19, 14–20. [Google Scholar] [CrossRef][Green Version]
- Carpita, A.; Canale, A.; Raffaelli, A.; Saba, A.; Benelli, G.; Raspi, A. (Z)-9-Tricosene Identified in Rectal Gland Extracts of Bactrocera oleae Males: First Evidence of a Male-Produced Female Attractant in Olive Fruit Fly. Naturwissenschaften 2012, 99, 77–81. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Acín, P.; Gómez-Zubiaur, A.; Corbella-Martorell, C.; Quero, C. A Shift in the Paradigm? A Male-Specific Lactone Increases the Response of Both Sexes of the Olive Fruit Fly Bactrocera oleae to the Food Lure Ammonium Bicarbonate. J. Pest Sci. 2024, 97, 965–978. [Google Scholar] [CrossRef]
- De Marzo, L.; Nuzzaci, G.; Solinas, M. Studio Anatomico, Istologico, Ultrastrutturale e Fisiologico Del Retto ed Osservazioni Etologiche in Relazione Alla Possibile Produzione di Feromoni Sessuali Nel Maschio di Dacus oleae Gmel. Entomologica 1978, 14, 203–266. [Google Scholar]
- Anderson, P.; Anton, S. Experience-based Modulation of Behavioural Responses to Plant Volatiles and Other Sensory Cues in Insect Herbivores. Plant. Cell Environ. 2014, 37, 1826–1835. [Google Scholar] [CrossRef]
- Judd, G.J.R.; Gardiner, M.G.T.; DeLury, N.C.; Karg, G. Reduced Antennal Sensitivity, Behavioural Response, and Attraction of Male Codling Moths, Cydia pomonella, to Their Pheromone (E,E)-8,10-dodecadien-1-ol Following Various Pre-exposure Regimes. Entomol. Exp. Appl. 2005, 114, 65–78. [Google Scholar] [CrossRef]
- Anderson, P.; Sadek, M.M.; Hansson, B.S. Pre-Exposure Modulates Attraction to Sex Pheromone in a Moth. Chem. Senses 2003, 28, 285–291. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Gut, L.J.; Vogel, K.J.; Miller, J.R. Behaviors of Naïve vs. Pheromone-Exposed Leafroller Moths in Plumes from High-Dosage Pheromone Dispensers in a Sustained-Flight Wind Tunnel: Implications for Mating Disruption of These Species. J. Insect Behav. 2004, 17, 533–554. [Google Scholar] [CrossRef]
- Dodson, G. Lek Mating System and Large Male Aggressive Advantage in a Gall-Forming Tephritid Fly (Diptera: Tephritidae). Ethology 1986, 72, 99–108. [Google Scholar] [CrossRef]
- Johny, J.; Nihad, M.; Alharbi, H.A.; AlSaleh, M.A.; Antony, B. Silencing Sensory Neuron Membrane Protein RferSNMPu1 Impairs Pheromone Detection in the Invasive Asian Palm Weevil. Sci. Rep. 2024, 14, 16541. [Google Scholar] [CrossRef]
- Hagen, K.S.; Santas, L.; Tsecouras, A. A Technique of Culturing the Olive Fly, Dacus oleae Gmel., on Synthetic Media under Xenic Conditions. In Proceedings of the Symposium on the Use and Application of Radioisotopes and Radiation in the Control of Plant and Animal Insect Pests, Athens, Greece, 22–26 April 1963; pp. 333–356. [Google Scholar]
- Manoukas, A.G.; Mazomenos, B. Effect of Antimicrobials upon Eggs and Larvae of Dacus oleae (Diptera, Tephritidae) and the Use of Propionates for Larval Diet Preservation. Ann. Zool. Ecol. Anim. 1977, 9, 277–285. [Google Scholar]
- Tsitsipis, J.A.; Kontos, A. Improved Solid Adult Diet for the Olive Fruit Fly, Dacus oleae. Entomol. Hell. 2017, 1, 24. [Google Scholar] [CrossRef]
- Kramer, W.L.; Mulla, M.S. Oviposition Attractants and Repellents of Mosquitoes: Oviposition Responses of Culex Mosquitoes to Organic Infusions. Environ. Entomol. 1979, 8, 1111–1117. [Google Scholar] [CrossRef]
- Gavara, A.; Navarro-Llopis, V.; Primo, J.; Vacas, S. Laboratory Investigation of Pheromone Pre-exposure in Lobesia botrana Males Indicates Minor Role of Desensitization in the Field. Physiol. Entomol. 2022, 47, 126–135. [Google Scholar] [CrossRef]
- Kuhns, E.H.; Pelz-Stelinski, K.; Stelinski, L.L. Reduced Mating Success of Female Tortricid Moths Following Intense Pheromone Auto-Exposure Varies with Sophistication of Mating System. J. Chem. Ecol. 2012, 38, 168–175. [Google Scholar] [CrossRef]
- Stelinski, L.; Holdcraft, R.; Rodriguez-Saona, C. Female Moth Calling and Flight Behavior Are Altered Hours Following Pheromone Autodetection: Possible Implications for Practical Management with Mating Disruption. Insects 2014, 5, 459–473. [Google Scholar] [CrossRef]
- Anderson, P.; Hansson, B.S.; Nilsson, U.; Han, Q.; Sjoholm, M.; Skals, N.; Anton, S. Increased Behavioral and Neuronal Sensitivity to Sex Pheromone after Brief Odor Experience in a Moth. Chem. Senses 2007, 32, 483–491. [Google Scholar] [CrossRef]
- Guerrieri, F.; Gemeno, C.; Monsempes, C.; Anton, S.; Jacquin-Joly, E.; Lucas, P.; Devaud, J.-M. Experience-Dependent Modulation of Antennal Sensitivity and Input to Antennal Lobes in Male Moths (Spodoptera littoralis) Pre-Exposed to Sex Pheromone. J. Exp. Biol. 2012, 215, 2334–2341. [Google Scholar] [CrossRef]
- D’Errico, G.; Faraone, N.; Rotundo, G.; De Cristofaro, A.; Trimble, R.M. Sensory Adaptation of Antennae and Sex Pheromone-Mediated Flight Behavior in Male Oriental Fruit Moths (Lepidoptera: Tortricidae) after Prolonged Exposure to Single and Tertiary Blends of Synthetic Sex Pheromone. Environ. Entomol. 2013, 42, 548–557. [Google Scholar] [CrossRef]
- Suckling, D.M.; Stringer, L.D.; Jiménez-Pérez, A.; Walter, G.H.; Sullivan, N.; El-Sayed, A.M. With or without Pheromone Habituation: Possible Differences between Insect Orders? Pest Manag. Sci. 2018, 74, 1259–1264. [Google Scholar] [CrossRef]
- Thackery, D.; Burman, J. The Effects of Synthetic Pheromone Exposure on Female Oviposition and Male Longevity in Zygaena filipendulae (Linnaeus, 1758) (Lepidoptera: Zygaenidae, Zygaeninae). Entomol. Gaz. 2016, 67, 249–256. [Google Scholar]
- Frechette, B.; Dixon, A.F.G.; Alauzet, C.; Hemptinne, J. Age and Experience Influence Patch Assessment for Oviposition by an Insect Predator. Ecol. Entomol. 2004, 29, 578–583. [Google Scholar] [CrossRef]
- Gazit, Y.; Akiva, R.; Kramer, R.; Yehezkel, A.; Yaacobi, G.; Nestel, D. Response of Male Bactrocera zonata (Diptera: Tephritidae) to Methyl Eugenol: Can They Be Desensitized? Fla. Entomol. 2024, 107, 20240071. [Google Scholar] [CrossRef]
- Būda, V.; Blažyte-Cereškiene, L.; Radžiutė, S.; Apšegaitė, V.; Stamm, P.; Schultz, S.; Aleknavičius, D.; Mozūraitis, R. Male-Produced (−)-δ-Heptalactone, Pheromone of Fruit Fly Rhagoletis batava (Diptera: Tephritidae), a Sea Buckthorn Berries Pest. Insects 2020, 11, 138. [Google Scholar] [CrossRef]
- Sarles, L.; Fassotte, B.; Boullis, A.; Lognay, G.; Verhaeghe, A.; Markó, I.; Verheggen, F.J. Improving the Monitoring of the Walnut Husk Fly (Diptera: Tephritidae) Using Male-Produced Lactones. J. Econ. Entomol. 2018, 111, 2032–2037. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R.; Jang, E.B.; Shelly, T.E. Pheromones, Male Lures, and Trapping of Tephritid Fruit Flies. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies: Lures, Area-Wide Programs, and Trade Implications; Shelly, T., Epsky, N., Jang, E., Reyes-Flores, J., Vargas, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 15–74. [Google Scholar]
- Gerofotis, C.D.; Ioannou, C.S.; Papadopoulos, N.T. Aromatized to Find Mates: α-Pinene Aroma Boosts the Mating Success of Adult Olive Fruit Flies. PLoS ONE 2013, 8, e81336. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Haniotakis, G.E. Male Olive Fruit Fly Attraction to Synthetic Sex Pheromone Components in Laboratory and Field Tests. J. Chem. Ecol. 1985, 11, 397–405. [Google Scholar] [CrossRef]
- Kokkari, A.I.; Milonas, P.G.; Anastasaki, E.; Floros, G.D.; Kouloussis, N.A.; Koveos, D.S. Determination of Volatile Substances in Olives and Their Effect on Reproduction of the Olive Fruit Fly. J. Appl. Entomol. 2021, 145, 841–855. [Google Scholar] [CrossRef]
- Kokkari, A.; Kouloussis, N.A.; Floros, G.; Koveos, D.S. Effect of Olive Fruit Volatiles on Landing, Egg Production, and Longevity of Bactrocera oleae Females under Different Temperatures. Insects 2024, 15, 728. [Google Scholar] [CrossRef]
- Kovaiou, S.K.; Kokkari, A.; Floros, G.; Kantiranis, N.; Kouloussis, N.A.; Filippidis, A.A.; Koveos, D.S. Oviposition-Deterrent Effect of a High-Quality Natural Zeolite on the Olive Fruit Fly Bactrocera oleae, under Different Conditions of Temperature and Relative Humidity. Insects 2024, 15, 256. [Google Scholar] [CrossRef]
- Checchia, I.; Perin, C.; Mori, N.; Mazzon, L. Oviposition Deterrent Activity of Fungicides and Low-Risk Substances for the Integrated Management of the Olive Fruit Fly Bactrocera oleae (Diptera, Tephritidae). Insects 2022, 13, 363. [Google Scholar] [CrossRef]
- Daher, E.; Cinosi, N.; Chierici, E.; Rondoni, G.; Famiani, F.; Conti, E. Field and Laboratory Efficacy of Low-Impact Commercial Products in Preventing Olive Fruit Fly, Bactrocera oleae, Infestation. Insects 2022, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Mojdehi, M.R.A.; Keyhanian, A.A.; Rafiei, B. Application of Oviposition Deterrent Compounds for the Control of Olive Fruit Fly, Bactrocera oleae Rossi (Dip. Tephritidae) Control. Int. J. Trop. Insect Sci. 2022, 42, 63–70. [Google Scholar] [CrossRef]
- Cardé, R.T.; Minks, A.K. Control of Moth Pests by Mating Disruption: Successes and Constraints. Annu. Rev. Entomol. 1995, 40, 559–585. [Google Scholar] [CrossRef]
- Miller, J.R.; Gut, L.J. Mating Disruption for the 21st Century: Matching Technology With Mechanism. Environ. Entomol. 2015, 44, 427–453. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.T.; Lisk, J.C.; Howse, P.E.; Baker, R.; Montiel Bueno, A.; Ramos, C. Mating Disruption of the Olive Fruit Fly (Dacus oleae) with the Major Component of Its Sex Pheromone. In Proceedings of the CEC/IOBC International Symposium, Fruit Flies of Economic Importance, Athens, Greece, 16–19 November 1983; pp. 500–505. [Google Scholar]
- Montiel-Bueno, A.; Simón-Mata, M.A. La Interrupción de La Comunicación Sexual de La Mosca Del Olivo (Dacus oleae Gmel.) Como Estrategia de Lucha Integrada En Olivar. Bol. Serv. Plagas 1985, 11, 11–23. [Google Scholar]
- Montiel, A.; Jones, O.T. Estado Actual Del Uso de Feromonas en El Manejo Integrado de Plagas Del Olivo. Bol. Sanid. Veg. Plagas 1989, 15, 161–173. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, S.; Corbella-Martorell, C.; Tarantino, E.; Quero, C. A Pre-Exposure to Male-Specific Compound γ-Hexalactone Reduces Oviposition in Bactrocera oleae (Rossi) (Diptera: Tephritidae) Under Laboratory Conditions. Insects 2025, 16, 147. https://doi.org/10.3390/insects16020147
López S, Corbella-Martorell C, Tarantino E, Quero C. A Pre-Exposure to Male-Specific Compound γ-Hexalactone Reduces Oviposition in Bactrocera oleae (Rossi) (Diptera: Tephritidae) Under Laboratory Conditions. Insects. 2025; 16(2):147. https://doi.org/10.3390/insects16020147
Chicago/Turabian StyleLópez, Sergio, Clàudia Corbella-Martorell, Elisa Tarantino, and Carmen Quero. 2025. "A Pre-Exposure to Male-Specific Compound γ-Hexalactone Reduces Oviposition in Bactrocera oleae (Rossi) (Diptera: Tephritidae) Under Laboratory Conditions" Insects 16, no. 2: 147. https://doi.org/10.3390/insects16020147
APA StyleLópez, S., Corbella-Martorell, C., Tarantino, E., & Quero, C. (2025). A Pre-Exposure to Male-Specific Compound γ-Hexalactone Reduces Oviposition in Bactrocera oleae (Rossi) (Diptera: Tephritidae) Under Laboratory Conditions. Insects, 16(2), 147. https://doi.org/10.3390/insects16020147






