Seasonal and Spatial Distribution of Fall Armyworm Larvae in Maize Fields: Implications for Integrated Pest Management
Simple Summary
Abstract
1. Introduction
2. Material and Methods
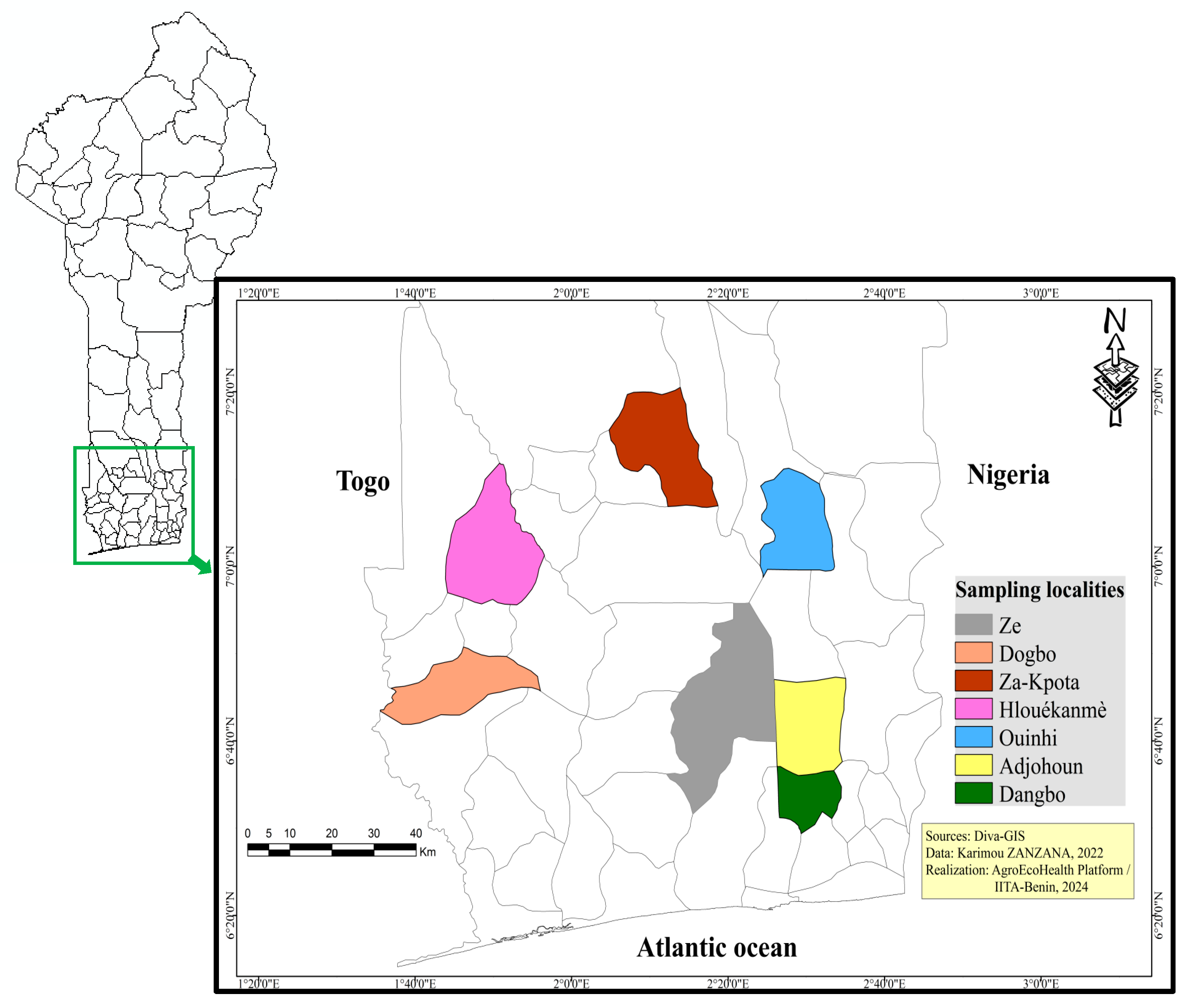
2.1. Study Area
2.2. Sampling of Fall Armyworm Larvae in Maize Fields
2.3. Parameters
2.4. Meteorological Data
2.5. Data Analysis
2.5.1. Larval Dispersion Model Analysis in Maize Fields
2.5.2. Statistical Analysis
3. Results
3.1. Larval Infestation of Maize by FAW in Dry and Rainy Seasons
3.2. Density of FAW Larvae During the Dry and Rainy Season
3.3. Percentage of Damaged Plants During the Dry and Rainy Seasons
3.4. Leaf Damage Severity During the Dry and Rainy Season
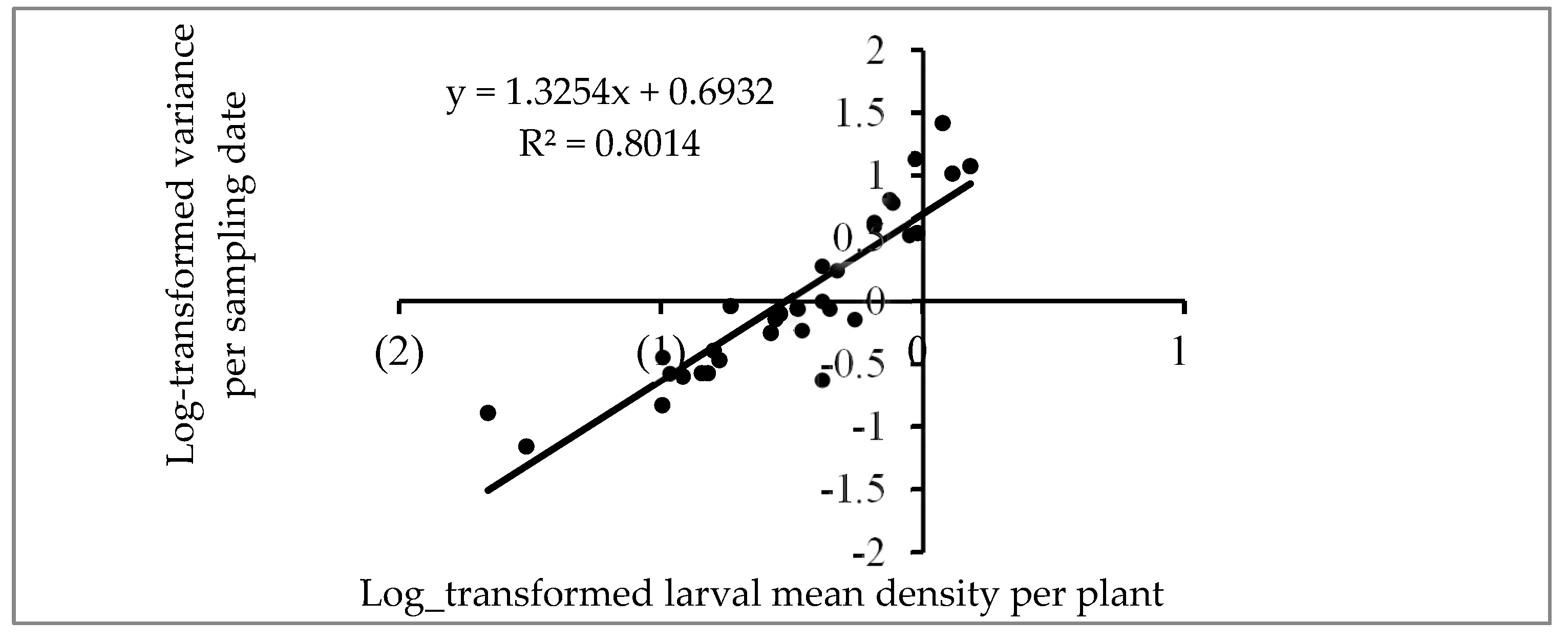
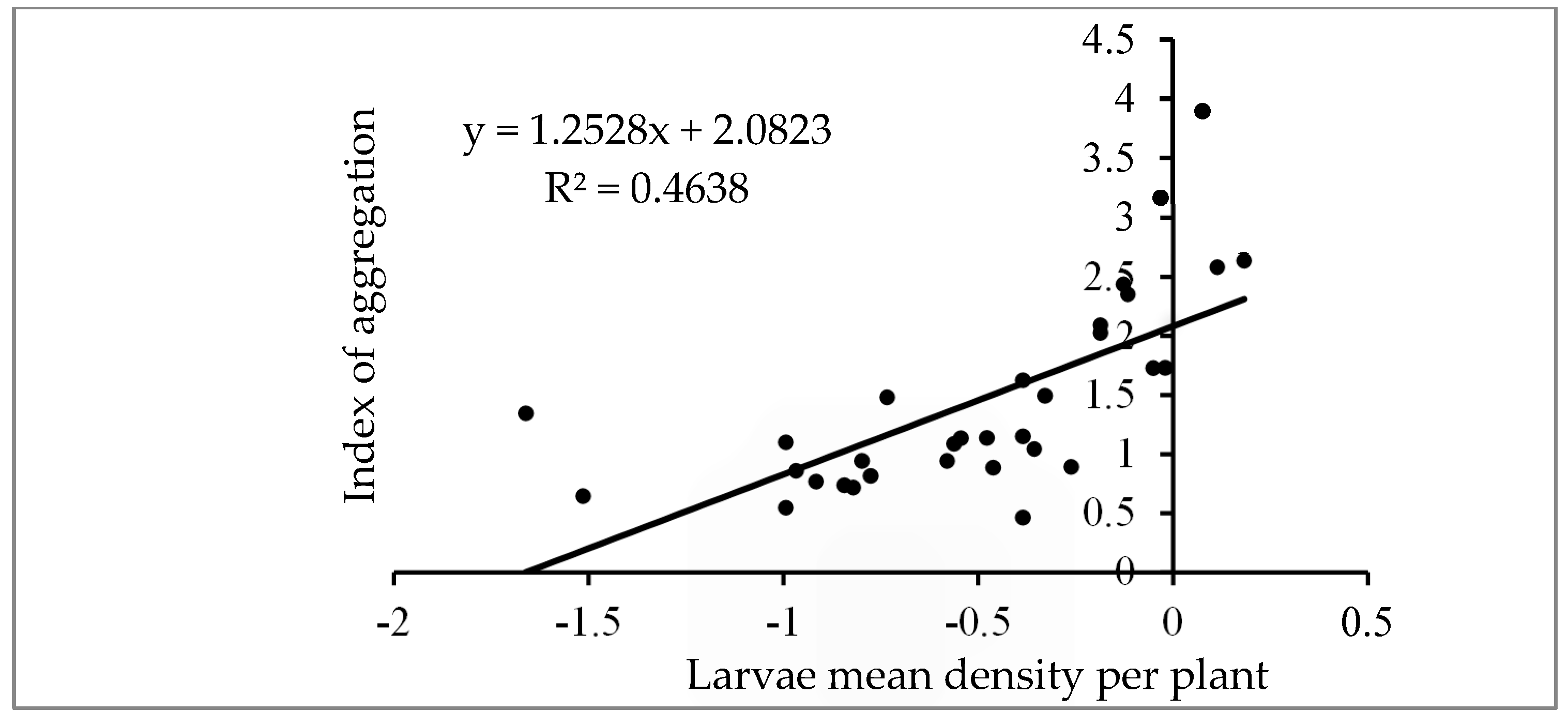
3.5. Dispersion Pattern of FAW Larvae
3.6. Temperature and Precipitation During the Survey
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, 0165632. [Google Scholar] [CrossRef]
- CABI. Spodoptera frugiperda (Fall Armyworm). Available online: https://www.cabi.org/isc/datasheet/29810 (accessed on 14 July 2024).
- Tao, W.C.; Zhang, X.Y.; Zhang, Y.; Deng, X.Y.; Zhang, H.L.; Zhang, Z.H.; Li, Q.; Jiang, C.X. Effects of the Host Plants of the Maize-Based Intercropping Systems on the Growth, Development and Preference of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2024, 15, 26. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M.; Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management, 1st ed.; CIMMYT: Mexico City, Mexico, 2018. [Google Scholar]
- Rwomushana, I.; Bateman, M.; Beale, T.; Beseh, P.; Cameron, K.; Chiluba, M.; Clottey, V.; Davis, T.; Day, R.; Early, R.; et al. Fall Armyworm: Impacts and Implications for Africa. CABI Evidence Note Update; CABI: Oxfordshire, UK, 2018. [Google Scholar]
- Toepfer, S.; Fallet, P.; Kajuga, J.; Bazagwira, D.; Mukundwa, I.P.; Szalai, M.; Turlings, T.C.J. Streamlining Leaf Damage Rating Scales for the Fall Armyworm on Maize. J. Pest. Sci. 2021, 94, 1075–1089. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall Armyworm: Impacts and Implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Hafeez, M.; Li, X.W.; Zhang, J.M.; Zhang, Z.J.; Huang, J.; Wang, L.K.; Khan, M.M.; Shah, S.; Fernández-Grandon, G.M.; Lu, Y. Bin Role of Digestive Protease Enzymes and Related Genes in Host Plant Adaptation of a Polyphagous Pest, Spodoptera frugiperda. Insect Sci. 2021, 28, 611–626. [Google Scholar] [CrossRef]
- Hafeez, M.; Li, X.; Chen, L.; Ullah, F.; Huang, J.; Zhang, Z.; Zhang, J.; Siddiqui, J.A.; Zhou, S.X.; Ren, X.Y.; et al. Molecular Characterization and Functional Analysis of Cytochrome P450-Mediated Detoxification CYP302A1 Gene Involved in Host Plant Adaptation in Spodoptera frugieprda. Front. Plant. Sci. 2023, 13, 1079442. [Google Scholar] [CrossRef]
- FAO. The Global Action for Fall Armyworm Control: Action Framework 2020–2022: Working Together to Tame the Global Threat; FAO: Rome, Italy, 2020. [Google Scholar]
- Han, S.-P.; Zhou, Y.-Y.; Wang, D.; Qin, Q.-J.; Song, P.; He, Y.-Z. Impact of Host Plants on Biological Characteristics and Vg/VgR Expression of Spodoptera frugiperda. J. Pest. Sci. 2022, 96, 1569–1577. [Google Scholar] [CrossRef]
- Koffi, D.; Kyerematen, R.; Eziah, V.Y.; Osei-Mensah, Y.O.; Afreh-Nuamah, K.; Aboagye, E.; Osae, M.; Meagher, R.L. Assessment of Impacts of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) on Maize Production in Ghana. J. Integr. Pest. Manag. 2021, 11. [Google Scholar] [CrossRef]
- Suby, S.B.; Soujanya, P.L.; Yadava, P.; Patil, J.; Subaharan, K.; Prasad, G.S.; Babu, K.S.; Jat, S.L.; Yathish, K.R.; Vadassery, J.; et al. Invasion of Fall Armyworm (Spodoptera frugiperda) in India: Nature, Distribution, Management and Potential Impact. Curr. Sci. 2020, 119, 44–51. [Google Scholar] [CrossRef]
- Houngbo, S.; Zannou, A.; Aoudji, A.; Sossou, H.C.; Sinzogan, A.; Sikirou, R.; Zossou, E.; Totin Vodounon, H.S.; Adomou, A.; Ahanchédé, A. Farmers’ Knowledge and Management Practices of Fall Armyworm, Spodoptera frugiperda (J.E. Smith) in Benin, West Africa. Agriculture 2020, 10, 430. [Google Scholar] [CrossRef]
- Kogan, M.; Herzog, D.C. Sampling Methods in Soybean Entomology; Springer Science & Business Media: New York, NY, USA, 1980. [Google Scholar]
- Rahmathull, V.K.; Sathyanara, K.; Angadi, B.S. Influence of Abiotic Factors on Population Dynamics of Major Insect Pests of Mulberry. Pak. J. Biol. Sci. 2015, 18, 215–223. [Google Scholar] [CrossRef]
- Cammell, M.E.; Knight, J.D. Effects of Climatic Change on the Population Dynamics of Crop Pests. Adv. Ecol. Res. 1992, 22, 117–162. [Google Scholar]
- Hamby, K.A.; Bellamy, D.E.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and Abiotic Factors Impacting Development, Behavior, Phenology, and Reproductive Biology of Drosophila suzukii. J. Pest. Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Omoregie, M.E.; Enobakhare, D.A.; Omoregie, A.O. Population Dynamics of the Fall Armyworm, Spodoptera frugiperda J. E. Smith (Lepidoptera: Noctuidae) on Early and Late Season Maize. Anim. Res. Int. 2023, 20, 4734–4740. [Google Scholar]
- Schowalter, T.D. Population Systems. In Insect Ecology: An Ecosystem Approach; Academic Press: Cambridge, MA, USA, 2011; pp. 129–156. [Google Scholar]
- Nboyine, J.A.; Kusi, F.; Abudulai, M.; Badii, B.K.; Zakaria, M.; Adu, G.B.; Haruna, A.; Seidu, A.; Osei, V.; Alhassan, S.; et al. A New Pest, Spodoptera frugiperda (J.E. Smith), in Tropical Africa: Its Seasonal Dynamics and Damage in Maize Fields in Northern Ghana. Crop Prot. 2020, 127, 104960. [Google Scholar] [CrossRef]
- Kuno, E. Sampling and Analysis of Insect Populations. Annu. Rev. Entomol. 1991, 36, 285–304. [Google Scholar] [CrossRef]
- Afshari, A.; Soleiman-Negadian, E.; Shishebor, P. Population Density and Spatial Distribution of Aphis gossypii Glover (Homoptera: Aphididae) on Cotton in Gorgan. J. Agric. Sci. Technol 2009, 11, 27–38. [Google Scholar]
- Báez, M.S.; Ibarra, J.E.; Villanueva, F. Distribución Espacial y Tamaño de Muestra de Los Gusanos: Cogollero Spodoptera frugiperda (Smith) y Elotero Heliothis Zea (Boddie) En Cultivo de Maíz. Folia Entomol. Mex. 1980, 45, 58–59. [Google Scholar]
- Farias, P.R.S.; Barbosa, J.C.; Busoli, A.C. Distribuição Espacial Da Lagarta-Do-Cartucho, Spodoptera frugiperda (J.E.Smith) (Lepidoptera: Noctuidae), Na Cultura Do Milho. Neotrop. Entomol. 2001, 30, 681–689. [Google Scholar] [CrossRef]
- Hernández-Mendoza, J.L.; López-Barbosa, E.C.; Garza-González, E.; Mayek-Pérez, N. Spatial Distribution of Spodoptera frugiperda (Lepidoptera: Noctuidae) in Maize Landraces Grown in Colima, Mexico. Int. J. Trop. Insect. Sci. 2008, 28, 126–129. [Google Scholar] [CrossRef]
- He, Y.; Wang, K.; Du, G.; Zhang, Q.; Li, B.; Zhao, L.; He, P.; Chen, B. Temporal and Spatial Distribution Patterns of Spodoptera frugiperda in Mountain Maize Fields in China. Insects 2022, 13, 938. [Google Scholar] [CrossRef]
- Elliott, N.C.; Kieckhefer, R.W.; Walgenbach, D.D. Binomial Sequential Sampling Methods for Cereal Aphids in Small Grains. J. Eco. Entomol. 1990, 83, 1381–1387. [Google Scholar] [CrossRef]
- Taylor, L.R.; Woiwod, I.P.; Perry, J.N. The Negative Binomial as a Dynamic Ecological Model for Aggregation, and the Density Dependence of k. J. Anim. Ecol. 1979, 48, 289–304. [Google Scholar] [CrossRef]
- Taylor, L.R. Assessing and Interpreting the Spatial Distributions of Insect Populations. Annu. Rev. Entomol. 1984, 29, 321–358. [Google Scholar] [CrossRef]
- Anandhi, S.; Saminathan, V.R.; Yasodha, P.; Roseleen, S.S.J.; Sharavanan, P.T.; Rajanbabu, V. Correlation of Fall Armyworm Spodoptera frugiperda (J.E. Smith) with Weather Parameters in Maize Ecosystem. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1213–1218. [Google Scholar] [CrossRef]
- Caniço, A.; Mexia, A.; Santos, L. Seasonal Dynamics of the Alien Invasive Insect Pest. Insects 2020, 11, 512. [Google Scholar] [CrossRef]
- Ramasamy, M.; Das, B.; Ramesh, R. Predicting Climate Change Impacts on Potential Worldwide Distribution of Fall Armyworm Based on CMIP6 Projections. J. Pest. Sci. 2021, 95, 841–854. [Google Scholar] [CrossRef]
- Aholoukpè, H.S.N.; Amadji, G.L.; Koussihouede, H.K.I. Stocks de Carbone Dans Les Sols Des Zones Agro-Écologiques Du Bénin. In Carbon des Sols en Afrique: Impact des Usages des Sols et des Pratiques Agricoles. Carbon des Sols en Afrique: Impact des Usages des Sols et des Pratiques Agricoles; Chevallier, T., Razafimbelo, T.M., Chapuis-Lardy, L., Brossard, M., Eds.; IRD Éditions; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; pp. 101–122. [Google Scholar]
- Davis, F.M.; Ng, S.S.; Williams, W.P. Visual Rating Scales for Screening Whorl-Stage Corn for Resistance to Fall Armyworm. In Technical bulletin (Mississippi Agricultural and Forestry Experiment Station); Department of Information Services, Division of Agriculture, Forestry, and Veterinary Medicine, Mississippi State University: Starkville, MI, USA, 1992; pp. 1–9. [Google Scholar]
- Atachi, P.; Dannon, E.A.; Arodokoun, Y.D.; Tamò, M. Distribution and Sampling of Maruca vitrata (Fabricius) (Lep., Pyralidae) Larvae on Lonchocarpus sericeus (Poir) H.B. and K. J. Appl. Entomol. 2002, 126, 188–193. [Google Scholar] [CrossRef]
- Iwao, S. A New Regression Method for Analyzing the Aggregation Pattern of Animal Populations. Res. Popul. Ecol. 1968, 10, 1–20. [Google Scholar] [CrossRef]
- Ruesink, W.G. Introduction to Sampling Theory. In Sampling Methods in Soybean Entomology; Springer: New York, NY, USA, 1980; pp. 61–78. [Google Scholar]
- Taylor, L.R. Aggregation, Variance and the Mean. Nature 1961, 189, 732–735. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Ecological Methods; Chapman & Hall: London, UK, 1978; p. 524. [Google Scholar]
- Lloyd, M. Mean Crowding. J. Anim. Ecol. 1967, 36, 1–30. [Google Scholar] [CrossRef]
- Zahner, P.J.B.; Delucchi, V.; Graf, B. Distribution and Sampling of Winter Populations of Panonychus ulmi Koch (Acarina: Tetranychidae) on Apple Tree. Oecol. Appl. 1985, 6, 99–110. [Google Scholar]
- Sharanabasappa, S.D.; Kalleshwaraswamy, C.M.; Maruthi, M.S.; Pavithra, H.B. Biology of Invasive Fall Army Worm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) on Maize. Indian J. Entomol. 2018, 80, 540. [Google Scholar] [CrossRef]
- Bakry, M.M.S.; Abdel-Baky, N.F. Population Density of the Fall Armyworm, Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) and Its Response to Some Ecological Phenomena in Maize Crop. Braz. J. Biol. 2023, 83, 1–17. [Google Scholar] [CrossRef]
- Murúa, G.; Molina-Ochoa, J.; Coviella, C. Population Dynamics of the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) and Its Parasitoids in Northwestern Argentina. Fla. Entomol. 2006, 89, 175–182. [Google Scholar] [CrossRef]
- Morrill, W.L.; Greene, G.L. Distribution of Fall Armyworm Larvae. 1. Regions of Field Corn Plants Infested by Larvae 1. Environ. Entomol. 1973, 2, 195–198. [Google Scholar] [CrossRef]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the Global Extent of Invasion of the Cereal Pest Spodoptera frugiperda, the Fall Armyworm. NeoBiota 2018, 50, 25–50. [Google Scholar] [CrossRef]
- van Huis, A. Integrated Pest Management in the Small Farmer’s Maize Crop in Nicaragua. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 1981. [Google Scholar]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Climate Change Increase in Crop Losses to Insect Pests in a Warming Climate. Science 2018, 361, 31. [Google Scholar] [CrossRef]
- Vicente, J.R.; Vaz, A.S.Q.; Isabel, A.; Buchadas, A.R.; Guisan, A.; Kueffer, C.; Marchante, E.; Marchante, H.; Cabral, J.A.; Nesper, M.; et al. Alien Plant Species: Environmental Risks in Agricultural and Agro-Forest Landscapes Under Climate Change. In Climate Change-Resilient Agriculture and Agroforestry: Ecosystem Services and Sustainability; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 215–234. [Google Scholar]
- Tepa-Yotto, G.T.; Tonnang, H.E.Z.; Goergen, G.; Subramanian, S.; Kimathi, E.; Abdel-Rahman, E.M.; Flø, D.; 532 Thunes, K.H.; Fiaboe, K.K.M.; Niassy, S.; et al. Global Habitat Suitability of Spodoptera frugiperda (JE Smith) 533 (Lepidoptera, Noctuidae): Key Parasitoids Considered for Its Biological Control. Insects 2021, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Vilarinho, E.C.; Fernandes, O.A.; Hunt, T.E.; Caixeta, D.F. Movement of “Spodoptera frugiperda” Adults (Lepidoptera: Noctuidae) in Maize in Brazil. Fla. Entomol. 2011, 94, 480–488. [Google Scholar] [CrossRef]
- Ahmad, S.; Arslan, M. Influence of Meteorological Factors on Population Dynamics of Fall Armyworm, Spodoptera frugiperda, Lepidoptera: Noctuidae and Its Varietal Susceptiblilty to FAW. In Proceedings of the 1st International Electronic Conference on Entomology, Virtual, 1–15 July 2021; pp. 1–15. [Google Scholar]
- Winsou, J.K.; Tepa-Yotto, G.T.; Thunes, K.H.; Meadow, R.; Tamò, M.; Sæthre, M.G. Seasonal Variations of Spodoptera frugiperda Host Plant Diversity and Parasitoid Complex in Southern and Central Benin. Insects 2022, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Boukari, S.A.; Sinzogan, A.A.C.; Sikirou, R.; Deguenon, J.M.; Amagnidé, G.A.Y.G.; Zossou, N.; Vodounon, H.S.T.; Adomou, A.C.; Ahanchédé, A. Influence of Agricultural Practices on Spodoptera frugiperda (JE Smith) Infestation, Natural Enemies and Biocontrol in Maize. J. Agric. Crop. Res. 2022, 10, 113–130. [Google Scholar]
- Dassou, A.G.; Idohou, R.; Azandémè-Hounmalon, G.Y.; Sabi-Sabi, A.; Houndété, J.; Silvie, P.; Dansi, A. Fall Armyworm, Spodoptera frugiperda (J.E. Smith) in Maize Cropping Systems in Benin: Abundance, Damage, Predatory Ants and Potential Control. Int. J. Trop. Insect. Sci. 2021, 41, 2627–2636. [Google Scholar] [CrossRef]
- Meagher, R.L.; Nuessly, G.S.; Nagoshi, R.N.; Hay-Roe, M.M. Parasitoids Attacking Fall Armyworm (Lepidoptera: Noctuidae) in Sweet Corn Habitats. Biol. Control 2016, 95, 66–72. [Google Scholar] [CrossRef]
- Kasoma, C.; Shimelis, H.; Laing, M.D. Fall Armyworm Invasion in Africa: Implications for Maize Production and Breeding. J. Crop. Improv. 2021, 35, 111–146. [Google Scholar] [CrossRef]
- Viana, P.A.; Guimaraes, P.D.O.; Goncalves, I.D.S.; Magalhaes, C.D.S. Resistência Nativa de Híbridos Experimentais de Milho à Spodoptera frugiperda. In Proceedings of the Congresso Nacional de Milho e Sorgo, Simpósio Sobre Lepdópteros Comuns a Milho, Soja e Algodão, 1., 2014, Salvador, Brazil, 25 September 2016. [Google Scholar]
- Kennedy, G.G.S.N.P. Life Systems of Polyphagous Arthropod Pests in Temporally Unstable Cropping Systems. Annu. Rev. Entomol. 2000, 45, 467–493. [Google Scholar] [CrossRef]
- Fiaboe, K.R.; Agboka, K.; Agnamba, A.O.; Teyo, K.L.; Amegah, A.L.; Koffi, D.; Kpadonou, G.E.; Agboka, K.M.; Gwokyalya, R.; Fening, K.O.; et al. Fertilizer-Bioinsecticide Synergy Improves Maize Resilience to Spodoptera frugiperda Infestation. Crop. Prot. 2024, 177, 106548. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Soil Fertility Management and Insect Pests: Harmonizing Soil and Plant Health in Agroecosystems. Soil Tillage Res 2003, 72, 203–211. [Google Scholar] [CrossRef]
- FAO. Integrated Management of the Fall Armyworm on Maize: A Guide for Farmer Field Schools in Africa. Available online: http://www.fao.org/family-farming/detail/en/c/1112643/ (accessed on 14 July 2024).
- Hailu, G.; Niassy, S.; Zeyaur, K.R.; Ochatum, N.; Subramanian, S. Maize–Legume Intercropping and Push–Pull for Management of Fall Armyworm, Stemborers, and Striga in Uganda. Agron. J. 2018, 110, 2513–2522. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.O.R.J. Population Dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and Associated Arthropod Natural Enemies in Honduran Subsistence Maize. Crop. Prot. 2006, 25, 1180–1190. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Perecin, D. Modelos Probabilísticos Para Distribuicão de Lagartas de Spodoptera frugiperda (JE Smith 1797) Na Cultura Do Milho. Cientifica 1982, 10, 181–191. [Google Scholar]
- Price, P.W. Insect Ecology, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Mitchell, F.L. Natural Control and Spatial Distribution of Fall Armyworm (Spodoptera frugiperda) Within Louisiana Corn Fields; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 1985. [Google Scholar]
- Melo, E.P.; Fernandes, M.G.; Degrande, P.E.; Cessa, R.M.A.; Salomão, J.L.; Nogueira, R.F. Distribuição Espacial de Plantas Infestadas Por Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) Na Cultura Do Milho. Neotrop. Entomol. 2006, 35, 689–697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sétamou, M.; Schulthess, F.; Poehling, H.M.; Borgemeister, C. Spatial Distribution of and Sampling Plans for Mussidia Nigrivenella (Lepidoptera: Pyralidae) on Cultivated and Wild Host Plants in Benin. Environ. Entomol. 2000, 29, 1216–1225. [Google Scholar] [CrossRef]
- Moradi Vajargah, M.; Golizadeh, A.; Dastjerdi, H.R.; Zalucki, M.P.; Hassanpour, M.; Naseri, B. Population Density and Spatial Distribution Pattern of Hypera postica (Coleoptera: Curculionidae) in Ardabil, Iran. Not. Bot. Horti. Agrobot. Cluj Napoca. 2011, 39, 42–48. [Google Scholar] [CrossRef]
- Guimapi, R.A.; Niassy, S.; Mudereri, B.T.; Abdel-Rahman, E.M.; Tepa-Yotto, G.T.; Subramanian, S.; Mohamed, S.A.; Thunes, K.H.; Kimathi, E.; Agboka, K.M.; et al. Harnessing Data Science to Improve Integrated Management of Invasive Pest Species across Africa: An Application to Fall Armyworm (Spodoptera frugiperda) (J.E. Smith) (Lepidoptera: Noctuidae). Glob. Ecol. Conserv. 2022, 35, e02056. [Google Scholar] [CrossRef]
- Sedaratian, A.; Fathipour, Y.; Talebi, A.A.; Farahani, S. Population Density and Spatial Distribution Pattern of Thrips tabaci (Thysanoptera: Thripidae) on Different Soybean Varieties. J. Agric. Sci. Technol. 2010, 12, 275–288. [Google Scholar]

| Score | Description |
|---|---|
| 1 | No visible leaf-feeding damage |
| 2 | Few pinholes on 1–2 older leaves |
| 3 | Several shot-hole injuries on a few leaves (<5 leaves) and small circular hole damage to leaves |
| 4 | Several shot-hole injuries on several leaves (6–8 leaves) or small lesions/pinholes, small circular lesions, and a few small elongated (rectangular-shaped) lesions up to 1.3 cm in length on whorl and furl leaves |
| 5 | Elongated lesions (>2.5 cm long) on 8–10 leaves, plus a few small- to mid-sized uniform to irregular-shaped holes (with basement membrane consumed) eaten from the whorl and/or furl leaves |
| 6 | Several large, elongated lesions on several whorl and furl leaves and/or several large uniform- to irregular-shaped holes eaten from furl and whorl leaves |
| 7 | Many elongated lesions of all sizes on several whorl and furl leaves plus several large uniform- to irregular-shaped holes eaten from the whorl and furl leaves |
| 8 | Many elongated lesions of all sizes on most whorl and furl leaves plus many mid- to large-sized uniform- to irregular-shaped holes eaten from the whorl and furl leaves |
| 9 | Whorl and furl leaves almost totally destroyed, with the plant dying as a result of extensive foliar damage |
| Source | DF | Mean Square | F Value | p |
|---|---|---|---|---|
| Zone | 1 | 0.15 | 4.35 | 0.0378 |
| Replicate | 59 | 0.04 | 1.21 | 0.1546 |
| Season | 1 | 1.67 | 48.20 | 0.0001 |
| Year | 1 | 0.28 | 8.07 | 0.0048 |
| Zone*Season | 1 | 0.11 | 3.36 | 0.0677 |
| Zone*Year | 1 | 0.00 | 0.19 | 0.6637 |
| Zone*Season*Year | 2 | 0.10 | 2.96 | 0.0531 |
| Season | Agroecological Zone | Larval Infestation Rate (%) (Mean ± SE) | ||
|---|---|---|---|---|
| Year 2021 | Year 2022 | |||
| Dry season | AEZ 6 | 36.94 ± 2.61 bB | 29.97 ± 2.25 aC | F3,129 = 11.69; p < 0.0001 |
| AEZ 8 | 36.50 ± 2.00 bB | 29.87 ± 2.27 aC | ||
| Rainy season | AEZ 6 | 42.40 ± 2.90 aB | 41.44 ± 2.32 aB | F3,129 = 3.80; p < 0.01 |
| AEZ 8 | 47.96 ± 2.87 abA | 50.94 ± 1.90 bA | ||
| F3,10 = 3.54; p < 0.01 | F3,17 = 21.49; p < 0.0001 | |||
| Source | DF | Mean Square | F Value | p |
|---|---|---|---|---|
| Zone | 1 | 60.77 | 53.01 | 0.0001 |
| Replicate | 599 | 1.11 | 0.97 | 0.6681 |
| Season | 1 | 270.33 | 235.79 | 0.0001 |
| Year | 1 | 26.47 | 23.09 | 0.0001 |
| Zone*Season | 1 | 76.41 | 66.65 | 0.0001 |
| Zone*Year | 1 | 2.11 | 1.84 | 0.1744 |
| Zone*Season*Year | 2 | 6.41 | 5.59 | 0.0037 |
| Season | Agroecological Zone | Sampling Week | Larval Density (Mean ± SE) | |
|---|---|---|---|---|
| Year 2021 | Year 2022 | |||
| Dry season | AEZ 6 | 1 | 0.88 ± 0.08 abc | 0.96 ± 0.09 abc |
| 2 | 0.58 ± 0.05 d | 0.68 ± 0.05 cde | ||
| 3 | 0.71 ± 0.06 cd | 0.67 ± 0.06 cde | ||
| 4 | - | 0.56 ± 0.05 ed | ||
| 5 | - | 0.43 ± 0.04 e | ||
| Overall mean | 0.73 ± 0.03 B | 0.66 ± 0.02 B | ||
| AEZ 8 | 1 | 1.11 ± 0.09 a | 1.07 ± 0.11 ab | |
| 2 | 0.82 ± 0.07 bc | 1.20 ± 0.09 a | ||
| 3 | 0.98 ± 0.70 ab | 0.95 ± 0.07 abc | ||
| 4 | - | 0.87 ± 0.08 bc | ||
| 5 | - | 0.82 ± 0.07 bcd | ||
| Overall mean | 0.98 ± 0.04 A | 0.99 ± 0.03 A | ||
| F5,1795 = 7.15; p < 0.0001 | F9,3230 = 10.42; p < 0.0001 | |||
| Rainy season | AEZ 6 | 1 | 0.44 ± 0.03 b | 0.18 ± 0.03 d |
| 2 | 0.62 ± 0.04 a | 0.42 ± 0.03 c | ||
| 3 | 0.69 ± 0.04 a | 0.36 ± 0.03 c | ||
| 4 | - | 0.38 ± 0.03 c | ||
| 5 | - | 0.77 ± 0.04 a | ||
| Overall mean | 0.59 ± 0.02 C | 0.43 ± 0.01 C | ||
| AEZ 8 | 1 | 0.48 ± 0.04 b | 0.37 ± 0.03 c | |
| 2 | 0.56 ± 0.04 a | 0.21 ± 0.02 d | ||
| 3 | 0.63 ± 0.04 a | 0.39 ± 0.03 c | ||
| 4 | - | 0.45 ± 0.03 c | ||
| 5 | - | 0.67 ± 0.03 b | ||
| Overall mean | 0.56 ± 0.02 C | 0.42 ± 0.01 C | ||
| F5,2995 = 6.38; p < 0.0001 | F9,5391 = 35.88; p < 0.0001 | |||
| Source | DF | Mean Square | F Value | p |
|---|---|---|---|---|
| Zone | 1 | 0.07 | 2.71 | 0.1010 |
| Replicate | 59 | 0.03 | 1.16 | 0.2095 |
| Season | 1 | 4.68 | 173.08 | 0.0001 |
| Year | 1 | 1.53 | 56.74 | 0.0001 |
| Zone*Season | 1 | 0.08 | 3.05 | 0.0819 |
| Zone*Year | 1 | 0.09 | 3.36 | 0.0679 |
| Zone*Season*Year | 2 | 0.18 | 6.93 | 0.0011 |
| Season | Agroecological Zones | Percentage of Damaged Plants (Mean ± SE) | ||
|---|---|---|---|---|
| Year 2021 | Year 2022 | |||
| Dry season | AEZ 6 | 58.33 ± 1.51 bB | 51.53 ± 2.31 aC | F3,129 = 8.09; p < 0.0005 |
| AEZ 8 | 55.72 ± 1.82 abB | 53.73 ± 2.35 aC | ||
| Rainy season | AEZ 6 | 80.92 ± 1.94 cA | 63.44 ± 2.06 aB | F3,129 = 16.61; p < 0.0001 |
| AEZ 8 | 82.22 ± 2.36 cA | 72.05 ± 1.86 bA | ||
| F3,10 = 66.42; p < 0.0001 | F3,17 = 19.91; p < 0.0001 | |||
| Source | DF | Mean Square | F Value | p |
|---|---|---|---|---|
| Zone | 1 | 4.06 | 1.71 | 0.1913 |
| Replicate | 599 | 2.49 | 1.05 | 0.1981 |
| Season | 1 | 1077.71 | 453.04 | 0.0001 |
| Year | 1 | 205.88 | 86.55 | 0.0001 |
| Zone*Season | 1 | 31.91 | 13.42 | 0.0003 |
| Zone*Year | 1 | 2.86 | 1.20 | 0.2724 |
| Zone*Season*Year | 2 | 434.31 | 182.58 | 0.0001 |
| Season | Agroecological Zone | Sampling Week | Plant Damage Score Per AEZ (Scale 1–9) (Mean ± SE) | |
|---|---|---|---|---|
| Year 2021 | Year 2022 | |||
| Dry season | AEZ 6 | 1 | 3.36 ± 0.10 b | 2.58 ± 0.07 b |
| 2 | 3.17 ± 0.09 bc | 2.25 ± 0.06 c | ||
| 3 | 3.30 ± 0.08 bc | 2.30 ± 0.06 c | ||
| 4 | - | 2.73 ± 0.07 ab | ||
| 5 | - | 2.24 ± 0.07 c | ||
| Overall mean | 3.28 ± 0.05 A | 2.42 ± 0.03 BC | ||
| AEZ 8 | 1 | 2.95 ± 0.08 c | 2.32 ± 0.07 c | |
| 2 | 3.13 ± 0.08 bc | 2.63 ± 0.07 ab | ||
| 3 | 3.82 ± 0.15 a | 2.63 ± 0.06 ab | ||
| 4 | - | 2.89 ± 0.07 a | ||
| 5 | - | 2.84 ± 0.07 a | ||
| Overall mean | 3.30 ± 0.06 A | 2.66 ± 0.03 A | ||
| F5,1795 = 8.60; p < 0.0001 | F9,3231 = 12.91; p < 0.0001 | |||
| Rainy season | AEZ 6 | 1 | 2.03 ± 0.05 b | 1.55 ± 0.04 f |
| 2 | 2.10 ± 0.05 b | 2.56 ± 0.07 b | ||
| 3 | 2.32 ± 0.05 a | 2.70 ± 0.08 b | ||
| 4 | - | 1.98 ± 0.05 e | ||
| 5 | - | 3.48 ± 0.08 a | ||
| Overall mean | 2.16 ± 0.02 B | 2.46 ± 0.03 B | ||
| AEZ 8 | 1 | 2.00 ± 0.05 b | 1.84 ± 0.04 e | |
| 2 | 2.10 ± 0.05 b | 1.79 ± 0.05 e | ||
| 3 | 2.32 ± 0.05 a | 2.45 ± 0.07 c | ||
| 4 | - | 2.23 ± 0.05 d | ||
| 5 | - | 3.41 ± 0.08 a | ||
| Overall mean | 2.14 ± 0.03 B | 2.35 ± 0.02 C | ||
| F5,2995 = 8.22; p < 0.0001 | F9,5391 = 110.90; p < 0.0001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanzana, K.; Sinzogan, A.; Tepa-Yotto, G.T.; Dannon, E.; Goergen, G.; Tamò, M. Seasonal and Spatial Distribution of Fall Armyworm Larvae in Maize Fields: Implications for Integrated Pest Management. Insects 2025, 16, 145. https://doi.org/10.3390/insects16020145
Zanzana K, Sinzogan A, Tepa-Yotto GT, Dannon E, Goergen G, Tamò M. Seasonal and Spatial Distribution of Fall Armyworm Larvae in Maize Fields: Implications for Integrated Pest Management. Insects. 2025; 16(2):145. https://doi.org/10.3390/insects16020145
Chicago/Turabian StyleZanzana, Karimou, Antonio Sinzogan, Ghislain T. Tepa-Yotto, Elie Dannon, Georg Goergen, and Manuele Tamò. 2025. "Seasonal and Spatial Distribution of Fall Armyworm Larvae in Maize Fields: Implications for Integrated Pest Management" Insects 16, no. 2: 145. https://doi.org/10.3390/insects16020145
APA StyleZanzana, K., Sinzogan, A., Tepa-Yotto, G. T., Dannon, E., Goergen, G., & Tamò, M. (2025). Seasonal and Spatial Distribution of Fall Armyworm Larvae in Maize Fields: Implications for Integrated Pest Management. Insects, 16(2), 145. https://doi.org/10.3390/insects16020145






