Simple Summary
Plant bugs’ locating of mates mainly relies on peculiar chemical signals known as sex pheromones, which can be utilized to develop environmentally friendly pest control methods. This study investigated the sex pheromones of Polymerus pekinensis, a phytophagous insect of alfalfa crops in East Asia. By analyzing chemical extracts from both male and female insects, we identified two antenna-active compounds: octyl acetate (OA) and decyl acetate (DA). Females produced higher amounts of OA, while males produced greater quantities of DA. Field experiments demonstrated that lures loaded with solely OA strongly attracted male P. pekinensis, making it a valuable tool for monitoring this pest. However, the addition of DA in larger amounts significantly decreased the attractivity of lures. Our findings offer new strategies for monitoring and managing these insects in a manner that does not harm non-target insects.
Abstract
Insect sex pheromones have been widely used in integrated pest control due to their efficiency, non-toxicity, specificity, and environmental sustainability. They are considered a key component of green pest management techniques. Polymerus pekinensis is a phytophagous plant bug on alfalfa (Medicago sativa L.) in East Asia. This study used gas chromatography–electroantennogram detection (GC–EAD) and gas chromatography–mass spectrometry (GC–MS) to analyze the whole-body extracts from male and female P. pekinensis. Octyl acetate (OA) and decyl acetate (DA) elicited the antennal response of males and were identified as the predominant components of female and male extracts, respectively. Subsequent field trials demonstrated that OA (>8 mg per lure) showed the strongest attraction to conspecific males. However, when DA was added in a lure (≥2 mg), a significant decline in captures occurred. These findings provide new insights into the understanding of sex pheromones in Miridae and benefit the development of sustainable management of P. pekinensis.
1. Introduction
Chemical communication is vital for insect survival and reproduction, facilitating both intraspecific and interspecific interactions through sex pheromones [1,2]. Since the identification of the first insect sex pheromone from the silkworm [3], over 3000 sex pheromone compounds have been isolated and identified from more than 90 families across nine orders of insects [4]. Due to their high species specificity and environmental sustainability, sex pheromones play essential roles in integrated pest management (IPM), e.g., pest monitoring, mass trapping, mating disruption, and biological control [5,6]. The effectiveness of pheromone-based approaches has been demonstrated across various insect orders, particularly in Lepidoptera and Hemiptera, which have become integral components of IPM [1,5,7].
The Miridae is one of the largest families in the order Heteroptera, containing over 11,000 species [7,8]. Many mirids feed on plant sap and are recognized as agricultural pests [7]. Chemical communication between sexes plays a vital role in mirid reproduction and species isolation [9]. In recent years, female sex pheromones in over 30 species were revealed, including Apolygus lucorum [10], Lygus pratensis [11], Sahlbergella singularis [12], Distantiella Theobroma [12], Helopeltis cinchonae [13], Apolygus spinolae [14], L. lineolaris [15], Lygocoris pabulinus [16], and L. rugulipennis [17]. These pheromones are primarily composed of saturated or mono-unsaturated unbranched ester or aldehyde compounds [9,10,11,13]. Representative components include hexyl butyrate, (E)-2-hexenyl butyrate, (E)-4-oxo-2-hexenal, and (E)-2-octenyl butyrate [7], which are likely the candidate components of sex pheromones in other Miridae species [9,11,12]. The pheromone compounds are produced in varying secretion sites among species. Metathoracic scent glands are the most frequently reported organs in many mirids like Adelphocoris suturalis [18], Phytocoris calli [19], A. spinolae [20], and Orthops Campestris [20]. Identification and functional verification of these pheromonal chemicals have provided the foundation for developing species-specific monitoring and control strategies against mirids.
The plant bug, Polymerus pekinensis Horváth (Hemiptera: Miridae) (NCBI: txid1929840), is widely distributed in East Asia, including China, Korea, and Japan (http://museum.ioz.ac.cn, (accessed on 29 December 2024)) [21,22]. Besides the main host plants, alfalfa (Medicago sativa L.) and Rubia cordifolia L., it also feeds on Phaseolus vulgaris L., Vicia faba L., Brassica napus, and potato Solanum tuberosum L. [21,22]. Both nymphs and adults pierce and suck plant juices, causing extensive chlorosis and defoliation in alfalfa, consequently leading to substantial economic losses [9,21,22]. Current management of P. pekinensis and other mirid bugs heavily relies on broad-spectrum insecticides such as chlorpyrifos, malathion, and cyhalothrin [7,22]. However, this chemical-based approach raises serious environmental concerns, including soil and groundwater contamination, and poses risks to beneficial non-targets [23,24]. The development of pheromone-based monitoring and control methods has shown promise in various mirid species [1,10], offering more environmentally friendly alternatives [1,25]. However, the sex pheromone of the Polymerus genus remains unknown. Based on our preliminary observations in the field, P. pekinensis females exhibited typical “sex calling” behavior in the scotophase like A. lucorum [10], L. pratensis [11], and Ph. calli [19], suggesting that female-produced sex pheromones played vital roles in the mating communication of this plant bug.
This study aimed to identify the sex pheromone produced by female P. pekinensis using gas chromatography–electroantennogram detection (GC–EAD) and gas chromatography–mass spectrometry (GC–MS), as well as to assess their attractiveness to P. pekinensis in field conditions. Identification of sex pheromones in the Polymerus genus will enhance our understanding of the chemical communication of insects and provide a foundation for developing monitoring strategies for P. pekinensis.
2. Materials and Methods
2.1. Insects and Pheromone Collection
P. pekinensis nymphs were collected from the farm of the Institute of Plant Protection, Hebei Academy of Agricultural and Forestry Sciences (38.95° N, 115.45° E), Baoding, China. The nymphs were reared in boxes (18 cm × 12 cm × 7 cm) at 26 ± 2 °C, 60–70% relative humidity (RH), and a 16:8 photoperiod (L:D). They were fed with fresh pods of Phaseolus vulgaris, which were replaced every two days. After adult emergence, individuals were separated by sex and maintained in cages (30 cm × 30 cm × 30 cm). Virgin sexual maturity adults (3–5 days old) were used for sex pheromone collection and electrophysiologic experiments [10].
Pheromone collection was conducted using the whole-body extraction method [10,11]. Briefly, a virgin female or male was separately closed in a 2 mL centrifuge tube and allowed to acclimate in the dark for 2 h. Subsequently, 500 μL of n-Hexane was added to immerse the individuals for pheromone collection. After a 5 min immersion, the supernatant was transferred to a sample vial and stored at −20 °C until subsequent GC–EAD and GC–MS analysis. The quantities of OA and DA in the extracts were determined using the external standard method with standards.
2.2. Chemicals, Lures, and Traps
Octyl acetate (OA) and decyl acetate (DA) (purity > 99%) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Polyethylene (PE) vials (25 mm× 6 mm× 0.25 mm; Pherobio Technology Co., Ltd., Beijing, China) were used as pheromone dispensers in the field experiments. The lures for field trapping were prepared by dissolving pheromones in 200 μL of sunflower oil (food grade) containing 1.0% butylated hydroxytoluene (BHT) and loading it into the PE vials [11]. After pheromone loading, the vials were heat-sealed immediately, allowing the compounds to diffuse and release through the PE wall at a slow rate. Triangle-shaped traps with removable sticky boards (Pherobio Technology Co., Ltd., Beijing, China) were used in the field experiments.
2.3. GC–EAD Recording
Electrophysiologic analyses of female and male extracts were conducted on a gas chromatograph (GC2030; Shimadzu, Kyoto, Japan) equipped with a DB-WAX column (30 m × 0.25 mm × 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA) and a 1:1 effluent splitter that allowed for simultaneous flame ionization detection (FID) and electroantennogram detection (EAD). The EAD section consisted of two electrodes, a data acquisition interface board (IDAC-2) and a CS-55 air stimulus controller (Syntech Ltd., Buchenbach, Germany). The carrier gas was nitrogen, and the injector temperature was set at 230 °C. The GC oven was initiated at 50 °C (held 1 min) and then increased to 120 °C (5 °C/min, held 1 min), and it subsequently increased to 230 °C (10 °C/min, held 5 min). The transfer tube for EAG preparation was constantly heated at 200 °C, with the outlet being positioned within a humidified airstream (300 mL/min) directed over an antenna. The antennae (with tips removed) were connected to electrodes using micro-glass capillaries filled with a 0.9% saline solution. Antennal signals were recorded and analyzed using a computer equipped with an IDAC interface box and GC–EAD software (version 4.0; Syntech Ltd., Buchenbach, Germany). A total of 20 female and 20 male P. pekinensis specimens were utilized for the GC–EAD analysis.
2.4. GC–MS Analyses
The mirid extracts were analyzed on an Agilent 7890A GC coupled with a 5975C mass spectrometer (Agilent Technologies). The ionization voltage was 70 eV, and the ion source temperature was 230 °C. The analysis employed an identical column and oven program as described in the GC–EAD experiment. The mass spectra were compared with those reported in the NIST14 database, and the identification of pheromone components was conducted by comparing the GC retention times and mass spectra with those of authentic samples.
2.5. Electroantennogram (EAG) Recordings
EAG system and test protocols were performed as described above. All chemicals were tested at doses of 0.01, 0.1, 1, 10, 100, and 1000 μg. Each dose was delivered by applying 10 μL of the corresponding solution (prepared in n-hexane) to a filter paper strip (0.5 cm × 5 cm), which was then placed in a Pasteur pipette (15 cm long). n-hexane (10 μL) was used as a solvent control. The apparatus maintained two parallel airflows: a continuous flow and a stimulating flow, each set at 500 mL·min−1. Upon initiating recording with a pedal press, the compensatory airflow was automatically shut off, and the stimulating airflow passed over the filter paper strip for 0.1 s. An IDAC-2 stimulus controller (Syntech Ltd., Buchenbach, Germany) was used to measure and record the resulting antennal voltage changes. Each stimulus puff was followed by a solvent control puff, and the compounds were tested in increasing concentrations. The EAG values were corrected by subtracting the solvent response. For each compound, ten replicates for each sex were tested.
2.6. Field Trials
The field experiments were conducted in an abandoned peach orchard in Baoding, China (38.86° N, 115.59° E). This area had high plant coverage, with various weeds such as Chenopodium album and Humulus scandens, as well as Rubia cordifolia, the main host plant of P. pekinensis. Triangular traps were hung on peach branches placed 20 cm above the canopy of plants, with a minimum spacing of 10 m between traps. All field experiments used a completely randomized block design with three replicates. The captures on the sticky boards were recorded every three days. The traps with renewed sticky boards were randomly reassigned after each check.
Field experiment 1 (2–17 September 2022) was conducted to evaluate the attractiveness of antenna-active components identified through GC–EAD screening. Based on the quantitative GC–MS analysis of female extracts, the treatments included OA (5 mg), DA (50 μg), and a binary blend (OA: 5 mg + DA: 50 μg), as well as a negative control (200 μL sunflower oil) and a positive control (five live virgin females, 5VF). Virgin females and fresh P. vulgaris were placed in rearing tubes (height 4.5 cm, diameter 2 cm) (with gauze caps) as 5VF lures. The females and their food were replaced daily.
Field experiment 2 (20 September–5 October 2022) was performed to optimize the dose of the pheromone candidate. Different doses of OA (0.1, 0.4, 0.8, 1.6, 2, 4, 6, 8, 10, 15, and 20 mg) dissolved in 200 μL of sunflower oil with 1.0% BHT were loaded in each PE vial. The doses of lures were optimized by comparing the captures in traps baited with a series of OA doses. The positive and negative controls were the same as in experiment 1.
Field experiment 3 (7–22 October 2022) aimed to investigate the role of DA in attracting male P. pekinensis. Based on the results of field experiment 2, different doses of DA (0.1, 1, 2, 4, 6, and 8 mg) were added into the OA lures (8 mg) to evaluate their influence on trapping. Positive and negative controls were also conducted.
2.7. Statistical Analysis
The amounts of pheromone components (between female and male extracts) and the corrected EAG values (between OA and DA at the same concentration) were compared by independent-sample t-tests. The EAG values among series concentrations and capture data from the field experiments were analyzed using one-way ANOVA tests, followed by Tukey’s test. A significance level of 0.05 was applied for all analyses.
3. Results
3.1. Pheromone Identification
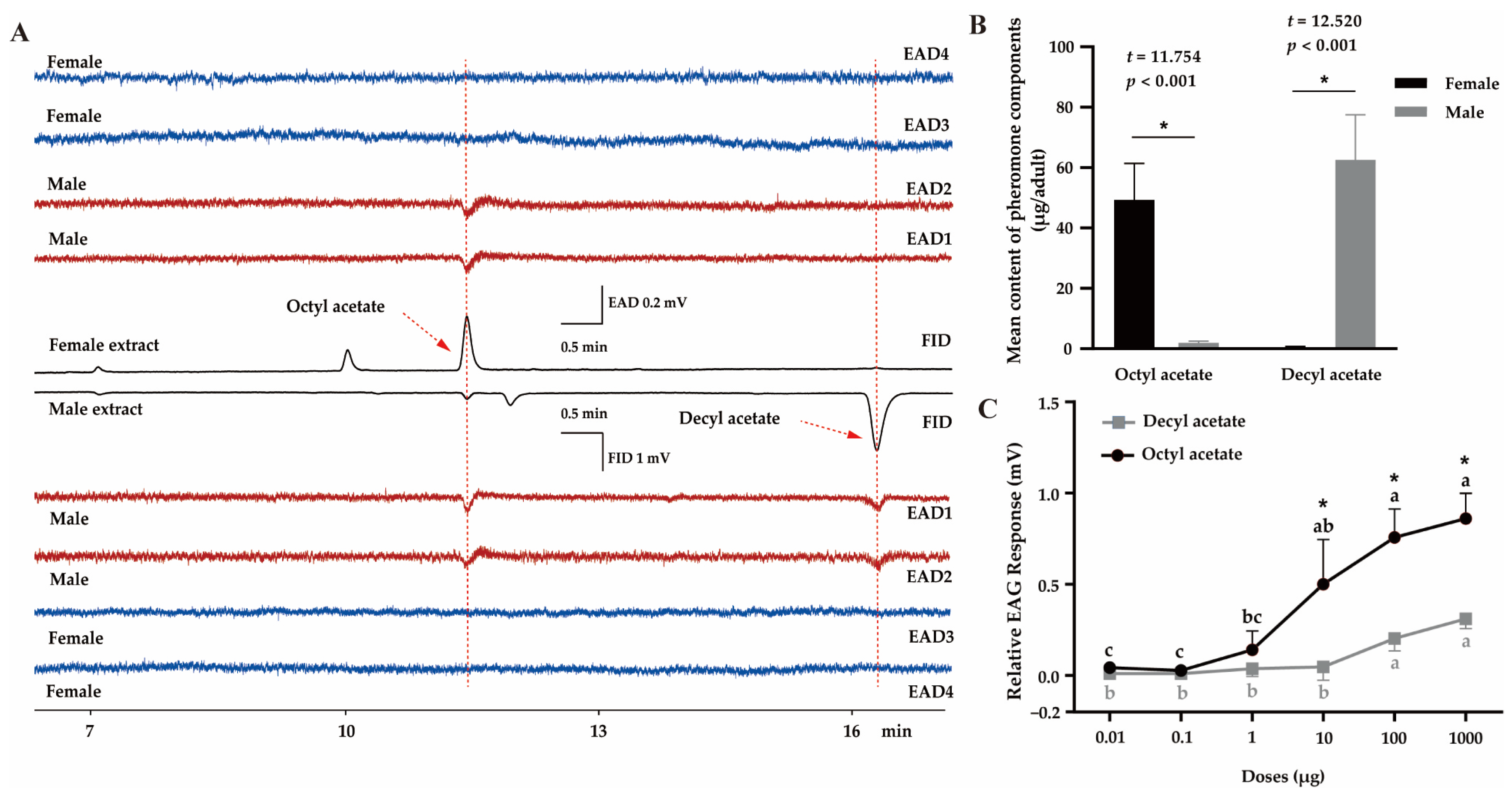
In the GC–EAD experiment, male antennae strongly responded to two compounds present in both female and male extracts, indicating that they were the potential candidates for sex pheromones (Figure 1). GC–MS results showed that the retention times and mass spectra of the two compounds highly corresponded with those of the authentic OA and DA, respectively (Figure 1A, Figures S1 and S2). In female extracts, OA was the major component (49.3 ± 12.1 μg per bug, n = 10), which was more abundant than that in the males (2.0 ± 0.6 μg per bug, n = 10) (t = 11.754, p < 0.001). In contrast, in males, the major component was DA (62.6 ± 14.9 μg, n = 10), which was significantly more than in the females (0.5 ± 0.2 μg, n = 10) (t = 12.520, p < 0.001) (Figure 1B). The GC–EAD results (Figure 1A) indicated that the contents of DA in female extracts and OA in male extracts were extremely low, with no response by male antennae. These findings predicted that the antennal response to OA and DA was associated with the chemical concentration. Further EAG results demonstrated that both OA and DA triggered significant dose-dependent EAG responses in male antennae (Figure 1C; OA: F5,12 = 21.334, p < 0.001; DA: F5,12 = 18.921, p < 0.001). However, OA elicited higher EAG responses than DA at the same dose, particularly 10 μg (t = 3.227, p = 0.032), 100 μg (t = 5.641, p = 0.005), and 1000 μg (t = 6.408, p = 0.003).
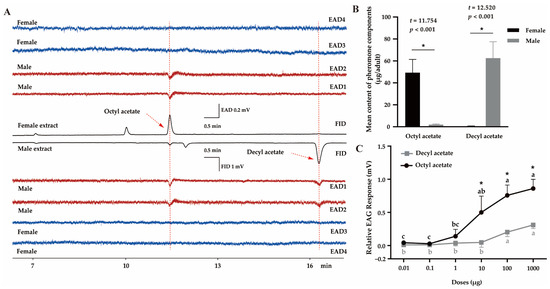
Figure 1.
Identification of the putative sex pheromone of Polymerus pekinensis. (A) GC–EAD responses of male (red) and female (blue) antennae to female and male extracts. FID, flame ionization detector; EAD, electroantennogram detector. (B) The content of each component in female and male P. pekinensis, calculated according to authentic standards. Each value represents the mean of ten replicates. Significant differences are denoted by asterisks (p < 0.05). (C) EAG responses of P. pekinensis males (n = 10) to synthetic octyl acetate (OA) and decyl acetate (DA). For each compound, means with different letters are significantly different among doses by Tukey’s test (p < 0.05). Meanwhile, asterisks represent significant differences between OA and DA at the same concentration.
3.2. Field Trapping
Throughout the entire field experiment, no P. pekinensis females were caught in any traps. In field experiment 1, OA and the binary mixture of OA and DA attracted significantly more male P. pekinensis, compared with the trap with five virgin females (F2,6 = 15.989, p = 0.004) (Figure 2). No significant difference was found between them (F1,4 = 1.316, p = 0.315), indicating that OA was presumably the dominant contributor to chemical communication in P. pekinensis. This result was consistent with the finding that DA alone had minimal effect on attracting males (Figure 2). Thus, OA appeared to be the primary component of the sex pheromone in P. pekinensis.
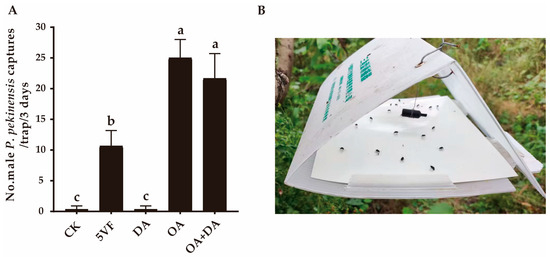
Figure 2.
Attractiveness of potential pheromone components to Polymerus pekinensis males. (A) Captures of Polymerus pekinensis males in traps baited with the binary blend of octyl acetate (OA, 5 mg) and decyl acetate (DA, 50 μg) or each single component. CK, sunflower oil; 5VF, 5 virgin females. Different letters on each column represent significantly different results (p < 0.05). (B) Field attraction of triangle-shaped trap baited with a single-component OA lure.
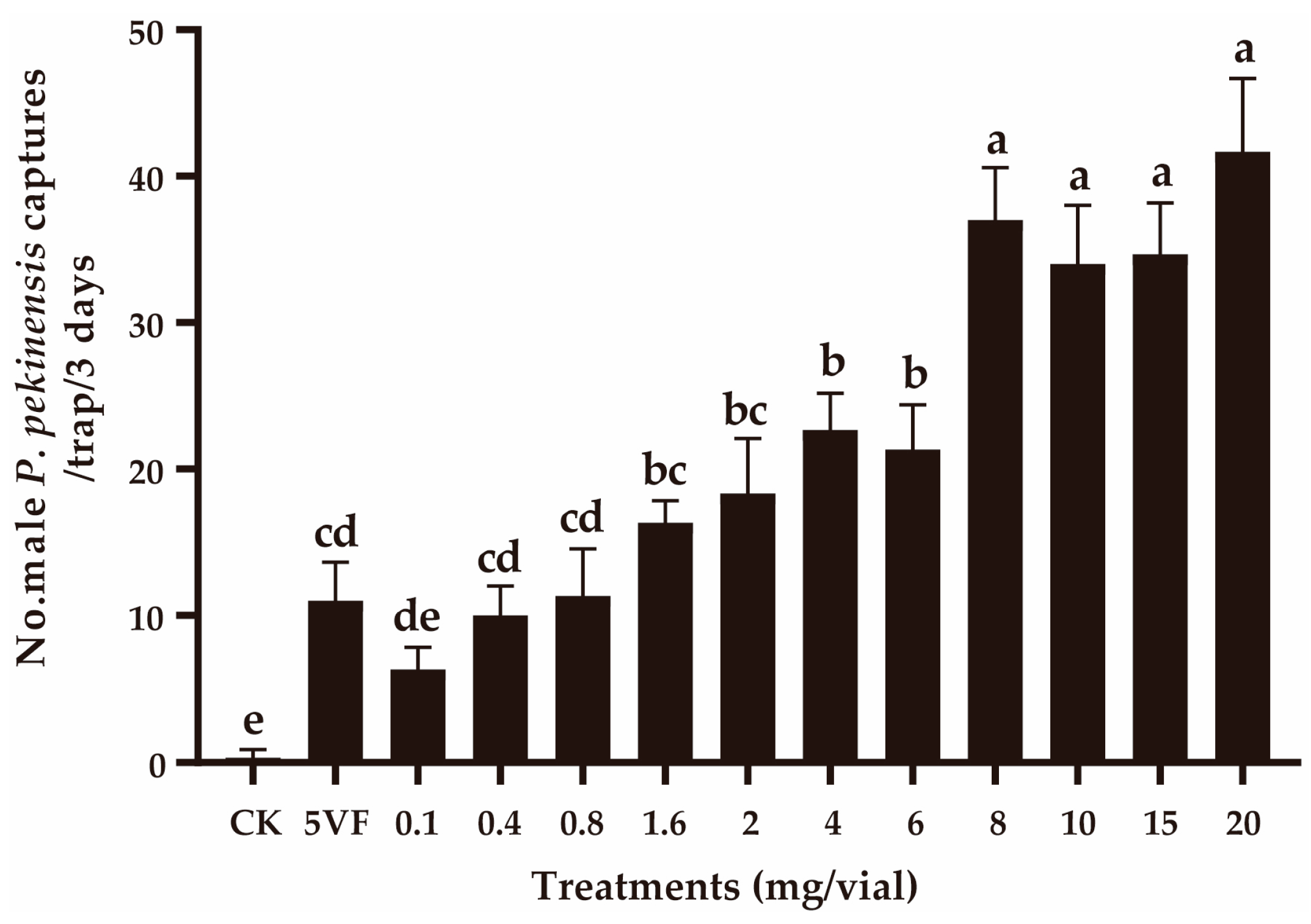
In field experiment 2, the number of captures was positively related with the pheromone dose within the 0.8–8 mg addition of OA (Figure 3). When the pheromone doses were further increased, no significant change in capture numbers was observed (F3,8 = 2.167, p = 0.170) (Figure 3). At lower doses (0.1–0.8 mg), the capture numbers were similar to those obtained with traps containing five virgin female P. pekinensis (F3,8 = 2.667, p = 0.119).
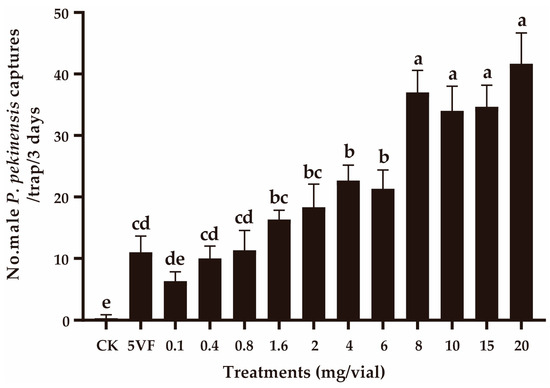
Figure 3.
Captures of Polymerus pekinensis males in traps baited with varying doses of octyl acetate (OA). CK, sunflower oil; 5VF, 5 virgin females. Different letters represent significantly different results (p < 0.05).
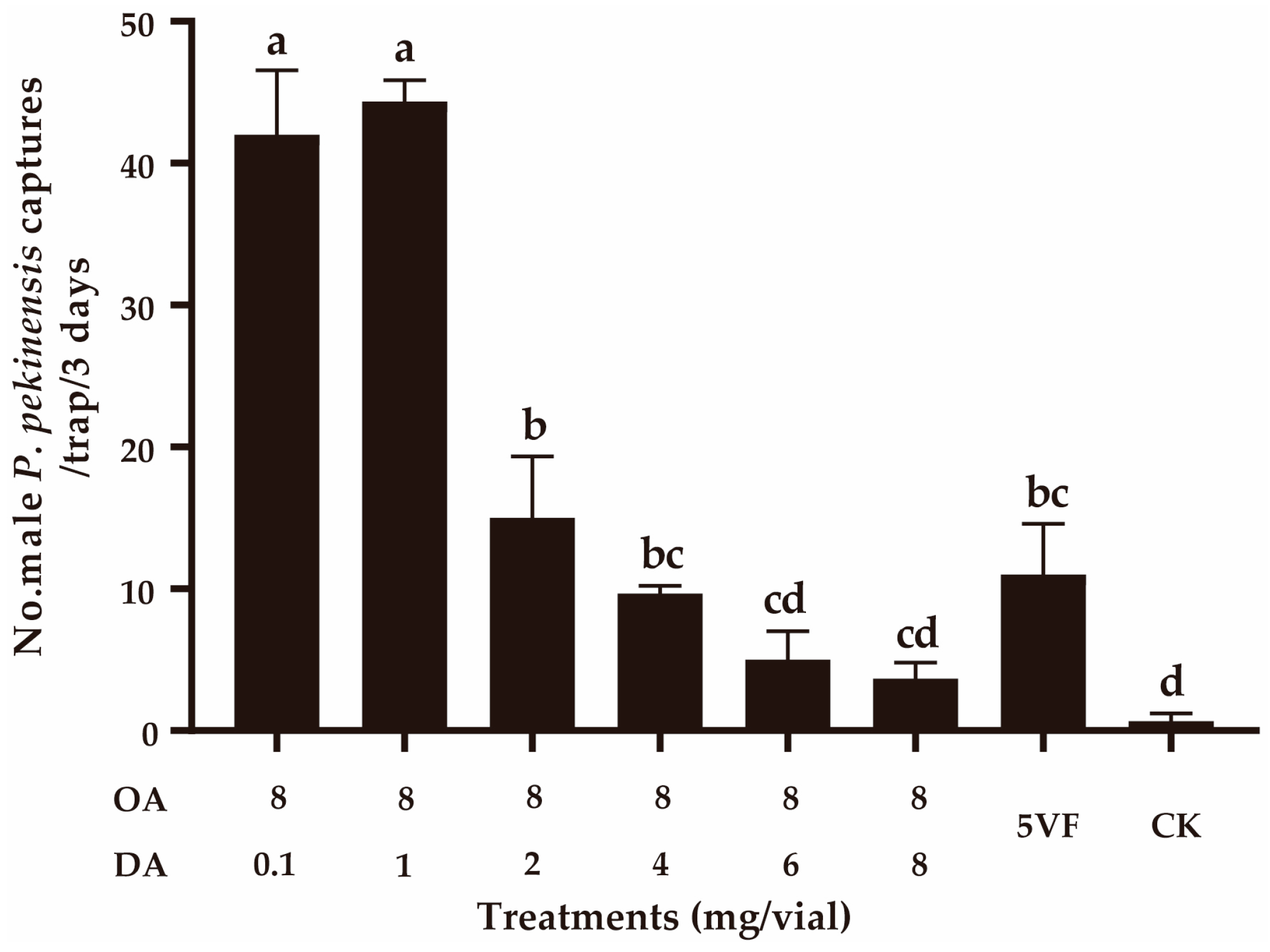
In field experiment 3, we confirmed that DA negatively functioned in the attractiveness by adding different doses (0.1–8 mg) of DA to the lure containing 8 mg of OA. With higher doses of DA being added (≥2 mg), the captures of OA lures significantly decreased (F5,12 = 127.474, p < 0.001) (Figure 4). When the OA:DA ratio was 1:1, capture numbers were not significantly different from those using the five virgin female P. pekinensis traps (F1,4 = 6.535, p = 0.065) (Figure 4). These results suggested that DA can function as a courtship inhibition pheromone in dosages higher than 1 mg.
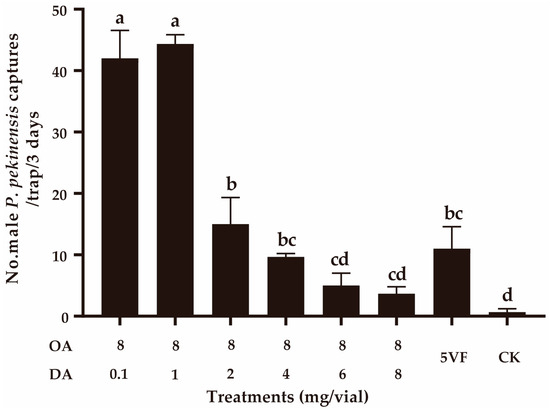
Figure 4.
Effect of addition of decyl acetate (DA) on the attractiveness of octyl acetate (OA). CK, sunflower oil; 5VF, 5 virgin females. Different letters represent significantly different results (p < 0.05).
4. Discussion
In the past decades, significant advancements have been made in the chemical ecology of mirids. For many mirids, sex attractants have demonstrated promising potential for monitoring and controlling mirid pests [9]. Notable examples include the identification and successful application of pheromones in managing A. lucorum [10], L. pratensis [11], L. lineolaris [15], S. singularis [12], and L. rugulipennis [26]. Through a comprehensive GC–EAD and GC–MS analyses of whole-body extracts from male and female P. pekinensis, we have successfully identified OA and DA as antenna-active components. Field trials have demonstrated that OA functioned as a primary attractant, while DA exhibited dose-dependent antagonistic effects. This study provides the first characterization of sex pheromone components in the Polymerus genus and provides new insight into the chemical communication of insects.
Traditionally, insect sex pheromone identification follows a stepwise approach: extraction of crude components, identification via GC–EAD and GC–MS analyses, behavioral screening (e.g., Y-tube olfactometer, cage tests, or wind tunnel experiments), and field trials [6,27]. In this study, we omitted the behavioral screening stage due to the specific behavioral characteristics of plant bugs. These insects require extended time to recover after mechanical disturbance [28]. Disturbed mirids commonly release sex pheromone and defensive compounds (such as hexyl butyrate, (E)-2-hexenal, and (E)-4-oxo-2-hexenal) [29,30] and exhibit abnormal locomotory patterns, e.g., rapid movements, excessive grooming, antenna waving, and wall-seeking behavior [9,31]. These traits will compromise the reliability of laboratory-based behavioral assays.
Although OA is detected in the extracts of several mirid species, such as Ph. calli [19] and Ph. relativus [32], it has not been identified as a sex pheromone component of plant bugs so far. Additionally, OA is also identified as an alarm pheromone component, herbivore-induced plant volatile (HIPV), or food-associated attractant that mimics fermented fruit volatiles [33,34,35]. The present study reported that OA solely served as the sex pheromone of P. pekinensis, which raises an intriguing concern: this plant bug might be attracted or confused by other organisms that contain and release OA. Actually, P. pekinensis successfully survives and flourishes with no disruption in chemical communication system by other organisms. An explanation is that the attractiveness of OA is easily affected or impaired by its analogs, like DA (Figure 4). In other mirids, OA is commonly detected as one component of gland secrets, while other components are its homologs or analogs. Taking Ph. calli for instance, the main female-specific components consist of hexyl acetate, (E)-2-hexenyl acetate, OA, and (E)-2-octenyl acetate, but the sex pheromone is identified as blend of hexyl acetate and (E)-2-octenyl acetate [19]. In the case of Ph. calli and P. pekinensis populations occurring in the same ecological niche, the large amount of hexyl acetate, (E)-2-hexenyl acetate, and (E)-2-octenyl acetate in Ph. calli females would contribute to preventing the attraction of P. pekinensis males. Similarly, (E)-2-octenyl acetate, an analog of DA, serves as an essential attractant component for A. lucorum and Taylorilygus apicalis males, while it strongly inhibits male attraction in other mirids, e.g., A. spinolae, O campestris, and Stenotus rubrovittatus [20]. Another reason that chemical communication of P. pekinensis is hardly disrupted by other OA-released organisms is that the OA amounts in females are relatively large for an insect, up to 49.3 ± 12.1 μg per individual (Figure 1B). OA from plant or fermented sources, as a comparison, is no more than 5 μg [34,35], and such trace amounts are unlikely to induce an electrophysiological (Figure 1) and behavioral (Figure 3) response of P. pekinensis males.
Many sex pheromone components are concurrently present in both females and males in several Miridae species [9], such as Ph. calli [19], A. spinolae [15], A. suturalis [36], and Ph. relativus [32]. Similarly, in P. pekinensis, OA and DA were detected in both sexes (Figure 1). While some pheromone compounds originate from males’ transfer during mating [37], the analyses of virgin adults in our study eliminate this possibility. Beyond sexual attraction, these compounds likely serve multiple ecological functions in Miridae, including aggregation, chemical defense, alarm signaling, and interspecific interactions [9]. Furthermore, certain pheromone components function as attractants of natural enemy behavior and inhibitions of entomopathogenic pathogen, suggesting an evolutionary advantage in insect survival [38,39]. This multifunctional nature of pheromone components may explain their presence in both sexes and reflect their broader ecological significance beyond reproductive communication [40].
The effectiveness of OA as a single-component attractant in P. pekinensis differs from the typical multi-component pheromone systems reported in most Miridae species [9,10,11,14,41]. For example, H. cinchonae uses a blend of 1-acetoxy-5-butyroxyhexane and hexyl 3-acetoxybutyrate in specific ratios [10], while L. pratensis employs hexyl butyrate, (E)-2-hexenyl butyrate, and (E)-4-oxo-2-hexenal for mate attraction [11]. Among Apolygus species, A. lucorum and A. spinolae share common components like (E)-4-oxo-2-hexenal, (E)-2-hexenyl butyrate, and hexyl butyrate but respond to different ratios, which contributes to reproductive isolation [15,20]. While multi-component systems often provide greater species specificity through unique compound ratios [5], our field experiment identified OA as the sole attractant in P. pekinensis. Interestingly, our results showed that DA did not exhibit antagonistic effects at lower dosages (0.1 and 1 mg per lure), suggesting that it had more nuanced roles beyond pheromone antagonism. This pattern parallels several well-documented cases in Lepidoptera, such as in Macdunnoughia crassisigna, where a blend of (Z)-7-dodecene acetate (Z7-12:OAc) and (Z)-9-tetradecene acetate (Z9-14:OAc) serves as the primary attractant at a 3:1 ratio, while (Z)-11-hexadecen-1-ol (Z11-16:OH) may contribute to species specificity [42]. Similarly, in Amyelois transitella, a four-component blend at a specific ratio functioned as the main sex attractant, while some minor components identified from female pheromone glands showed no effect on attraction [43]. From a practical perspective, a single-component system could streamline monitoring procedures and reduce synthesis costs for pest management [44]. However, the use of a single compound raises concerns about species specificity, particularly in areas where multiple mirid species coexist [9,20]. During our field trials, we did not trap other mirid species, which could be mainly due to the absence of other species with shared pheromone components during the study period [42], the species-specific feature of OA for P. pekinensis, or the presence of additional compounds or ecological factors that we have not yet identified [2,5,9].
Beyond the role of OA as an attractant, our study revealed an antagonistic effect of DA on pheromone response. In this study, despite showing lower antennal activity than OA, DA significantly weakened pheromone effectiveness, especially in higher concentrations. This inhibitory pattern mirrors observations in other mirid species through various mechanisms. For example, male-specific compounds in A. lucorum compete with attractants at antennal receptor sites [45], while in L. hesperus, male-produced compounds reduce female attractiveness after mating [16,46]. In P. pekinensis, a higher abundance of DA in males than females, combined with its antagonistic effects, suggested its potential role as an anti-pheromone [10,47].
The application potential of pheromone-based management for P. pekinensis warrants careful consideration. Although our traps efficiently attracted males, the impact on population dynamics still necessitates further investigation. As observed in other species, residual males may continue to achieve sufficient mating frequencies, thereby sustaining the population [48]. Future research should focus on the influence of pheromone attractants on the population dynamics of P. pekinensis and other mirids, the negative effects on natural enemies of mirids, and the optimization of application parameters to refine management strategies.
5. Conclusions
This study reported the first identification of sex pheromone components in the Polymerus genus. Based on the results of electrophysiology and field experiments, OA was identified as the sex pheromone of P. pekinensis, while DA was considered as an antagonist. The potential for using these compounds in monitoring systems and mating disruption strategies offers promising alternatives for sustainable P. pekinensis monitoring.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects16020111/s1, Figure S1: Mass spectra of octyl acetate (OA). A shows the standards of OA, while B displays the Polymerus pekinensis extract of OA; Figure S2: Mass spectra of decyl acetate (DA). A shows the standards of DA, while B displays the Polymerus pekinensis extract of DA.
Author Contributions
Conceptualization, T.Z. and L.W.; methodology, T.Z. and L.W.; software, L.W.; validation, Y.W., X.Z., T.Z. and L.W.; formal analysis, Y.W., L.W. and X.M.; investigation, X.Z. and L.W.; resources, Y.W., M.F. and T.Z.; data curation, M.F. and L.W.; writing—original draft preparation, L.W.; writing—review and editing, L.W.; visualization, T.Z. and L.W.; supervision, T.Z.; project administration, T.Z.; funding acquisition, X.M. and T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hebei Natural Science Foundation (C2023301126), HAAFS Science and Technology Innovation Special Project (2022KJCXZX-ZBS-3), and Shandong Province Science and Technology-based Small and Medium-sized Enterprises Innovation Capacity Enhancement Project (2022TSGC2293).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rizvi, S.A.H.; George, J.; Reddy, G.V.P.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones. Curr. Biol. 2017, 27, R739–R743. [Google Scholar] [CrossRef] [PubMed]
- Butenandt, V.A. Uber den sexsual-lockstoff des seidenspinners Bombyx mori. Reindarstellung Und Konstitution. Z. Naturforschg B 1959, 14, 283. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Vogt, R.G. Production and reception of insect pheromones. In Insect Pheromone Biochemistry and Molecular Biology; Academic Press: London, UK, 2021; pp. 1–12. [Google Scholar]
- Ando, T.; Yamamoto, M. Semiochemicals containing lepidopteran sex pheromones: Wonderland for a natural product chemist. J. Pestic. Sci. 2020, 45, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Yew, J.Y.; Chung, H. Insect pheromones: An overview of function, form, and discovery. Prog. Lipid. Res. 2015, 59, 88–105. [Google Scholar] [CrossRef]
- Lu, Y.; Wyckhuys, K.A.G.; Wu, K. Pest status, bio-ecology, and area-wide management of mirids in East Asia. Annu. Rev. Entomol. 2024, 69, 393–413. [Google Scholar] [CrossRef]
- Herczek, A.; Popov, Y.A. An unusual new species of Hallodapomimus Herczek, 2000 from the Eocene Baltic amber (Hemiptera, Heteroptera, Miridae, Phylinae). Zookeys 2015, 489, 25–32. [Google Scholar] [CrossRef]
- Mnguni, S.; Peter Heshula, L.U. A Review of Chemically Based Communication in Miridae, with a Focus on Two Sympatric Species of Eccritotarsus. J. Entomol. Sci. 2023, 58, 277–293. [Google Scholar] [CrossRef]
- Zhang, T.; Mei, X.; Zhang, X.; Lu, Y.; Ning, J.; Wu, K. Identification and field evaluation of the sex pheromone of Apolygus lucorum (Hemiptera: Miridae) in China. Pest. Manag. Sci. 2020, 76, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, X.; Wyckhuys, K.A.G.; Yao, Y.; Li, H.; Lu, W.; Lu, Y. Optimization and field demonstration of the Lygus pratensis (Hemiptera: Miridae) sex pheromone. Pest. Manag. Sci. 2021, 77, 817–823. [Google Scholar] [CrossRef]
- Mahob, R.J.; Babin, R.; ten Hoopen, G.M.; Dibog, L.; Yede; Hall, D.R.; Bilong Bilong, C.F. Field evaluation of synthetic sex pheromone traps for the cocoa mirid Sahlbergella singularis (Hemiptera: Miridae). Pest. Manag. Sci. 2011, 67, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Magsi, F.H.; Cai, X.; Luo, Z.; Li, Z.; Bian, L.; Xiu, C.; Fu, N.; Li, J.; Hall, D.R.; Chen, Z. Identification, synthesis, and field evaluation of components of the female-produced sex pheromone of Helopeltis cinchonae (Hemiptera: Miridae), an emerging pest of tea. Pest. Manag. Sci. 2024, 80, 4243–4252. [Google Scholar] [CrossRef]
- Yang, C.Y.; Kim, J.; Ahn, S.; Kim, D.; Cho, M.R. Identification of the female-produced sex pheromone of the plant bug Apolygus spinolae. J. Chem. Ecol. 2014, 40, 244–249. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Reddy, G.V.P.; Little, N.; Arnold, S.E.J.; Hall, D.R. Combining visual cues and pheromone blends for monitoring and management of the tarnished plant bug Lygus lineolaris (Hemiptera: Miridae). Pest. Manag. Sci. 2023, 79, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Fountain, M.; Jåstad, G.; Hall, D.; Douglas, P.; Farman, D.; Cross, J. Further studies on sex pheromones of female lygus and related bugs: Development of effective lures and investigation of species-specificity. J. Chem. Ecol. 2014, 40, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Van Tol, R.W.; Diaz Rodriguez, C.M.; de Bruin, A.; Yang, D.; Taparia, T.; Griepink, F.C. Visual attraction of the European tarnished plant bug Lygus rugulipennis (Hemiptera: Miridae) to a water trap with LED light in chrysanthemum greenhouses and olfactory attraction to novel compounds in Y-tube tests. Pest. Manag. Sci. 2022, 78, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, J.; Wang, Y.; Chen, L.; Chen, L.; Lei, C. Morphology and chemical analysis of the metathoracic scent glands system in Adelphocoris suturalis (Hemiptera: Miridae). J. Insect Sci. 2014, 14, 293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Aldrich, J.R. Sex pheromone of the plant bug, Phytocoris calli Knight. J. Chem. Ecol. 2008, 34, 719–724. [Google Scholar] [CrossRef]
- Yang, C.Y.; Kim, S.; Kim, J.; Kang, T.; Ahn, S. Sex pheromones and reproductive isolation in five mirid species. PLoS ONE 2015, 10, e0127051. [Google Scholar] [CrossRef]
- Leyi, Z. Fauna Sinica; Science Press: Beijing, China, 2004; Volume 113, p. 656. [Google Scholar]
- Qin, D. Investigation of Insect Pests of Lucern and Their Natural Enemies in Kangbao County of Hebei Province. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The buzz on insecticides: A review of uses, molecular structures, targets, adverse effects, and alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wyckhuys, K.A.G.; Yang, L.; Liu, B.; Zeng, J.; Jiang, Y.; Desneux, N.; Zhang, W.; Wu, K. Bt cotton area contraction drives regional pest resurgence, crop loss, and pesticide use. Plant Biotechnol. J. 2022, 20, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, L.; Zhu, X.; Zhang, J.; Li, N.; Fan, J.; Li, H.; Sun, X.; Zhang, L.; Lin, Y.; et al. Large-area field application confirms the effectiveness of toxicant-infused bait for managing (Hübner) in maize fields. Pest. Manag. Sci. 2023, 79, 5405–5417. [Google Scholar] [CrossRef]
- Fountain, M.T.; Deakin, G.; Farman, D.; Hall, D.; Jay, C.; Shaw, B.; Walker, A. An effective ‘push–pull’ control strategy for European tarnished plant bug, Lygus rugulipennis (Heteroptera: Miridae), in strawberry using synthetic semiochemicals. Pest. Manag. Sci. 2021, 77, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, C.; Guo, B.; She, D.; Mei, X.; Yang, X.; Ning, J. Research progress and application prospects on insect sex pheromone. Chin. J. Pestic. Sci. 2022, 24, 997–1016. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Mei, X.; Li, Y.; Gao, Z.; Ning, J. Sex pheromone of the jumping plant bug, Halticus minutus Reuter (Hemiptera: Miridae). J. Asia-Pac. Entomol. 2017, 20, 319–323. [Google Scholar] [CrossRef]
- Noge, K.; Prudic, K.L.; Becerra, J.X. Defensive Roles of (E)-2-Alkenals and Related Compounds in Heteroptera. J. Chem. Ecol. 2012, 38, 1050–1056. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, J.; Li, F.; Yan, Q.; Meng, L.; Li, B. Chemical polymorphism regulates the attractiveness to nymphs in the bean bug Riptortus pedestris. J. Pest. Sci. 2021, 94, 463–472. [Google Scholar] [CrossRef]
- Mcbrien, H.; Millar, J. Pheromones of Non-Lepidopteran Insects Associated with Agricultural Plants; CABI Publishers: Wallingford, UK, 1999. [Google Scholar]
- Millar, J.G.; Rice, R.E.; Wang, Q. Sex pheromone of the mirid bug Phytocoris relativus. J. Chem. Ecol. 1997, 23, 1743–1754. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, K. Honey Bee Alarm Pheromone mediates communication in plant–pollinator–predator interactions. Insects 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hedo, M.; Rambla, J.L.; Granell, A.; Urbaneja, A. Biological activity and specificity of Miridae-induced plant volatiles. BioControl 2018, 63, 203–213. [Google Scholar] [CrossRef]
- Zheng, S.; Cai, J.; Huang, P.; Wang, Y.; Yang, Z.; Yu, Y. Determination of volatile profiles of woodland strawberry (Fragaria vesca) during fruit maturation by HS-SPME GC–MS. J. Agric. Food Chem. 2023, 103, 7455–7468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, T.; Zhang, A.; Luo, J.; Chen, L.; Wang, M.; Ning, J.; Lei, C. Identification and field verification of sex pheromone from the mirid bug, Adelphocoris suturalis. Chemoecology 2016, 26, 25–31. [Google Scholar] [CrossRef]
- Thomas, M.L. Detection of female mating status using chemical signals and cues. Biol. Rev. 2011, 86, 1–13. [Google Scholar] [CrossRef]
- Farias, L.R.; Schimmelpfeng, P.H.C.; Togawa, R.C.; Costa, M.M.C.; Grynberg, P.; Martins, N.F.; Borges, M.; Blassioli-Moraes, M.C.; Laumann, R.A.; Báo, S.N.; et al. Transcriptome-based identification of highly similar odorant-binding proteins among neotropical stink bugs and their egg parasitoid. PLoS ONE 2015, 10, e0132286. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, L.; Ruther, J.; Hofferberth, J.; Stökl, J. Interference of chemical defence and sexual communication can shape the evolution of chemical signals. Sci. Rep. 2018, 8, 321. [Google Scholar] [CrossRef]
- Löfstedt, C.; Wahlberg, N.; Millar, J.G. Evolutionary Patterns of Pheromone Diversity in Lepidoptera; University of California Press: Oakland, CA, USA, 2016; Volume 1, pp. 43–82. [Google Scholar] [CrossRef]
- Hall, D.R.; Serrano, J.; Yokota, G.Y.; Nieto, D.J.; Farman, D.I.; McElfresh, J.S.; Del Pozo-Valdivia, A.I.; Millar, J.G.; Daane, K.M. Development of practical pheromone lures for Lygus hesperus and Lygus elisus (Heteroptera: Miridae). J. Econ. Entomol. 2024, toae266. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, C.; Mei, X.; Guo, B.; Yang, X.; Zhang, T.; Ning, J. Identification of sex pheromone in Macdunnoughia crassisigna Warren (Lepidoptera: Noctuidae) and field optimization of the sex attractant. Pest. Manag. Sci. 2024, 80, 577–585. [Google Scholar] [CrossRef]
- Kuenen, L.P.S.; Steven McElfresh, J.; Millar, J.G. Identification of Critical Secondary Components of the Sex Pheromone of the Navel Orangeworm (Lepidoptera: Pyralidae). J. Chem. Ecol. 2010, 103, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, B.; Dai, J.; Nazarenus, T.J.; Borges, R.; Mafra-Neto, A.; Cahoon, E.B.; Hofvander, P.; Stymne, S.; Löfstedt, C. Insect pest management with sex pheromone precursors from engineered oilseed plants. Nat. Sustain. 2022, 5, 981–990. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, S.; Zhang, Z.; Cao, S.; Li, B.; Liu, Y.; Wang, G. Identification and functional characterization of sex pheromone receptors in mirid bugs (Heteroptera: Miridae). Insect Biochem. Mol. 2021, 136, 103621. [Google Scholar] [CrossRef]
- Brent, C.S.; Byers, J.A. Female attractiveness modulated by a male-derived antiaphrodisiac pheromone in a plant bug. Anim. Behav. 2011, 82, 937–943. [Google Scholar] [CrossRef]
- Zhang, Q.; Aldrich, J.R. Male-produced anti-sex pheromone in a plant bug. Naturwissenschaften 2003, 90, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Sadek, M.M. Polyandry in field-collected Spodoptera littoralis moths and laboratory assessment of the effects of male mating history. Entomol. Exp. Appl. 2001, 98, 165–172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).