Chemical Components, Emission Dynamics, and External Immune Functions of Red Palm Weevil Larval Volatiles in Response to Changes in Developmental Stages and Pathogen Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Rearing
2.2. Microbe Preparation for Infection and Inhibition Assays
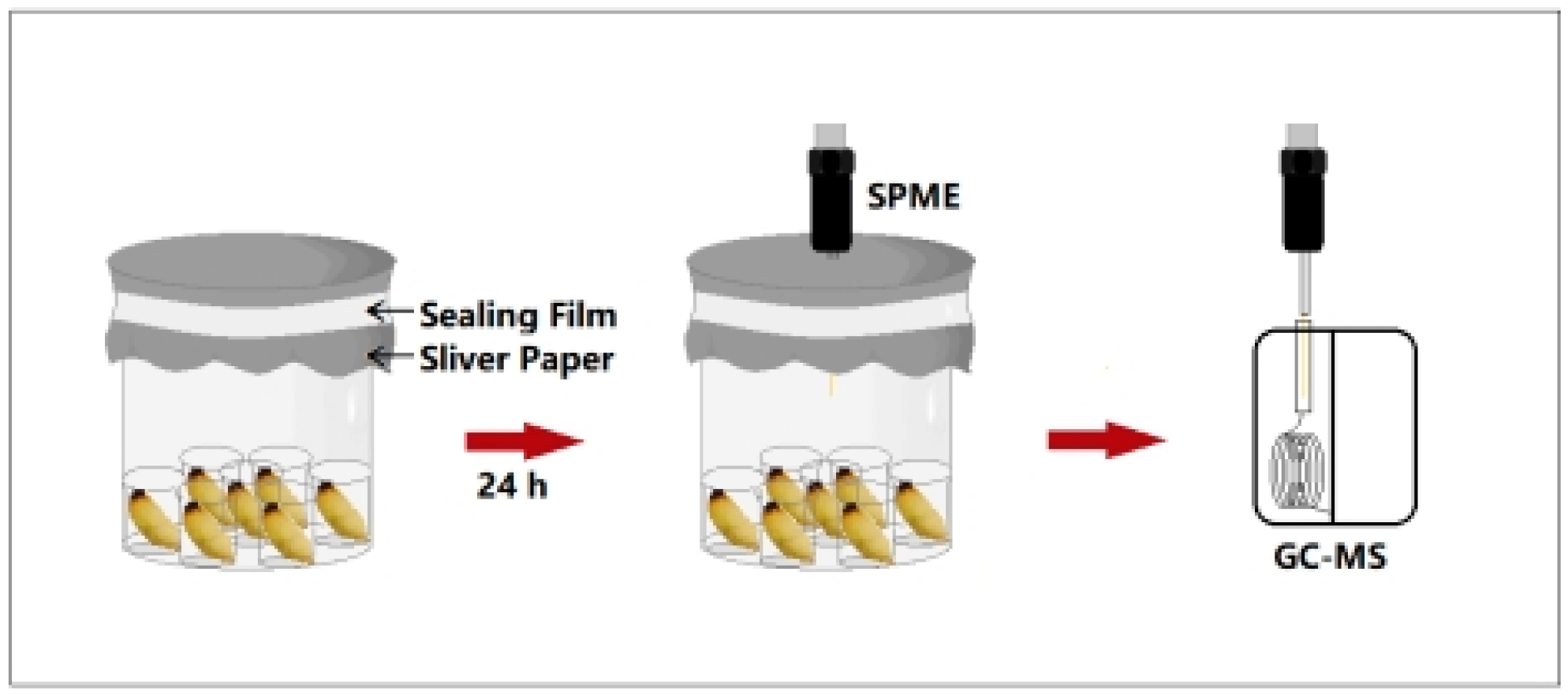
2.3. Collection of RPW Larval Volatiles at Different Developmental Stages
2.4. Assays on the Inhibitory Ability of Volatiles Against Pathogens
2.5. Qualitative and Quantitative Analysis of Volatile Components
2.6. Detection of Volatiles Following Challenge of Larvae with External Pathogens
2.7. Screening of Immunocompetent Compounds in Volatiles
2.8. Statistical Analysis
3. Results
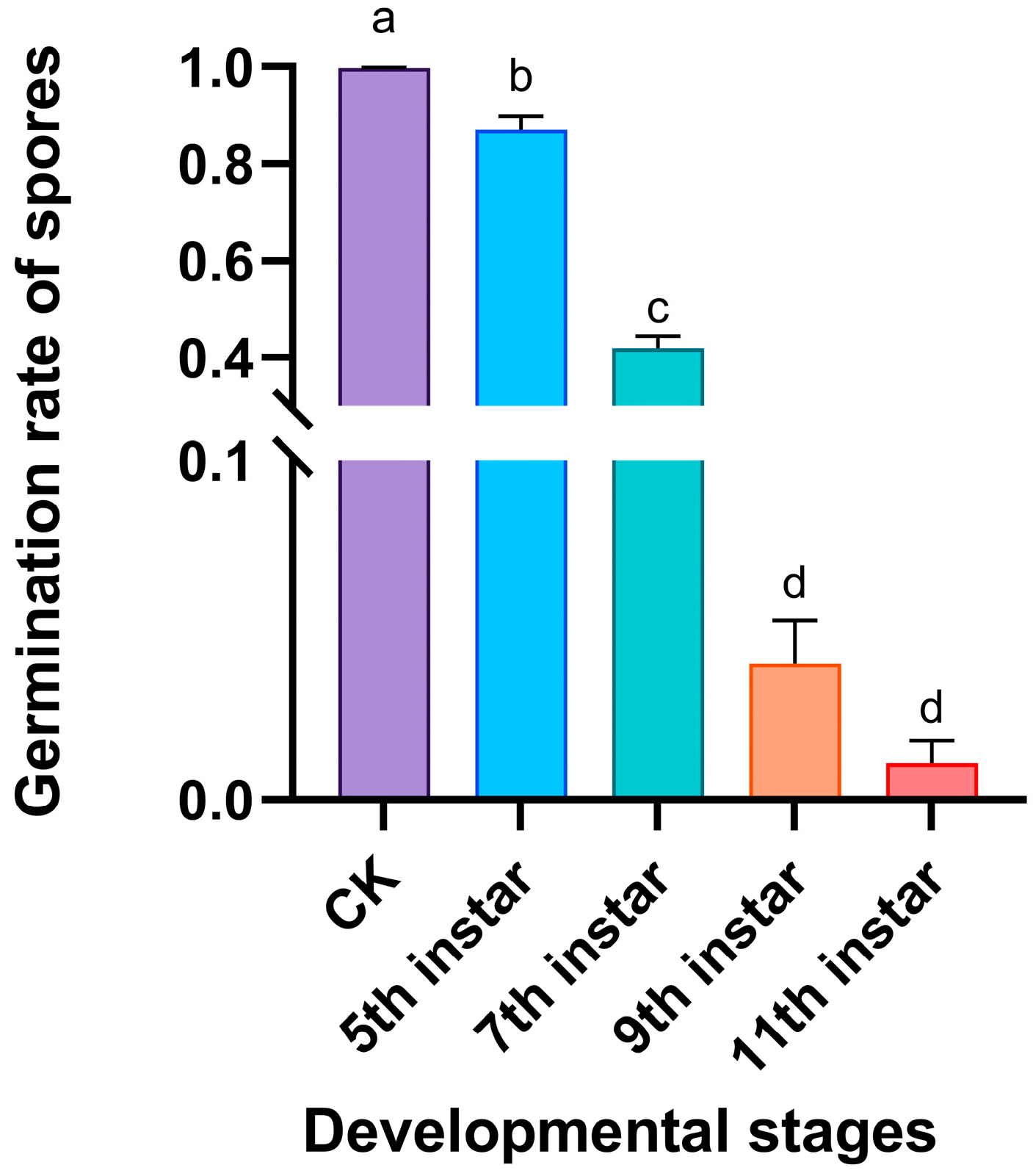
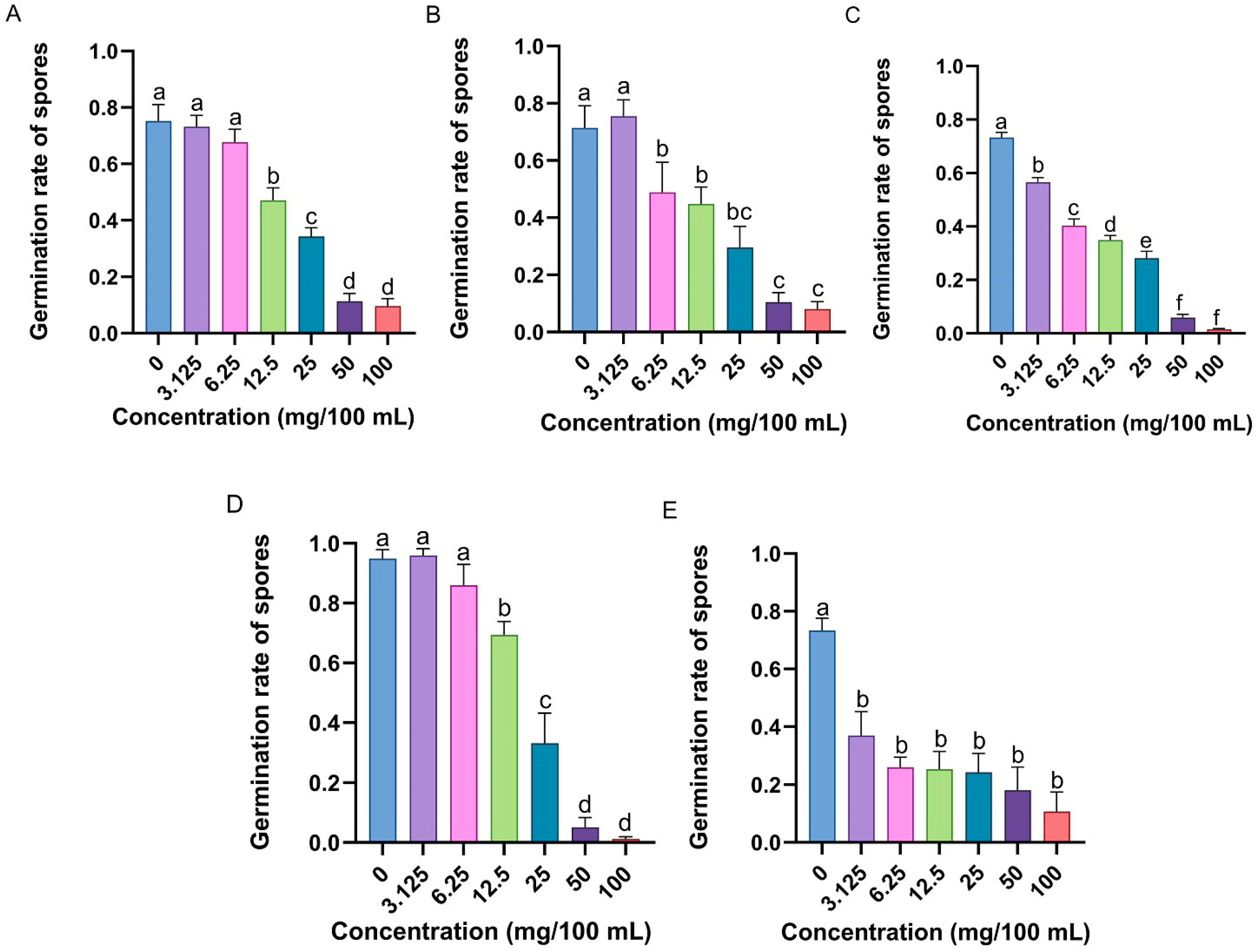
3.1. Immune Defensive Efficacy of RPW Larval Volatiles
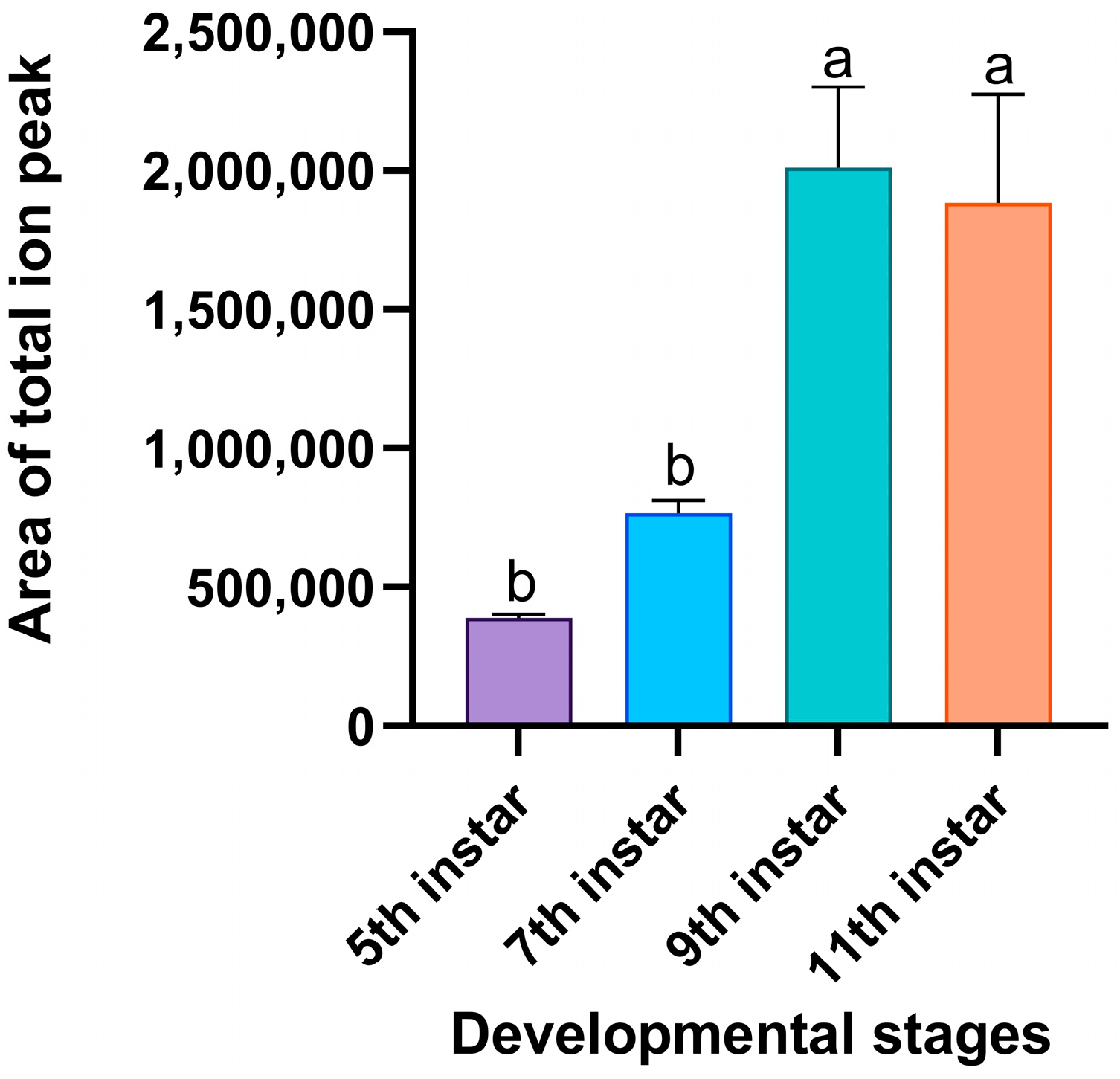
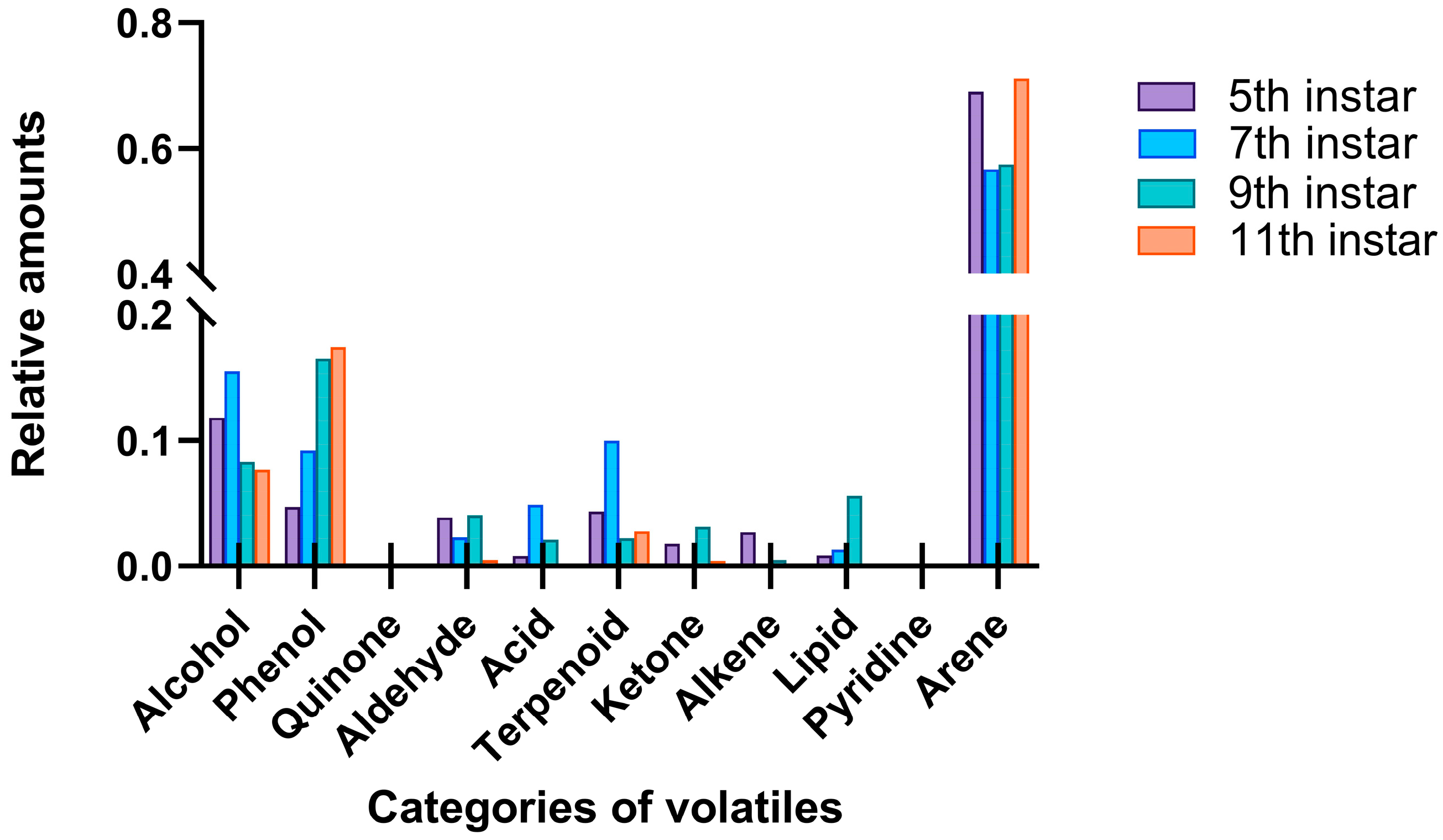
3.2. Volatiles and Emission Dynamics of RPW Larvae Across Developmental Stages
3.3. Effects of Pathogen Stress on Volatile Chemicals in RPW Larvae
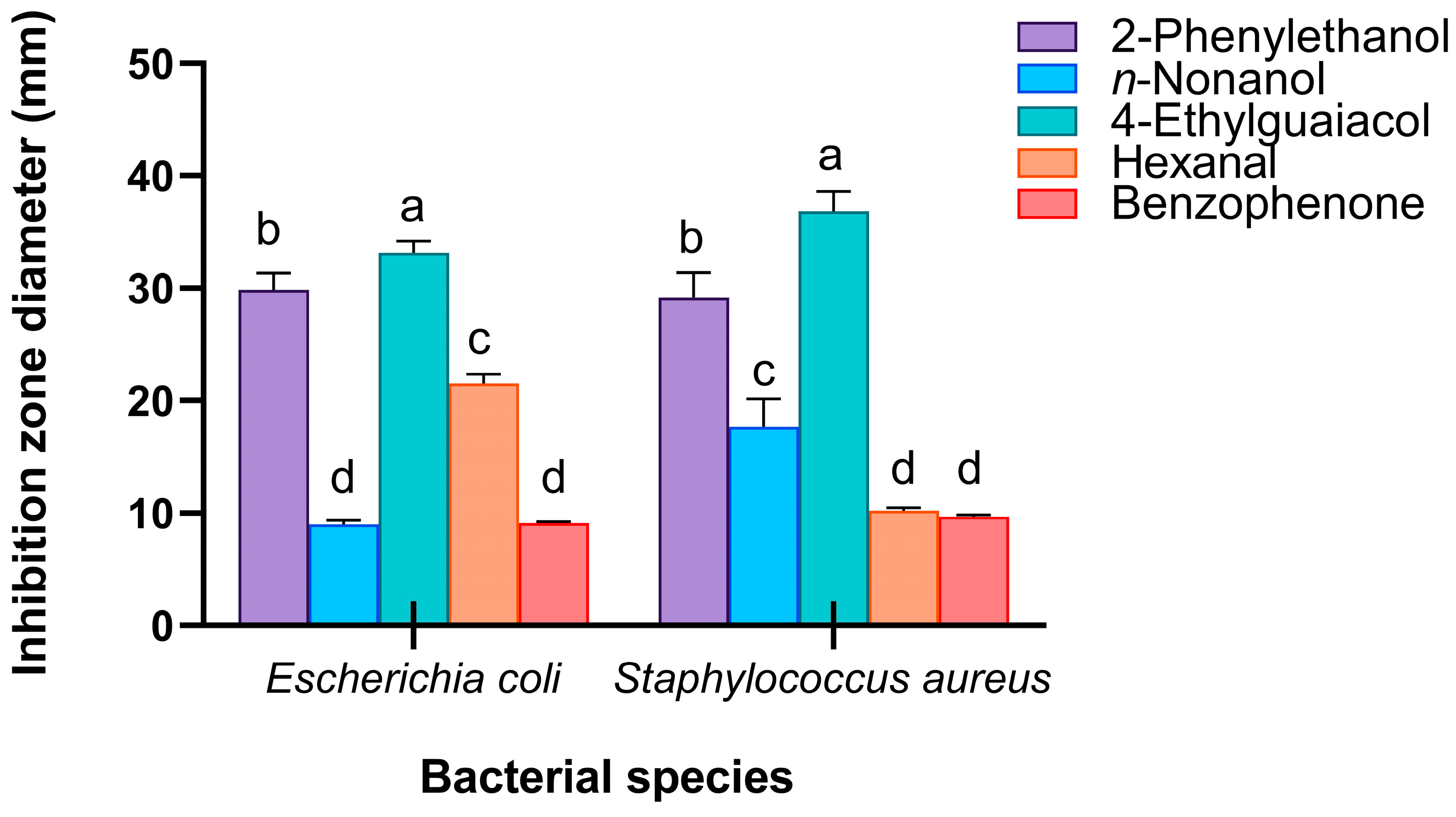
3.4. Antimicrobial Functions of Potential Immunocompetent Chemicals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, L.; Hou, Y.M. Red palm weevil Rhynchophorus ferrugineus (Olivier). In Biological Invasions and Its Management in China; Wan, F.H., Jiang, M.X., Zhan, A.B., Eds.; Springer Press: Berlin, Germany, 2017; Volume 1, pp. 245–256. [Google Scholar]
- Li, Y. Investigation and control measures of Rhychophorus ferrugineus in Xiamen, Fujian. Subtrop. Plant Sci. 2021, 50, 147–154. [Google Scholar]
- Lefroy, H.M. The More Important Insects Injurious to Indian Agriculture; Government of India Press: Calcutta, India, 1906. [Google Scholar]
- Wang, G.H.; Zhang, X.; Hou, Y.M.; Tang, B.Z. Analysis of the population genetic structure of Rhynchophorus ferrugineus in Fujian, China, revealed by microsatellite loci and mitochondrial COI sequences. Entomol. Exp. Appl. 2015, 155, 28–38. [Google Scholar] [CrossRef]
- Al-Dosary, N.M.N.; Al-Dobai, S.; Faleiro, J.R. Review on the management of red palm weevil Rhynchophorus ferrugineus Olivier in date palm Phoenix dactylifera L. Emir. J. Food Agric. 2016, 28, 34–44. [Google Scholar]
- Mendel, Z.; Voet, H.; Nazarian, I.; Dobrinin, S.; Ment, D. Comprehensive analysis of management strategies for red palm weevil in date palm settings, emphasizing sensor-based infestation detection. Agriculture 2024, 14, e260. [Google Scholar] [CrossRef]
- Vacas, S.; Abad-Payá, M.; Primo, J.; Navarro-Llopis, V. Identification of pheromone synergists for Rhynchophorus ferrugineus trapping systems from Phoenix canariensis palm volatiles. J. Agric. Food Chem. 2014, 62, 6053–6064. [Google Scholar] [CrossRef]
- Li, C.X.; Yan, W.; Huang, S.C.; Liu, L.; Qin, W.Q.; Wei, J. The efficacy of 10 pesticides against red palm weevil Rhychophorus ferrugineus (Oliver). China For. Sci. Technol. 2013, 27, 72–74. (In Chinese) [Google Scholar]
- Massa, R.; Panariello, G.; Pinchera, D.; Schettino, F.; Caprio, E.; Griffo, R.; Migliore, M.D. Experimental and numerical evaluations on palm microwave heating for red palm weevil pest control. Sci. Rep. 2017, 7, e45299. [Google Scholar] [CrossRef]
- Ishak, I.; Ng, L.C.; Haris-Hussain, M.; Jalinas, J.; Idris, A.B.; Azlina, Z.; Samsudin, A.; Wahizatul, A.A. Pathogenicity of an indigenous strain of the entomopathogenic fungus Metarhizium anisopliae (Hypocreales: Clavicipitaceae) (MET-GRA4 Strain) as a potential biological control agent against the red palm weevil (Coleoptera: Dryophthoridae). J. Econ. Entomol. 2020, 113, 43–49. [Google Scholar] [CrossRef]
- Husain, M.; Rasool, K.G.; Sutanto, K.D.; Omer, A.O.; Tufail, M.; Aldawood, A.S. Laboratory evaluation of indigenous and commercial entomopathogenic nematodes against red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects 2024, 15, e290. [Google Scholar] [CrossRef]
- Mastore, M.; Arizza, V.; Manachini, B.; Brivio, M.F. Modulation of immune responses of Rhynchophorus ferrugineus (Insecta: Coleoptera) induced by the entomopathogenic nematode Steinernema carpocapsae (Nematoda: Rhabditida). Insect Sci. 2015, 22, 748–760. [Google Scholar] [CrossRef]
- Pu, Y.C.; Ma, T.L.; Hou, Y.M.; Sun, M. An entomopathogenic bacterium strain, Bacillus thuringiensis, as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Pest Manag. Sci. 2017, 73, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Ahmed, S.; Al-Jabr, A.M. Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl 2015, 60, 849–859. [Google Scholar] [CrossRef]
- Mohamed, E.; Hasan, M.; Yaseen, T.; Moflih, M.; Hasan, A.; Almatni, W. The efficacy of some local isolates of the fungus Beauveria bassiana (Ascomycota: Hypocreales) on the red palm weevil (RPW) Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionida). Acta Hortic. 2023, 1371, 87–94. [Google Scholar] [CrossRef]
- Lei, C.J.; Ahmad, R.H.I.R.; Halim, N.A.; Asib, N.; Zakaria, A.; Azmi, W.A. Bioefficacy of an oil-emulsion formulation of entomopathogenic fungus, Metarhizium anisopliae against adult red palm weevil, Rhynchophorus ferrugineus. Insects 2023, 14, e482. [Google Scholar] [CrossRef]
- Pu, Y.C.; Zheng, Z.W.; Ding, C.H.; Chen, X.D. Development of potential microbial agents with two new entomopathogenic fungal strains to control the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Egypt. J. Biol. Pest Control 2023, 33, e107. [Google Scholar] [CrossRef]
- Sun, L.N.; Meng, J.Y.; Wang, Z.; Lin, S.Y.; Shen, J.; Yan, S. Research progress of aphid immunity system: Potential effective target for green pest management. Insect Sci. 2024, 31, 1662–1674. [Google Scholar] [CrossRef]
- Dong, W.; Flaven-Pouchon, J.; Gao, Y.H.; Song, C.Y.; El Wakil, A.; Zhang, J.Z.; Moussian, B. Chitinase 6 is required for procuticle thickening and organ shape in Drosophila wing. Insect Sci. 2023, 30, 268–278. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Huang, D.; Zhang, C.; Yuan, F.; Chen, Q.; Liu, T. Chemical proteomics reveals that camptothecin weakens insect immunity against bacteria by suppressing antimicrobial peptide expression. J. Agric. Food Chem. 2025, 73, 289–297. [Google Scholar] [CrossRef]
- Pu, Y.C.; Wang, R.; Liu, H.H.; Lu, S.P.; Tang, F.X.; Hou, Y.M. Immunosenescence along with direct physiological allocation trade-offs between life history and immunity in the red palm weevil Rhynchophorus ferrugineus. Dev. Comp. Immunol. 2021, 123, e104143. [Google Scholar] [CrossRef]
- Otti, O.; Tragust, S.; Feldhaar, H. Unifying external and internal immune defences. Trends Ecol. Evol. 2014, 29, 625–634. [Google Scholar] [CrossRef]
- Rafaluk, C.; Yang, W.T.; Mitschke, A.; Rosenstiel, P.; Schulenburg, H.; Joop, G. Highly potent host external immunity acts as a strong selective force enhancing rapid parasite virulence evolution. Environ. Microbiol. 2017, 19, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Luo, J.; Li, C.; Eleftherianos, I.; Zhang, W.; Xu, L. A life-and-death struggle: Interaction of insects with entomopathogenic fungi across various infection stages. Front. Immunol. 2024, 14, e1329843. [Google Scholar] [CrossRef] [PubMed]
- Muchoney, N.D.; Bowers, M.D.; Carper, A.L.; Mason, P.A.; Teglas, M.B.; Smilanich, A.M. Use of an exotic host plant shifts immunity, chemical defense, and viral burden in wild populations of a specialist insect herbivore. Ecol. Evol. 2022, 12, e8723. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.L.; Bai, C.; Cao, J.; Liu, P.; Han, W.X.; Gong, L.S.; Dong, Y. Research on chemical defense of Carabus augustus. Mod. Agric. Sci. Technol. 2019, 17, 117–119. [Google Scholar]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Li, J.; Lehmann, S.; Weißbecker, B.; Naharros, I.O.; Schütz, S.; Joop, G.; Wimmer, E.A. Odoriferous defensive stink gand transcriptome to identify novel genes necessary for quinone synthesis in the red flour beetle, Tribolium castaneum. PLoS Genet. 2013, 9, e1003596. [Google Scholar] [CrossRef]
- Gross, J.; Podsiadlowski, L.; Hilker, M. Antimicrobial activity of exocrine glandular secretion of Chrysomela larvae. J. Chem. Ecol. 2002, 28, 317–331. [Google Scholar] [CrossRef]
- Mazza, G.; Arizza, V.; Baracchi, D.; Barzanti, G.P.; Benvenuti, C.; Francardi, V.; Frandi, A.; Gherardi, F.; Longo, S.; Manachini, B.; et al. Antimicrobial activity of the red palm weevil Rhynchophorus ferrugineus. Bull. Insectology 2011, 64, 33–41. [Google Scholar]
- Wei, J.; Shao, W.; Wang, X.; Ge, J.; Chen, X.; Yu, D.; Kang, L. Composition and emission dynamics of migratory locust volatiles in response to changes in developmental stages and population density. Insect Sci. 2017, 24, 60–72. [Google Scholar] [CrossRef]
- Fennnie, C.; Favaro, R.; Khomenko, I.; Biasioli, F.; Cappellin, L.; Angeli, S. Diel rhythm of volatile emissions from males and females of the olive fruit fly Bactrocera oleae using PTR-ToF and GC-MS. J. Insect Physiol. 2024, 153, e104596. [Google Scholar] [CrossRef]
- Gross, J.; Schumacher, K.; Schmidtberg, H.; Vilcinskas, A. Protected by fumigants: Beetle perfumes in antimicrobial defense. J. Chem. Ecol. 2008, 34, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Eben, A.; Ina, M.; Wensing, A. A well protected intruder: The effective antimicrobial defense of the invasive ladybird Harmonia axyridis. J. Chem. Ecol. 2010, 36, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.X.; Yi, T.Q.; He, Z.C. Study on inhibition of fungi in aviation kerosene. Chem. Eng. Oil Gas 2010, 39, 35–37. [Google Scholar]
- Gao, J.G.; Wang, R.P.; Hao, Q.Y.; Huang, W.G. Comparison of antibacterial effect between biochemical fulvic acid and common antibacterial drugs. Humic Acid 2009, 5, 18–23. [Google Scholar]
- Li, H.X.; Dong, Z.; Meng, R.X.; Wei, C.G.; Fen, S.J.; Meng, H.W.; Shi, L. The chemical analysis of volatiles from live adults of Oedaleus asiaticus B. Bienko (Orthoptera: Acrididae). J. Inn. Mong. Agric. Univ. 2011, 32, 68–71. [Google Scholar]
- Zhang, Y.; Tan, Y.; Reymick, O.O.; Ouyang, Q.; Tao, N. γ-Cyclodextrin-encapsulated cinnamaldehyde for citrus preservation and its potential mechanisms against Penicillium digitatum. J. Fungi 2022, 8, e1199. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, D.X.; Wang, A.P.; Wang, Y.J. Analysis of body volatiles of the banana corm weevil, Cosmopolites sordidus (Germar) by solid-phase microextraction-gas chromatography-mass spectrometry. Chin. Agric. Sci. Bull. 2010, 26, 314–318. [Google Scholar]
- Bonacci, T.; Brandmayr, P.; Zetto, T.; Perrotta, I.D.; Guarino, S.; Peri, E.; Colazza, S. Volatile compounds released by disturbed and undisturbed adults of Anchomenus dorsalis (Coleoptera, Carabidae, Platynini) and structure of the pygidial gland. ZooKeys 2011, 81, 13–25. [Google Scholar] [CrossRef]
- Whitman, D.W.; Jones, C.G.; Blum, M.S. Defensive secretion production in lubber grasshoppers (Orthoptera: Romaleidae): Influence of age, sex, diet, and discharge frequency. Ann. Entomol. Soc. Am. 1992, 85, 96–102. [Google Scholar] [CrossRef]
- Sun, X.; Yan, W.; Qin, W.; Zhang, J.; Niu, X.; Ma, G.; Li, F. Screening of tropical isolates of Metarhizium anisopliae for virulence to the red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae). SpringerPlus 2016, 5, e1100. [Google Scholar] [CrossRef]
- Habineza, P.; Muhammad, A.; Ji, T.; Xiao, R.; Yin, X.; Hou, Y.; Shi, Z. The promoting effect of gut microbiota on growth and development of red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Dryophthoridae) by modulating its nutritional metabolism. Front. Microbiol. 2019, 10, e1212. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.C.; Xiang, H.J.; Liang, X.Y.; Wang, Y.; Hou, Y.M.; Fu, L.; Wang, R. External immune inhibitory efficiency of external secretions and their metabolic profiling in red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Front. Physiol. 2020, 10, e1624. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.C.; Silva, P.R.R.; França, S.M.; Saleh, I.A.; Neto, F.A.; El-Tayeb, M.A.; Matos, K.S.; Abdel-Maksoud, M.A.; Guimarães, S.S.C.; Zuffo, A.M.; et al. Metarhizium mendonceae sp. Nov.: An important biological control agent for insect pests. PLoS ONE 2025, 20, e0310548. [Google Scholar]
- Senthilkumar, T.; Jayas, D.S.; White, N.D.G.; Freund, M.S.; Shafai, C.; Thomson, D.J. Characterization of volatile organic compounds released by granivorous insects in stored wheat. J. Stored Prod. Res. 2012, 48, 91–96. [Google Scholar] [CrossRef]
- Xia, Z.D.; Mao, X.Z.; Luo, Y.H. Study on antifungal mechanism of α-pinene. Bull. Hunan Med. Univ. 1999, 24, 507–509. [Google Scholar]
- Liang, H.Y.; Wang, G.C.; Qin, X.F.; Wu, L.M. Bacteriostatic effects of six aromatic volatile organic compounds on Aspergillus flavus. J. Environ. Health 2013, 30, 143–145. (In Chinese) [Google Scholar]
- Zhang, Z.Z.; Li, Y.B.; Qi, L.; Wan, X.C. Antifungal activities of major tea leaf volatile constituents toward Colletorichum camelliae Massea. J. Agric. Food Chem. 2006, 54, 3936–3940. [Google Scholar] [CrossRef]
- Kosalec, I.; Klarić, M.Š.; Pepeljnjak, S. Antifungal activity of 2-phenylethanol and levomenthol against molds from indoor air and damp dwellings. Planta Med. 2007, 73, 859–860. [Google Scholar] [CrossRef]
- Gardini, F.; Lanciotti, R.; Caccioni, D.R.L.; Guerzoni, M.E. Antifungal activity of hexanal as dependent on its vapor pressure. J. Agric. Food Chem. 1997, 45, 4297–4302. [Google Scholar] [CrossRef]
- Fan, L.; Song, J.; Beaudry, R.M.; Hildebrand, P.D. Effect of hexanal vapor on spore viability of Penicillium expansum, lesion development on whole apples and fruit volatile biosynthesis. J. Food Sci. 2006, 71, M105–M109. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, Y.Z.; Ma, G.Q.; Cui, H.Q.; Gao, Y.; Shu, J.C. Synthesis and antifungal activities of benzophenones. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 60–64. [Google Scholar]
- Hallett, R.H.; Gries, G.; Gries, R.; Gries, G.; Cameron-Oehlschlager, A.; Borden, J.H. Aggregation pheromones of two asian palm weevils, Rhynchophorus ferrugineus and R. vulneratus. Naturwissenschaften 1993, 80, 328–331. [Google Scholar] [CrossRef]



| No. | Chemical Compounds a | Molecular Formula | Average Relative Amounts (%) | |||
|---|---|---|---|---|---|---|
| 5th-Instar | 7th-Instar | 9th-Instar | 11th-Instar | |||
| 1 | Verbenol | C10H16O | ND b | 0.16 | 0.02 | ND |
| 2 | n-Nonanol | C9H20O | 1.63 | 0.30 | 0.27 | 0.35 |
| 3 | Linalool | C10H18O | ND | 0.25 | ND | ND |
| 4 | Geraniol | C10H18O | ND | ND | ND | 0.33 |
| 5 | Geosmin | C14H7OF3Cl2 | 0.22 | ND | ND | ND |
| 6 | Benzyl alcohol | C7H8O | ND | ND | 0.01 | ND |
| 7 | α-Terpineol | C10H18O | ND | 0.40 | ND | 0.03 |
| 8 | 2-Phenylethanol | C5H12O2 | 7.55 | 5.08 | 7.26 | 6.82 |
| 9 | 2-Ethyl-1-hexanol | C10H16O2 | 1.60 | 1.39 | 0.75 | ND |
| 10 | 1-Tetradecanol | C14H30O | ND | 0.77 | ND | ND |
| 11 | 1-Pentanol | C5H12O | ND | 4.07 | ND | ND |
| 12 | 1-Dodecanol | C50H57N5O13S5 | 0.81 | 0.12 | ND | 0.03 |
| 13 | 1-Undecanol | C11H24O | ND | 2.93 | ND | 0.10 |
| 14 | Phenol | C6H6O | 0.78 | 0.07 | 8.22 | ND |
| 15 | 4-Ethylguaiacol | C9H12O2 | 0.39 | 1.17 | 0.19 | 3.92 |
| 16 | Methyl eugenol | C11H14O2 | ND | ND | ND | 0.08 |
| 17 | Isoeugenol | C10H12O2 | ND | ND | ND | 0.47 |
| 18 | Guaiacol | C7H8O2 | 3.04 | 7.85 | 7.82 | 2.66 |
| 19 | 4-Ethylphenol | C20H12Br2O2 | 0.39 | 0.13 | 0.21 | 5.88 |
| 20 | 2,3-Xylenol | C11H10N4Cl2 | 0.12 | ND | 0.07 | 4.29 |
| 21 | Eugenol | C10H12O2 | ND | ND | ND | 0.11 |
| 22 | Camphorquinone | C10H14O2 | 0.08 | ND | ND | ND |
| 23 | Vanillin | C8H8O3 | 1.38 | ND | ND | ND |
| 24 | Phenylacetaldehyde | C8H8O | 0.12 | 0.94 | 0.12 | 0.05 |
| 25 | Octanal | C8H16O | 0.17 | ND | ND | ND |
| 26 | Hexanal | C6H12O | 1.01 | 0.23 | 3.63 | 0.19 |
| 27 | Benzaldehyde | C7H6O | 0.81 | 0.99 | 0.27 | 0.22 |
| 28 | n-Decanal | C10H20O | 0.40 | 0.13 | 0.02 | 0.01 |
| 29 | Isovaleric acid | C5H10O2 | 0.57 | 4.89 | 2.12 | ND |
| 30 | Hexanoic acid | C6H12O2 | 0.24 | ND | ND | ND |
| 31 | β-Pinene | C10H16 | ND | ND | 1.03 | 1.38 |
| 32 | α-Pinene | C10H16 | 4.33 | 6.73 | 1.16 | 1.35 |
| 33 | Borneol | C10H18O | ND | 2.49 | 0.04 | ND |
| 34 | 2-Methylisoborneol | C11H20O | ND | 0.73 | ND | 0.04 |
| 35 | Benzophenone | C13H10O | 0.59 | ND | 0.13 | 0.26 |
| 36 | Acetoin | C4H8O2 | ND | ND | 0.78 | ND |
| 37 | 2-Heptanone | C7H14O | 1.21 | ND | 2.21 | 0.17 |
| 38 | Limonene | C10H16 | 2.68 | 0.18 | 0.49 | ND |
| 39 | n-Hexyl acetate | C8H16O2 | 0.63 | 0.85 | 0.12 | 0.09 |
| 40 | Methyl salicylate | C8H8O3 | 0.23 | 0.46 | ND | ND |
| 41 | γ-Octalactone | C7H7N3 | ND | ND | 4.90 | ND |
| 42 | γ-Decanolactone | C10H18O2 | ND | ND | 0.16 | ND |
| 43 | Ethyl-2-methylbutyrate | C7H14O2 | ND | ND | 0.43 | ND |
| 44 | 3-Amino-4-methylpyridine | C6H8N2 | ND | ND | ND | 0.09 |
| 45 | 2-n-Propylpyridine | C8H11N | ND | ND | 0.04 | ND |
| 46 | Toluene | C7H8 | 5.04 | 0.61 | 4.58 | ND |
| 47 | Styrene | C8H8 | 27.09 | 28.04 | 38.60 | 64.00 |
| 48 | p-Xylene | C8H10 | 8.32 | 8.21 | 4.76 | 0.45 |
| 49 | p-Dichlorobenzene | C6H4Cl2 | 0.04 | 0.18 | 0.04 | 0.08 |
| 50 | o-Xylene | C8H10 | 3.00 | 2.44 | 1.84 | 0.52 |
| 51 | Naphthalene | C10H8 | 3.75 | 4.54 | 0.30 | 0.47 |
| 52 | m-Xylene | C8H10 | 7.36 | 6.98 | 4.22 | 0.36 |
| 53 | Indole | C9H8NBr | 8.06 | 0.29 | ND | 1.25 |
| 54 | Ethylbenzene | C10H15NO2 | 2.88 | 2.33 | 2.50 | 0.45 |
| 55 | Acetophenone | C8H8O | 2.13 | 1.92 | 0.60 | 3.45 |
| 56 | 1,2,4,5-Tetramethylbenzene | C10H14 | 0.21 | 0.32 | ND | 0.06 |
| 57 | Butylated hydroxytoluene | C15H24O | 1.14 | 0.63 | 0.05 | 0.01 |
| 58 | 2-Methylnaphthalene | C11H10 | ND | 0.19 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.-H.; You, W.-Q.; Zheng, Z.-W.; Pu, Y.-C.; Xu, L.-N.; Hou, Y.-M.; Zhang, Y.; Ou-Yang, C. Chemical Components, Emission Dynamics, and External Immune Functions of Red Palm Weevil Larval Volatiles in Response to Changes in Developmental Stages and Pathogen Stress. Insects 2025, 16, 1266. https://doi.org/10.3390/insects16121266
Ding C-H, You W-Q, Zheng Z-W, Pu Y-C, Xu L-N, Hou Y-M, Zhang Y, Ou-Yang C. Chemical Components, Emission Dynamics, and External Immune Functions of Red Palm Weevil Larval Volatiles in Response to Changes in Developmental Stages and Pathogen Stress. Insects. 2025; 16(12):1266. https://doi.org/10.3390/insects16121266
Chicago/Turabian StyleDing, Can-Hui, Wen-Qing You, Zong-Wei Zheng, Yu-Chen Pu, Li-Na Xu, You-Ming Hou, Yue Zhang, and Cong Ou-Yang. 2025. "Chemical Components, Emission Dynamics, and External Immune Functions of Red Palm Weevil Larval Volatiles in Response to Changes in Developmental Stages and Pathogen Stress" Insects 16, no. 12: 1266. https://doi.org/10.3390/insects16121266
APA StyleDing, C.-H., You, W.-Q., Zheng, Z.-W., Pu, Y.-C., Xu, L.-N., Hou, Y.-M., Zhang, Y., & Ou-Yang, C. (2025). Chemical Components, Emission Dynamics, and External Immune Functions of Red Palm Weevil Larval Volatiles in Response to Changes in Developmental Stages and Pathogen Stress. Insects, 16(12), 1266. https://doi.org/10.3390/insects16121266







