Identification and Evaluation of Alfalfa Volatiles for Monitoring and Management of Odontothrips loti and Frankliniella occidentalis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Collection of Volatiles of Alfalfa with and Without O. loti
2.3. Identification of Alfalfa Headspace Volatiles
2.4. Olfactometer Assays with Alfalfa Volatiles
2.5. Effect of p-Menth-8-en-2-one Concentration on the Behavioral and Electrophysiological Responses of Thrips
2.5.1. Behavioral Responses
2.5.2. Effect of Lure Type and p-Menth-8-en-2-one on the Behavioral Response of O. loti
2.5.3. Electroantennography Assays
2.5.4. Statistical Analysis
3. Results
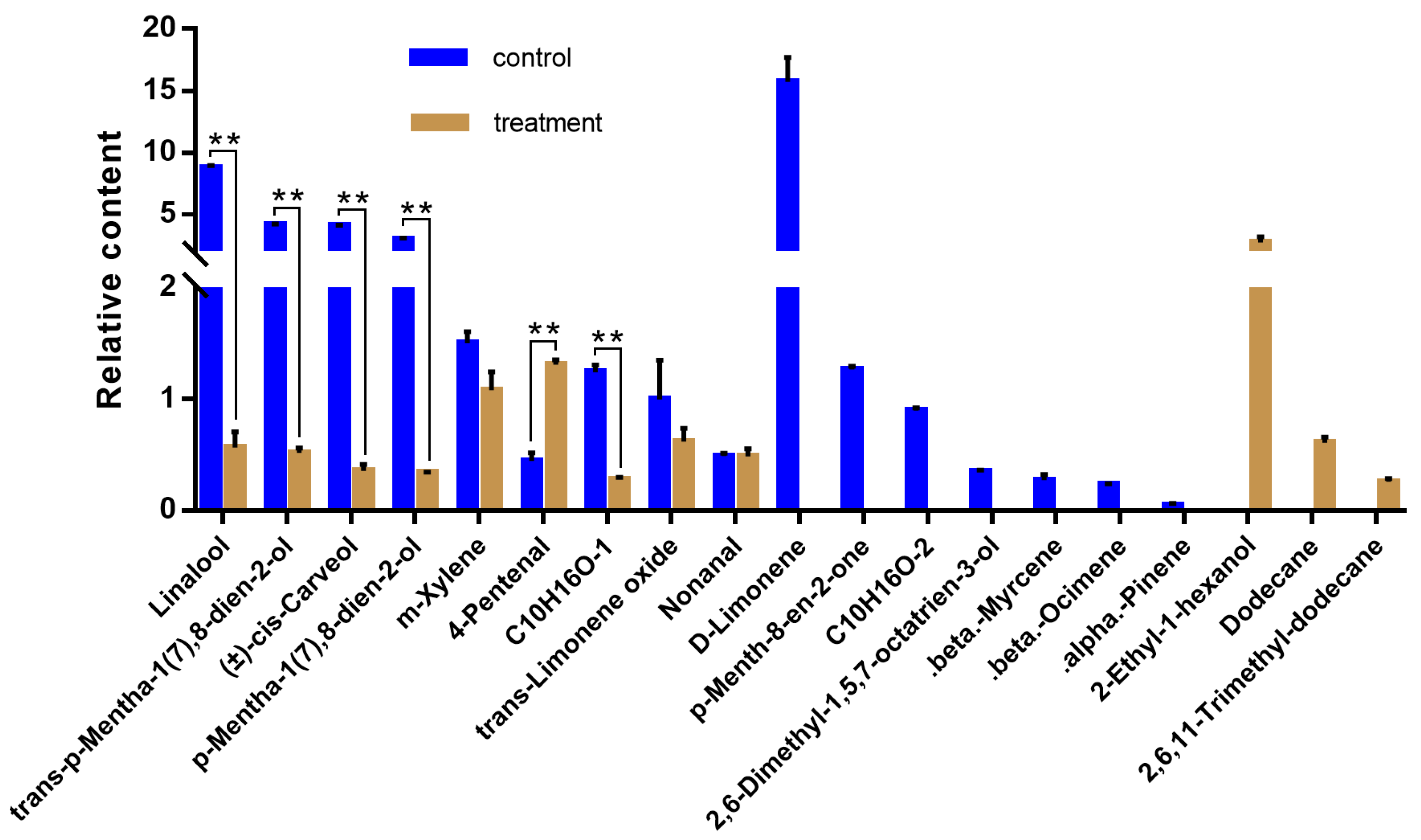
3.1. Composition and Relative Content of Volatiles of Alfalfa with and Without O. loti
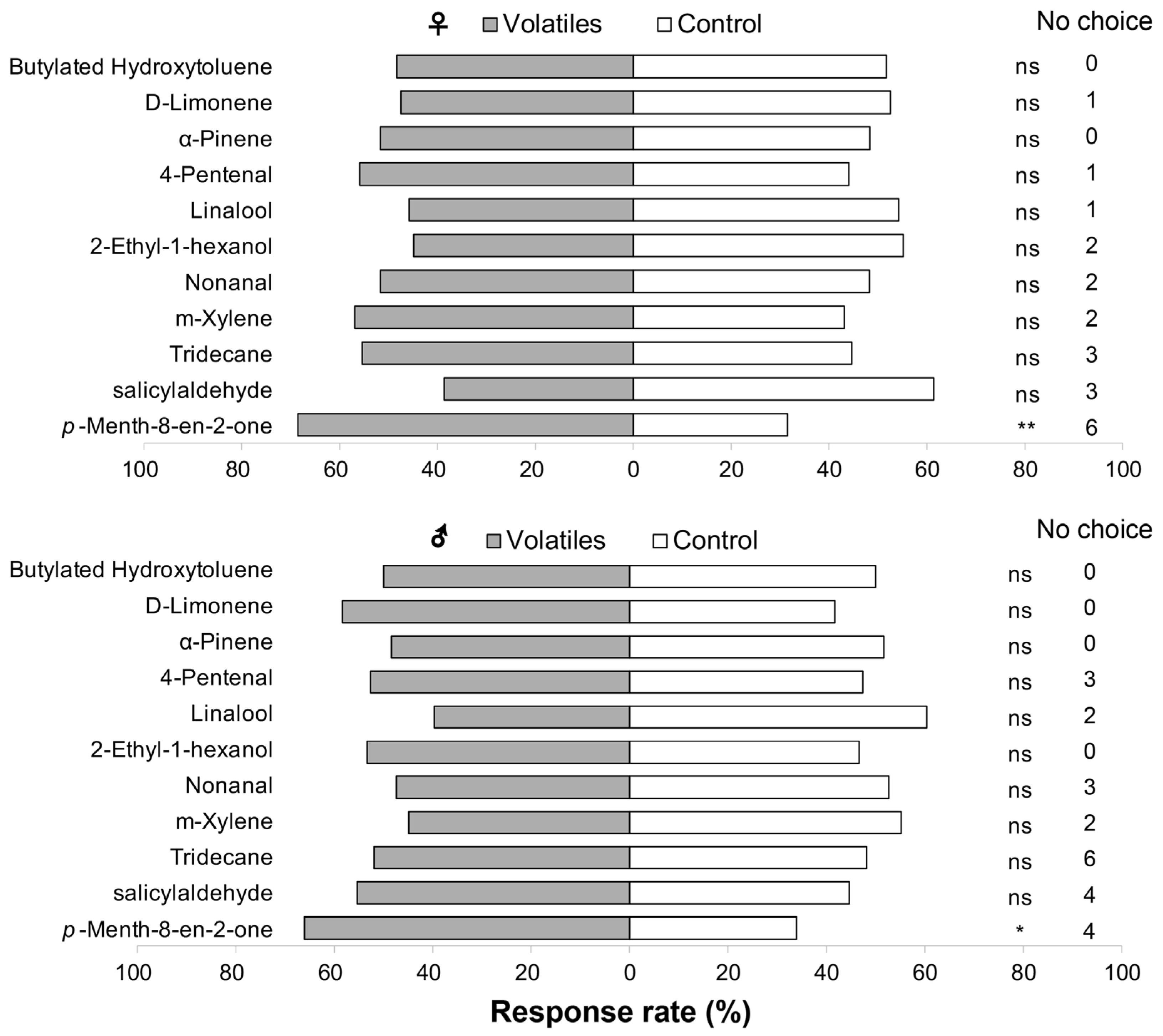
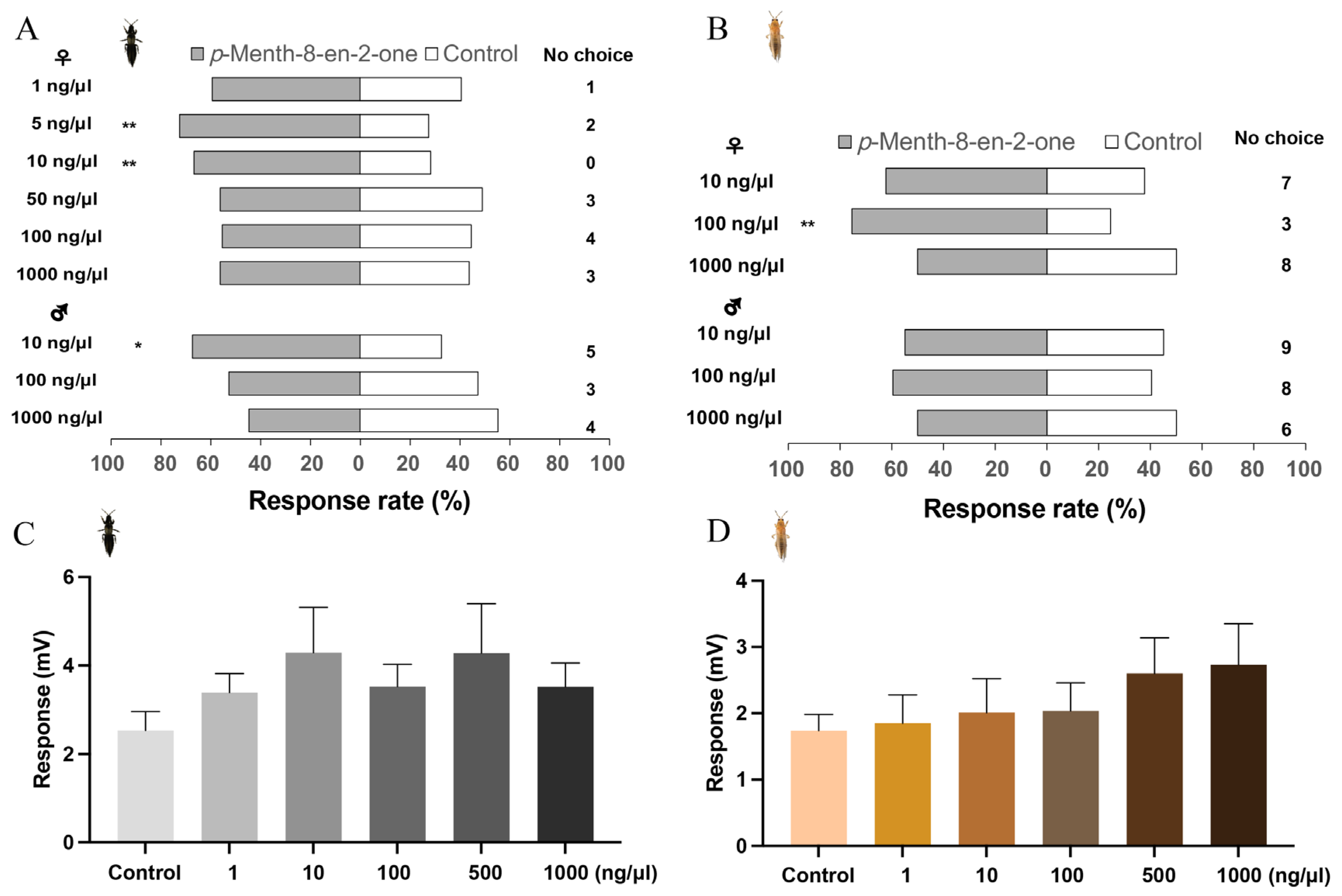
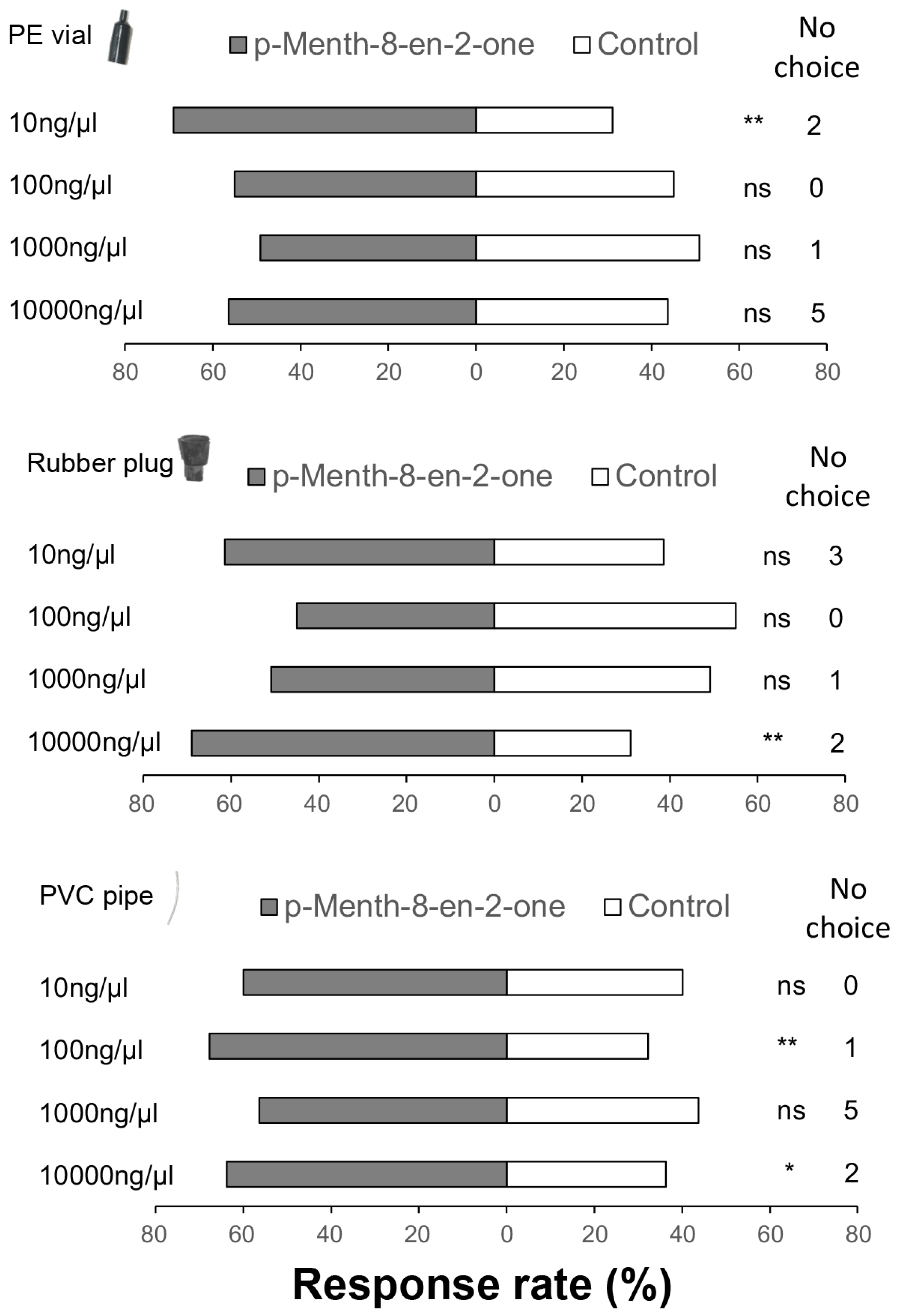
3.2. Behavioral and Electroantennographic Responses of Thrips to Alfalfa Volatiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Badieritakis, E.G.; Thanopoulos, R.C.; Fantinou, A.A.; Emmanouel, N.G. A Qualitative and Quantitative Study of Thrips (Thysanoptera) on Alfalfa and Records of Thrips Species on Cultivated and Wild Medicago Species of Greece. Biologia 2015, 70, 504–511. [Google Scholar] [CrossRef]
- Ábrahám, R. First Investigation of Species Composition of Thysanoptera Inhabiting Alfalfa Based on Their Second Stage Larvae. Acta Phytopathol. Entomol. Hung. 2012, 47, 81–86. [Google Scholar] [CrossRef]
- Ripa, R.; Funderburk, J.; Rodriguez, F.; Espinoza, F.; Mound, L. Population Abundance of Frankliniella occidentalis (Thysanoptera: ThriPidae) and Natural Enemies on Plant Hosts in Central Chile. Environ. Entomol. 2009, 38, 333–344. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Luo, Y.; Du, L.; Ban, L. Antennal Transcriptome Analysis of Olfactory Genes and Characterization of Odorant Binding Proteins in Odontothrips loti (Thysanoptera: Thripidae). Int. J. Mol. Sci. 2023, 24, 5284. [Google Scholar] [CrossRef]
- Rotenberg, D.; Baumann, A.A.; Ben-Mahmoud, S.; Christiaens, O.; Dermauw, W.; Ioannidis, P.; Jacobs, C.G.C.; Vargas Jentzsch, I.M.; Oliver, J.E.; Poelchau, M.F.; et al. Genome-Enabled Insights into the Biology of Thrips as Crop Pests. BMC Biol. 2020, 18, 142. [Google Scholar] [CrossRef]
- He, Z.; Guo, J.-F.; Reitz, S.R.; Lei, Z.-R.; Wu, S.-Y. A Global Invasion by the Thrip, Frankliniella occidentalis: Current Virus Vector Status and Its Management. Insect Sci. 2020, 27, 626–645. [Google Scholar] [CrossRef]
- Li, X.; Cheng, J.; Han, H.; Kirk, W.D.J.; O’Brien, M.; Wang, L.; Chen, L.; Zhang, H.; Zhang, Z.; Ullah, F.; et al. Identification of Aggregation Pheromone as an Attractant for Odontothrips loti, a Serious Thrips Pest on Alfalfa. J. Chem. Ecol. 2024, 50, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Yang, C.-L.; Wang, S.-S.; Hu, G.-X. Changes of Phenols and Lignin Contents in Alfalfa Leaf Damaged by Odontothrips loti. J. Appl. Ecol. 2014, 25, 1688–1692. [Google Scholar] [CrossRef]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.J.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion Biology, Ecology, and Management of Western Flower Thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef]
- Li, J.; Shang, Q.; Luo, Y.; Wei, S.; Zhao, C.; Ban, L. Transmission from Seed to Seedling and Elimination of Alfalfa Viruses. Front. Plant Sci. 2024, 15, 1330219. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.R.; Sagers, J. Developing Economic Injury Levels for Thrips (Frankliniella occidentalis) in Idaho Alfalfa under Controlled Pest Populations. J. Ext. 2024, 61, 1. [Google Scholar] [CrossRef]
- Findlay, J.R.; Hogge, J.; Leslie, M.; Reitz, S.; Sagers, J.; Thomas, J. Evaluating Thrips Damage on Idaho Alfalfa Crops. J. NACAA 2020, 13. Available online: https://www.nacaa.com/file.ashx?id=fe6cf15c-119c-40e4-895e-861e1db179f1 (accessed on 22 January 2025).
- Zhang, R.; Ma, J.; Wang, J.; Ren, X. The occurrence and control strategy of alfalfa disease and insects in Ninxia. Pratacult. Sci. 2003, 20, 40–44. [Google Scholar] [CrossRef]
- Guo, S.; Cao, L.; Song, W.; Shi, P.; Gao, Y.; Gong, Y.; Chen, J.; Hoffmann, A.A.; Wei, S. Chromosome-Level Assembly of the Melon Thrips Genome Yields Insights into Evolution of a Sap-sucking Lifestyle and Pesticide Resistance. Mol. Ecol. Resour. 2020, 20, 1110–1125. [Google Scholar] [CrossRef]
- Santos, J.L.; Pereira, P.S.; Reis, K.H.B.; Freitas, D.R.; Picanço Filho, M.C.; Peluzio, J.M.; Sarmento, R.A.; Guedes, R.N.C.; Picanço, M.C. Decision-Making for Thrips Control in Soybean Fields Using Precision a Griculture Principles. J. Appl. Entomol. 2024, 148, 140–149. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral Scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Beyaert, I.; Hilker, M. Plant Odour Plumes as Mediators of Plant–Insect Interactions. Biol. Rev. 2014, 89, 68–81. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of Plant Volatiles in Defence against Microbial Pathogens and Microbial Exploitation of Volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Paudel Timilsena, B.; Seidl-Adams, I.; Tumlinson, J.H. Herbivore-Specific Plant Volatiles Prime Neighboring Plants for Nonspecific Defense Responses. Plant Cell Environ. 2020, 43, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Fabisch, T.; Gershenzon, J.; Unsicker, S.B. Specificity of Herbivore Defense Responses in a Woody Plant, Black Poplar (Populus nigra). J. Chem. Ecol. 2019, 45, 162–177. [Google Scholar] [CrossRef]
- Xiu, C.; Pan, H.; Zhang, F.; Luo, Z.; Bian, L.; Li, Z.; Fu, N.; Zhou, L.; Magsi, F.H.; Cai, X.; et al. Identification of Aggregation Pheromones Released by the Stick Tea Thrips (Dendrothrips minowai) Larvae and Their Application for Controlling Thrips in Tea Plantations. Pest Manag. Sci. 2024, 80, 2528–2538. [Google Scholar] [CrossRef]
- Qian, Q.; Cui, J.; Miao, Y.; Xu, X.; Gao, H.; Xu, H.; Lu, Z.; Zhu, P. The Plant Volatile-Sensing Mechanism of Insects and Its Utilization. Plants 2024, 13, 185. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Mitchell, V.J.; McLaren, G.F.; Manning, L.M.; Bunn, B.; Suckling, D.M. Attraction of New Zealand Flower Thrips, Thrips obscuratus, to Cis-Jasmone, a Volatile Identified from Japanese Honeysuckle Flowers. J. Chem. Ecol. 2009, 35, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Maekawa, M.; Murai, T. Attractiveness of Methyl Anthranilate and Its Related Compounds to the Flower Thrips, Thrips hawaiiensis (Morgan), T. coloratus Schmutz, T. flavus Schrank and Megalurothrips distalis (Karny) (Thysanoptera: Thripidae). Appl. Entomol. Zool. 2001, 36, 475–478. [Google Scholar] [CrossRef]
- Diabate, S.; Martin, T.; Murungi, L.K.; Fiaboe, K.K.M.; Subramanian, S.; Wesonga, J.; Deletre, E. Repellent Activity of Ccymbopogon citratus and Tagetes Minuta and Their Specific Volatiles against Megalurothrips sjostedti. J. Appl. Entomol. 2019, 143, 855–866. [Google Scholar] [CrossRef]
- Thomas, G.W.C.; Dohmen, E.; Hughes, D.S.T.; Murali, S.C.; Poelchau, M.; Glastad, K.; Anstead, C.A.; Ayoub, N.A.; Bellair, M.; Binford, G.J.; et al. The Genomic Basis of Arthropod Diversity. BioRxiv 2019. [Google Scholar] [CrossRef]
- Patt, J.M.; Sétamou, M.; Moreno, A.T.; Markle, L.; Stelinski, L.; George, J.; Rivera, M. Field Evaluation of Attract-and-Kill Devices for Control of Asian Citrus Psyllid (Hemiptera: Liviidae) in Urban Landscapes. Fla. Entomol. 2023, 106, 248–256. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Dorogi, B.; Gulyás, A.; Nagy, P.; Rozgonyi, Z. Male and Female Noctuid Moths Attracted to Synthetic Lures in Europe. J. Chem. Ecol. 2010, 36, 592–598. [Google Scholar] [CrossRef]
- Park, J.; Mostafiz, M.M.; Hwang, H.-S.; Jung, D.-O.; Lee, K.-Y. Comparing the Life Table and Population Projection of Gaeolaelaps aculeifer and Stratiolaelaps scimitus (Acari: Laelapidae) Based on the Age-Stage, Two-Sex Life Table Theory. Agronomy 2021, 11, 1062. [Google Scholar] [CrossRef]
- Toledo-Hernández, R.A.; Lasa, R.; Montoya, P.; Liedo, P.; Rodríguez, D.; Sánchez, A.; Toledo, J. Efficacy of Food-Based Attractants for Monitoring Drosophila suzukii (Diptera: Drosophilidae) in Berry Crops. Crop Prot. 2021, 150, 105797. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, T.; Jiang, Y.; Chen, P.; Tang, J.; Wang, J.; Jin, D.; Guo, J. Research of Synergistic Substances on Tobacco Beetle [Lasioderma serricorne (Fabricius) (Coleoptera: Anobiidae)] Adults Attractants. Front. Chem. 2022, 10, 921113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, G.; Qiu, Y.; Luo, Z.; Cai, X.; Li, Z.; Bian, L.; Fu, N.; Zhou, L.; Magsi, F.H.; et al. Mixture of Synthetic Plant Volatiles Attracts More Stick Tea Thrips Dendrothrips minowai Priesner (Thysanoptera: Thripidae) and the Application as an Attractant in Tea Plantations. Plants 2024, 13, 1944. [Google Scholar] [CrossRef]
- Tu, X.; Fan, Y.; Ji, M.; Liu, Z.; Xie, N.; Liu, Z.; Zhang, Z. Improving a Method for Evaluating Alfalfa Cultivar Resistance to Thrips. J. Integr. Agric. 2016, 15, 600–607. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, X.; Wang, Y.; Chen, Z.; Sun, J.; Wan, F.; Yuan, Z. Field Comparison of the Resistance of 33 Alfalfa Varieties to Thrips. Acta Pratacult. Sin. 2015, 24, 187. [Google Scholar] [CrossRef]
- González-Orellana, J.; López-Guillén, G.; Malo, E.A.; Goldarazena, A.; Cruz-López, L. Behavioural and Electrophysiological Responses of Liothrips jatrophae (Thysanoptera: Phlaeothripidae) to Conspecific Extracts and Some of Its Identified Compounds. Physiol. Entomol. 2022, 47, 11–19. [Google Scholar] [CrossRef]
- Koschier, E.H.; De Kogel, W.J.; Visser, J.H. Assessing the Attractiveness of Volatile Plant Compounds to Western Flower Thrips Frankliniella occidentalis. J. Chem. Ecol. 2000, 26, 2643–2655. [Google Scholar] [CrossRef]
- Li, X.-W.; Zhang, Z.-J.; Hafeez, M.; Huang, J.; Zhang, J.-M.; Wang, L.-K.; Lu, Y.-B. Rosmarinus officinialis L. (Lamiales: Lamiaceae), a Promising Repellent Plant for Thrips Management. J. Econ. Entomol. 2021, 114, 131–141. [Google Scholar] [CrossRef]
- Blackmer, J.L.; Rodriguez-Saona, C.; Byers, J.A.; Shope, K.L.; Smith, J.P. Behavioral Response of Lygus Hesperus to Conspecifics and Headspace Volatiles of Alfalfa in a Y-Tube Olfactometer. J. Chem. Ecol. 2004, 30, 1547–1564. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, X.; Xi, B.; Hu, G.; Shan, S.; Peng, B.; Shi, X. Olfactory Behavior Responses of Odontothrips loti Female Adults to Four Leguminous Plants and Their Volatiles. Chin. J. Biol. Control 2023, 39, 824–833. [Google Scholar] [CrossRef]
- Anthony, S.J.; Zuchowski, W.; Setzer, W.N. Composition of the Floral Essential Oil of Brugmansia suaveolens. Rec. Nat. Prod. 2009, 3, 76–81. [Google Scholar] [CrossRef]
- Pei, T.-H.; Zhao, Y.-J.; Wang, S.-Y.; Li, X.-F.; Sun, C.-Q.; Shi, S.-S.; Xu, M.-L.; Gao, Y. Preliminary Study on Insecticidal Potential and Chemical Composition of Five Rutaceae Essential Oils against Thrips flavus (Thysanoptera: Thripidae). Molecules 2023, 28, 2998. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, C.; Wan, M.; Liu, Z.; Zheng, L.; Jin, F.; Jin, M.; She, Y.; Wang, J.; Wang, S. Multiresidue Determination of Six Pesticide Adjuvants in Characteristi c Minor Crops Using QuEChERS Method and Gas Chromatography-Mass Spectrometry. Chemistryselect 2019, 4, 66–70. [Google Scholar] [CrossRef]
- Calvet, C.; Pinochet, J.; Camprubí, A.; Estaún, V.; Rodríguez-Kábana, R. Evaluation of Natural Chemical Compounds against Root-Lesion and Root- Knot Nematodes and Side-Effects on the Infectivity of Arbuscular Mycorrhizal Fungi. Eur. J. Plant Pathol. 2001, 107, 601–605. [Google Scholar] [CrossRef]
- Kim, H.-K.; Kim, J.-R.; Ahn, Y.-J. Acaricidal Activity of Cinnamaldehyde and its Congeners against Tyrphagus putrescentiae (Acari: Acaridae). J. Stored Prod. Res. 2004, 40, 55–63. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and Use of Potential Bacterial Organic Antifungal Volatiles in Biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Costa, J.G.; Pires, E.V.; Riffel, A.; Birkett, M.A.; Bleicher, E.; Sant’Ana, A.E.G. Differential Preference of Capsicum spp. Cultivars by Aphis gossypii is Conferred by Variation in Volatile Semiochemistry. Euphytica 2011, 177, 299–307. [Google Scholar] [CrossRef]
- Kirk, W.D.J.; de Kogel, W.J.; Koschier, E.H.; Teulon, D.A.J. Semiochemicals for Thrips and Their Use in Pest Management. Annu. Rev. Entomol. 2021, 66, 101–119. [Google Scholar] [CrossRef]
- Guarino, S.; Peri, E.; Colazza, S.; Luchi, N.; Michelozzi, M.; Loreto, F. Impact of the Invasive Painted Bug Bagrada hilaris on Physiological Traits of Its Host Brassica oleracea Var Botrytis. Arthropod-Plant Interact. 2017, 11, 649–658. [Google Scholar] [CrossRef]
- Qiu, H.; Zhao, D.; Fox, E.G.P.; Ling, S.; Qin, C.; Xu, J. Chemical Cues Used by the Weevil Curculio chinensis in Attacking the Host Oil Plant Camellia oleifera. Diversity 2022, 14, 951. [Google Scholar] [CrossRef]
- Nwanade, C.F.; Wang, M.; Pei, T.; Meng, J.; Yu, Z.; Liu, J. Toxicity and Enzymatic Mechanism of Citrus spp. Essential Oils and Major Constituents on Haemaphysalis longicornis (Acari: Ixodidae) and Non-Target Harmonia axyridis (Coleoptera: Coccinellidae). Pestic. Biochem. Physiol. 2024, 204, 106113. [Google Scholar] [CrossRef]
- Diabate, S.; Deletre, E.; Murungi, L.K.; Fiaboe, K.K.M.; Subramanian, S.; Wesonga, J.; Martin, T. Behavioural Responses of Bean Flower Thrips (Megalurothrips sjostedti) to Vegetative and Floral Volatiles from Different Cowpea Cultivars. Chemoecology 2019, 29, 73–88. [Google Scholar] [CrossRef]
- Zhu, J.; Park, K.-C. Methyl Salicylate, a Soybean Aphid-Induced Plant Volatile Attractive to the Predator Coccinella septempunctata. J. Chem. Ecol. 2005, 31, 1733–1746. [Google Scholar] [CrossRef]
- Song, B.; Liang, Y.; Liu, S.; Zhang, L.; Tang, G.; Ma, T.; Yao, Y. Behavioral Responses of Aphis citricola (Hemiptera: Aphididae) and its Natural Enemy Harmonia axyridis (Coleoptera: Coccinellidae) to Non-Host Plant Volatiles. Fla. Entomol. 2017, 100, 411–421. [Google Scholar] [CrossRef]
- Su, X.; Lu, Y.-P.; Chen, K.-L. Analysis on Volatile Components in Mentha haplocalyx of Special Herbs in Qinghai Province. Asian J. Chem. 2014, 26, 555–558. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Chen, Y.; Li, Z.-J.; Li, X.; Fan, G. Bioactive Properties of the Aromatic Molecules of Spearmint (Mentha spicata L.) Essential Oil: A Review. Food Funct. 2022, 13, 3110–3132. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Leitão, A.J.; Neves, B.M.; Judas, F.; Cavaleiro, C.; Mendes, A.F. Standardised Comparison of Limonene-derived Monoterpenes Identifies Structural Determinants of Anti-inflammatory Activity. Sci. Rep. 2020, 10, 7199. [Google Scholar] [CrossRef]
- Pickett, J.A.; Woodcock, C.M.; Midega, C.A.; Khan, Z.R. Push–pull Farming Systems. Curr. Opin. Biotechnol. 2014, 26, 125–132. [Google Scholar] [CrossRef]
- Zhang, T.; Mei, X.; Zhang, X.; Lu, Y.; Ning, J.; Wu, K. Identification and Field Evaluation of the Sex Pheromone of Apolygusl ucorum (Hemiptera: Miridae) in China. Pest Manag. Sci. 2020, 76, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Liu, C.; Liu, Y.; Mei, X.; Wang, Z.; Zhang, T. Identification and Field Verification of an Aggregation Pheromone from the White-Spotted Flower Chafer, Protaetia brevitarsis Lewis (Coleoptera: Scarabaeidae). Sci. Rep. 2021, 11, 22362. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Han, C.; Tu, X.; McNeill, M.R.; Ban, L. Evaluation of Trap Systems for Monitoring of Odontothrips loti and Frankliniella occidentalis: A Pilot Field Trial. Insects, 2025; submitted. [Google Scholar]
- Guo, Z.-G.; Wang, M.-X.; Cui, L.; Han, B.-Y. Advance in Insect Phototaxis and the Development and Application of Colored Sticky Boards. J. Appl. Ecol. 2019, 30, 3615–3626. [Google Scholar] [CrossRef]
- Friedli, M.; Kirchgessner, N.; Grieder, C.; Liebisch, F.; Mannale, M.; Walter, A. Terrestrial 3D Laser Scanning to Track the Increase in Canopy Height of Both Monocot and Dicot Crop Species under Field Conditions. Plant Methods 2016, 12, 9. [Google Scholar] [CrossRef]
- Teulon, D.A.J.; Butler, R.C.; James, D.E.; Davidson, M.M. Odour-baited Traps Influence Thrips Capture in Proximal Unbaited Traps in the Field. Entomol. Exp. Appl. 2007, 123, 253–262. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Wei, S.; Tang, F.; McNeill, M.R.; Tu, X.; Liu, Y.; Han, C.; Qu, C.; Yin, X.; Ban, L. Identification and Evaluation of Alfalfa Volatiles for Monitoring and Management of Odontothrips loti and Frankliniella occidentalis. Insects 2025, 16, 1207. https://doi.org/10.3390/insects16121207
Luo Y, Wei S, Tang F, McNeill MR, Tu X, Liu Y, Han C, Qu C, Yin X, Ban L. Identification and Evaluation of Alfalfa Volatiles for Monitoring and Management of Odontothrips loti and Frankliniella occidentalis. Insects. 2025; 16(12):1207. https://doi.org/10.3390/insects16121207
Chicago/Turabian StyleLuo, Yingning, Shuhua Wei, Fang Tang, Mark R. McNeill, Xiongbing Tu, Yanqi Liu, Chen Han, Changqing Qu, Xuewei Yin, and Liping Ban. 2025. "Identification and Evaluation of Alfalfa Volatiles for Monitoring and Management of Odontothrips loti and Frankliniella occidentalis" Insects 16, no. 12: 1207. https://doi.org/10.3390/insects16121207
APA StyleLuo, Y., Wei, S., Tang, F., McNeill, M. R., Tu, X., Liu, Y., Han, C., Qu, C., Yin, X., & Ban, L. (2025). Identification and Evaluation of Alfalfa Volatiles for Monitoring and Management of Odontothrips loti and Frankliniella occidentalis. Insects, 16(12), 1207. https://doi.org/10.3390/insects16121207





