Cuticular Pegs near Wing Bases in Aphids of the Subfamily Eriosomatinae Kirkaldy, 1905 s. str. (Insecta, Aphididae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Light Microscopy
2.2. Scanning Electron Microscopy
3. Results
| Species Name | Number of Studied Specimens | Number of Tegulas Available for Study | Number of Pegs on Tegula (±SD) |
|---|---|---|---|
| Tetraneura ulmi, fundatrigenia | 2 | 3 | 17.33 ± 2.62 |
| Tetraneura ulmi, sexupara | 5 | 8 | 16.75 ± 4.18 |
| Tetraneura nigriabdominalis, sexupara | 3 | 5 | 22.60 ± 2.87 |
| Eriosoma ulmi, fundatrigenia | 3 | 5 | 27.40 ± 3.32 |
| Eriosoma ulmi, sexupara | 9 | 9 | 14.11 ± 2.60 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gettrup, E. Sensory Mechanisms in Locomotion: The Campaniform Sensilla of the Insect Wing and their Function during Flight. Cold Spring Harb. Symp. Quant. Biol. 1965, 30, 615–622. [Google Scholar] [CrossRef]
- Pass, G. Maintaining insect wing functionality—A review of the role played by the circulatory and tracheal systems. Arthropod Struct. Dev. 2018, 47, 391–407. [Google Scholar] [CrossRef]
- Snodgrass, R.E. The morphology of insect sense organs and the sensory nervous system. Smithson. Misc. Collect. 1926, 77, 1–80. [Google Scholar]
- McIver, S.B. Structure of cuticular mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Shields, V.D.C. High resolution ultrastructural investigation of insect sensory organs using field emission scanning electron microscopy. In Microscopy: Science, Technology, Applications and Education; Mendez, V.A., Diaz, J., Eds.; Formatex: Badajoz, Spain, 2010; pp. 321–328. [Google Scholar]
- Hartenstein, V. Development of insect sensilla. Comp. Mol. Insect Sci. 2005, 1, 379–419. [Google Scholar]
- Taylor, G.K.; Krapp, H.G. Sensory Systems and Flight Stability: What do Insects Measure and Why? Adv. Insect Physiol. 2007, 34, 231–316. [Google Scholar] [CrossRef]
- Yoshida, A.; Emoto, J. Sensory Scales Along the Wing Margin of Pieris rapae (Lepidoptera: Pieridae). Ann. Entomol. Soc. Am. 2010, 103, 988–992. [Google Scholar] [CrossRef]
- Hartenstein, V.; Posakony, J.W. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 1989, 107, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Ai, H. Sensors and Sensory Processing for Airborne Vibrations in Silk Moths and Honeybees. Sensors 2013, 13, 9344–9363. [Google Scholar] [CrossRef]
- Field, L.H.; Matheson, T. Chordotonal Organs of Insects. Adv. Insect Physiol. 1998, 27, 1–228. [Google Scholar] [CrossRef]
- Yack, J.E. The structure and function of auditory chordotonal organs in insects. Microsc. Res. Tech. 2004, 63, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.F. The organization of the chemosensory system in Drosophila melanogaster: A review. Cell Tissue Res. 1994, 275, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Raad, H.; Ferveur, J.F.; Ledger, N.; Capovilla, M.; Robichon, A. Functional Gustatory Role of Chemoreceptors in Drosophila Wings. Cell Rep. 2016, 15, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, H.; Wasserthal, L.T. Antennal thermoreceptors and wing- thermosensitivity of heliotherm butterflies: Their possible role in thermoregulatory behavior. J. Insect Physiol. 1993, 39, 1007–1019. [Google Scholar] [CrossRef]
- Crampton, G.C. On the misuse of the terms parapteron, hypopteron, tegula, squamula, patagium and scapula. J. N. Y. Entomol. Soc. 1914, 22, 248–261. [Google Scholar]
- Matsuda, R. Morphology and evolution of the insect thorax. Mem. Entomol. Soc. Can. 1970, 102, 5–431. [Google Scholar] [CrossRef]
- Büschges, A.; Ramirez, J.M.; Driesang, R.; Pearson, K.G. Connections of the forewing tegulae in the locust flight system and their modification following partial deafferentation. J. Neurobiol. 1992, 23, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Franielczyk-Pietyra, B.; Bernas, T.; Sas-Nowosielska, H.; Węgierek, P. Is there a relationship between the morphology of the forewing axillary sclerites and the way the wing folds in aphids (Aphidomorpha, Sternorrhyncha, Hemiptera)? Zoomorphology 2018, 137, 105–117. [Google Scholar] [CrossRef]
- Snodgrass, R.E. Principles of Insect Morphology; McGraw-Hill Book Company Inc.: New York, NY, USA, 1935. [Google Scholar]
- Wolf, H. The Locust Tegula: Significance for flight rhythm generation, wing movement control and aerodynamic force production. J. Exp. Biol. 1993, 182, 229–253. [Google Scholar] [CrossRef]
- Kristensen, N.P. Lepidoptera, Moths and Butterflies: Volume 2: Morphology, Physiology, and Development; Walter de Gruyter & Co.: Berlin, Germany, 2012. [Google Scholar]
- Grünert, U.; Gnatzy, W. Campaniform sensilla of Calliphora vicina (Insecta, Diptera). Zoomorphology 1987, 160, 320–328. [Google Scholar] [CrossRef]
- Kutsch, W.; Hanloser, H.; Reinecke, M. Light- and electron-microscopic analysis of a complex sensory organ: The tegula of Locusta migratoria. Cell Tissue Res. 1980, 210, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Knyazeva, N.I. Morphofunctional features of stretch receptors and chordotonal organs in the wing apparatus of the locust Locusta migratoria. Zh. Evol. Biokhim. Fiziol. 1986, 22, 47–54. [Google Scholar]
- Heie, O. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark—I. Fauna Ent. Scand. 1980, 9, 236. [Google Scholar]
- Favret, C. Aphid Species File. 2025. Available online: http://aphid.speciesfile.org/ (accessed on 14 October 2025).
- Franielczyk-Pietyra, B.; Inbar, M.; Hutyra, P.; Depa, Ł. Wings as Part of the Sensory System in the Aphid Subfamily Eriosomatinae s. lat. (Insecta, Hemiptera). Insects 2025, 16, 828. [Google Scholar] [CrossRef]
- Montagano, L.; Favret, C. The Distribution of Campaniform Sensilla on the Appendages of Mindarus Species (Hemiptera: Aphididae). Entomol. News 2016, 126, 196–203. [Google Scholar] [CrossRef]
- Kanturski, M.; Ali Akbar, S.; Favret, C. Morphology and sensilla of the enigmatic Bhutan pine aphid Pseudessigella brachychaeta Hille Ris Lambers (Hemiptera: Aphididae)—A SEM study. Zool. Anz. 2017, 266, 1–13. [Google Scholar] [CrossRef]
- Kanturski, M.; Lee, Y. Miyalachnus—A New Lachninae Aphid Genus from Japan (Insecta, Hemiptera, Aphididae). Insects 2024, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, W.; Depa, Ł.; Halgoš, J.; Matečný, I.; Lukáš, J.; Kanturski, M. Aphids of Slovakia. Distributional Catalogue, Checklist, Keys and List of Host Plants; Comenius University in Bratislava: Bratislava, Slovakia, 2016; p. 346. [Google Scholar]
- Weber, H. Kopf und Thorax von Psylla mali Schmidb. Z. Morph. Ökol. Tiere 1929, 14, 60–165. [Google Scholar] [CrossRef]
- Drohojowska, J. Thorax Morphology and Its Importance in Establishing Relationships Within Psylloidea (Hemiptera, Sternorrhyncha); Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2015; p. 167. [Google Scholar]
- Taszakowski, A.; Jindra, Z.; Wolski, A. Gryllofulvius gibbosus Taszakowski gen. et sp. nov.—A remarkable, flightless and stridulating plant bug (Heteroptera: Miridae: Cylapinae) from Madagascar. Eur. J. Taxon. 2025, 976, 171–181. [Google Scholar] [CrossRef]
- Kanturski, M.; Barjadze, S.; Glumac, A.; Kaszyca-Taszakowska, N. Stridulating Species of Aphids of the Genus Uroleucon (Hemiptera: Aphididae) with Descriptions of a New Species from Iran. Insects 2025, 16, 68. [Google Scholar] [CrossRef]
- Broughton, W.B.; Harris, K.M. First recording of the sound produced by the black citrus aphid, Toxoptera aurantii (Boy.). Bull. Entomol. Res. 1971, 60, 559–563. [Google Scholar] [CrossRef] [PubMed]
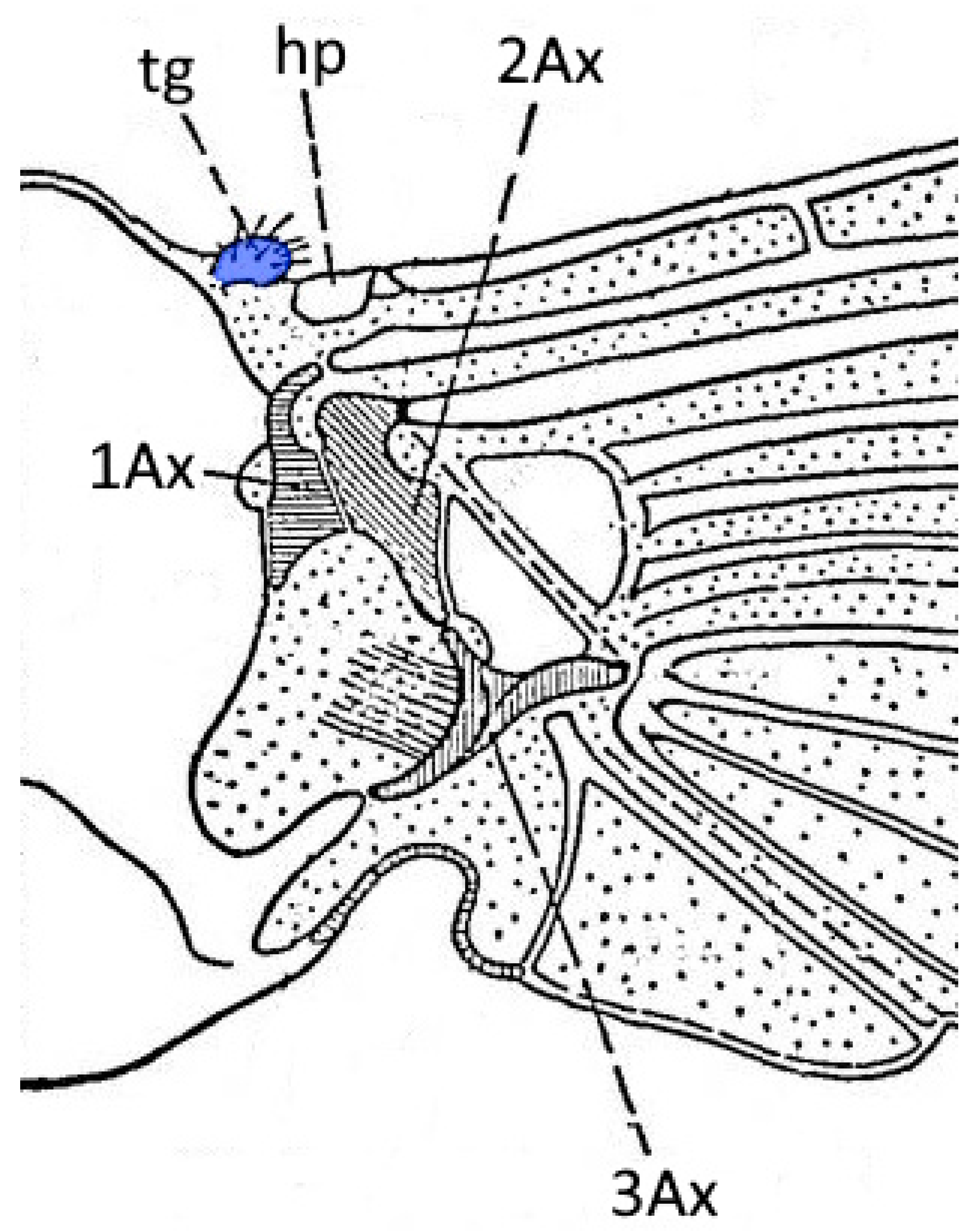
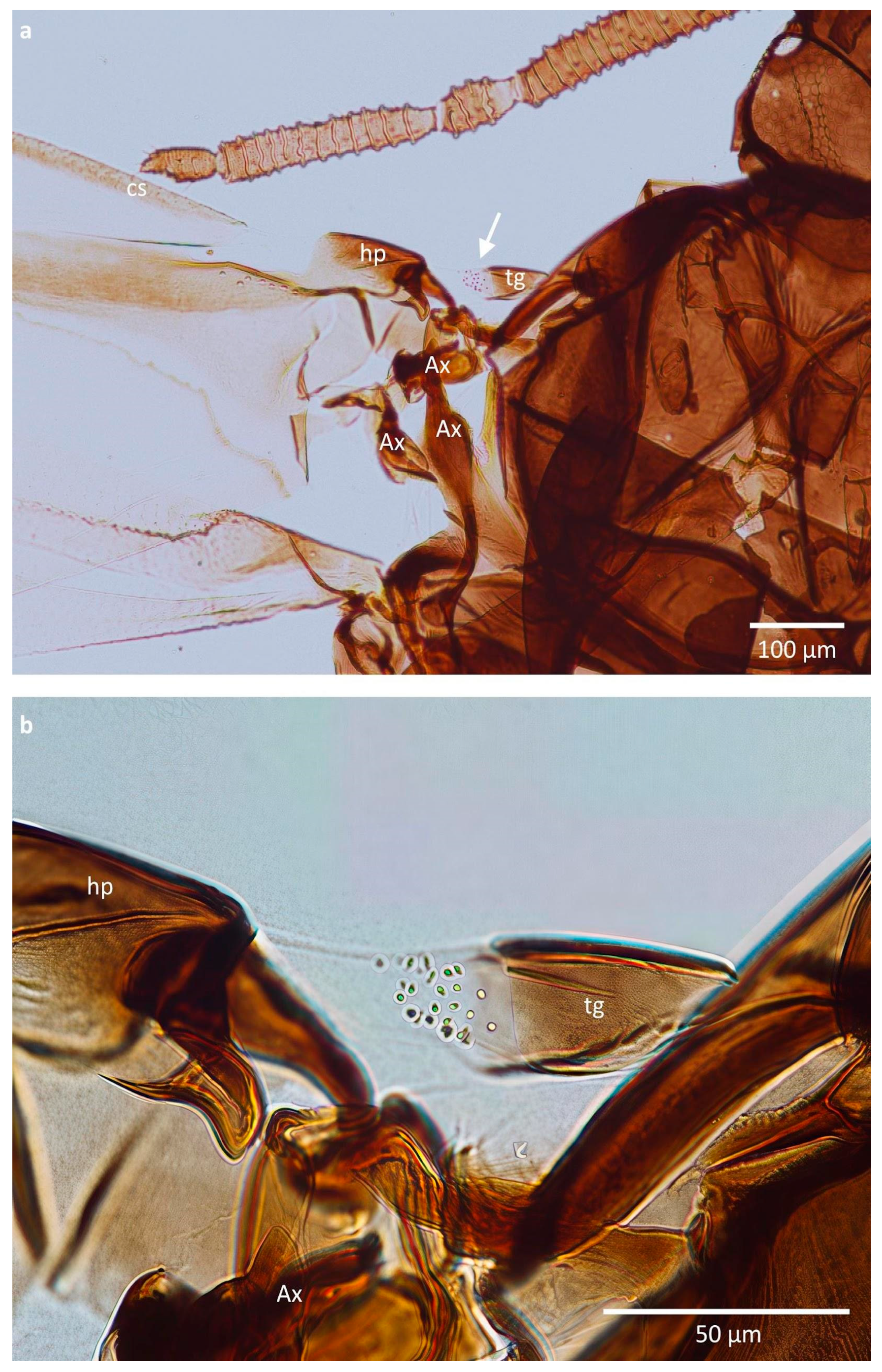
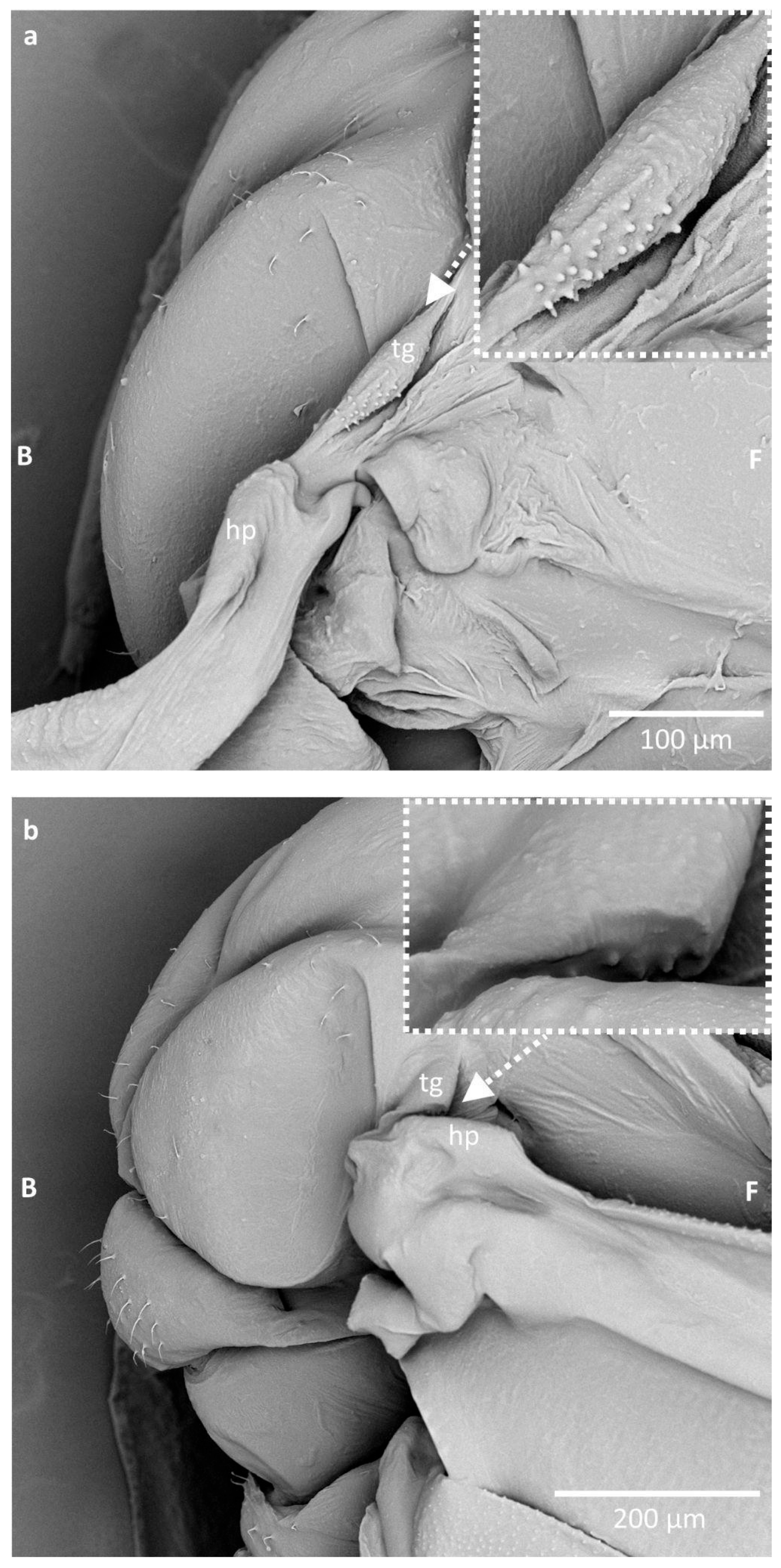

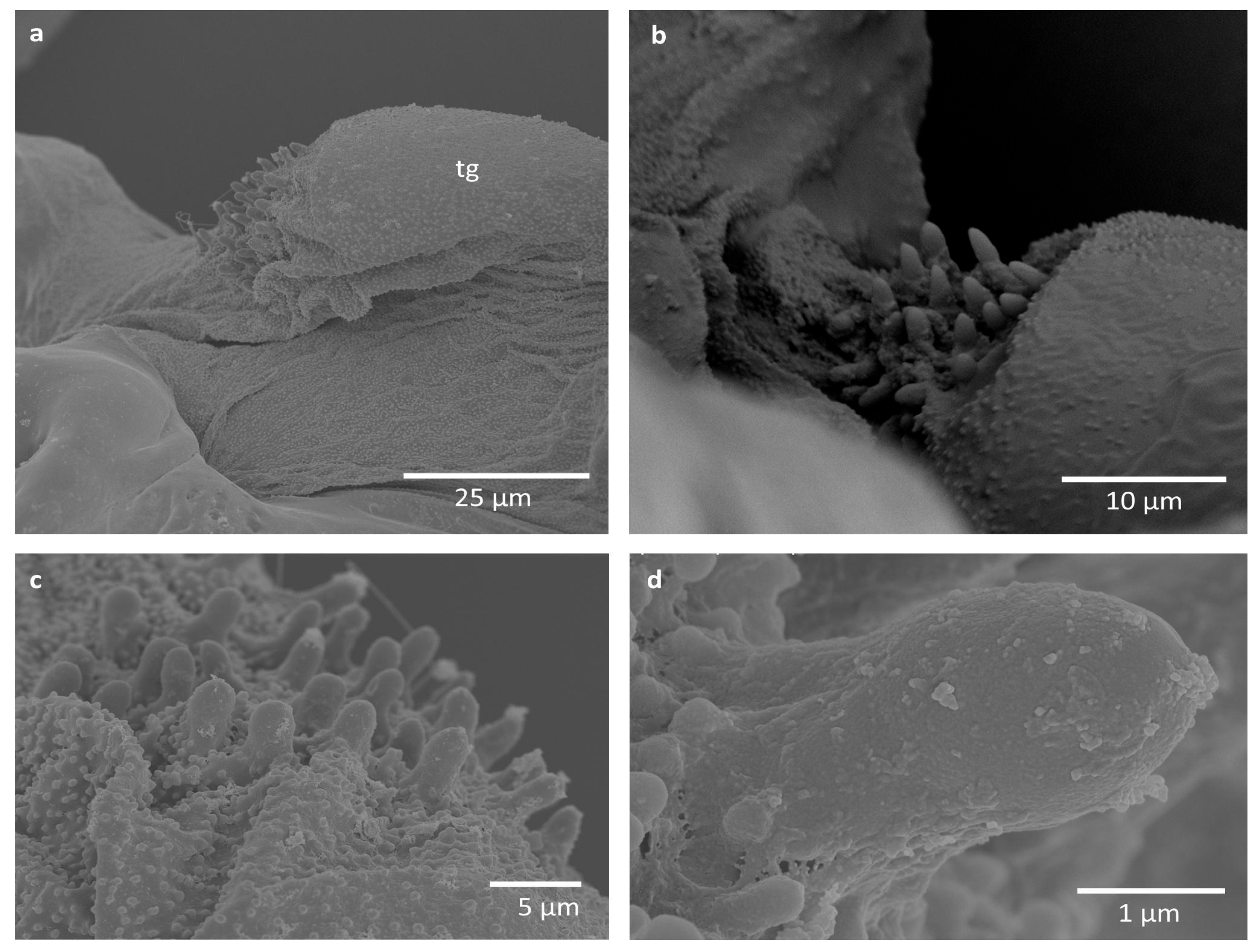

| Species Name | Average Height of Cuticular Pegs (µm) (±SD) | Average Width of Cuticular Pegs (µm) (±SD) | External Appearance |
|---|---|---|---|
| Eriosoma ulmi | 3.83 ± 0.64 | 1.74 ± 0.23 | Covered with cuticular grains |
| Tetraneura ulmi | 2.92 ± 0.36 | 1.26 ± 0.20 | Smooth |
| Tetraneura nigriabdominalis | 3.69 ± 0.38 | 1.39 ± 0.21 | Smooth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowińska, A.; Depa, Ł. Cuticular Pegs near Wing Bases in Aphids of the Subfamily Eriosomatinae Kirkaldy, 1905 s. str. (Insecta, Aphididae). Insects 2025, 16, 1200. https://doi.org/10.3390/insects16121200
Nowińska A, Depa Ł. Cuticular Pegs near Wing Bases in Aphids of the Subfamily Eriosomatinae Kirkaldy, 1905 s. str. (Insecta, Aphididae). Insects. 2025; 16(12):1200. https://doi.org/10.3390/insects16121200
Chicago/Turabian StyleNowińska, Agnieszka, and Łukasz Depa. 2025. "Cuticular Pegs near Wing Bases in Aphids of the Subfamily Eriosomatinae Kirkaldy, 1905 s. str. (Insecta, Aphididae)" Insects 16, no. 12: 1200. https://doi.org/10.3390/insects16121200
APA StyleNowińska, A., & Depa, Ł. (2025). Cuticular Pegs near Wing Bases in Aphids of the Subfamily Eriosomatinae Kirkaldy, 1905 s. str. (Insecta, Aphididae). Insects, 16(12), 1200. https://doi.org/10.3390/insects16121200







