Range Dynamics of Spongy Moth (Lymantria dispar L.) in Northern European Russia over the Past Two Centuries
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Historical Data Analysis
2.2. Current Monitoring of the Northern Range Border of L. dispar in Europe
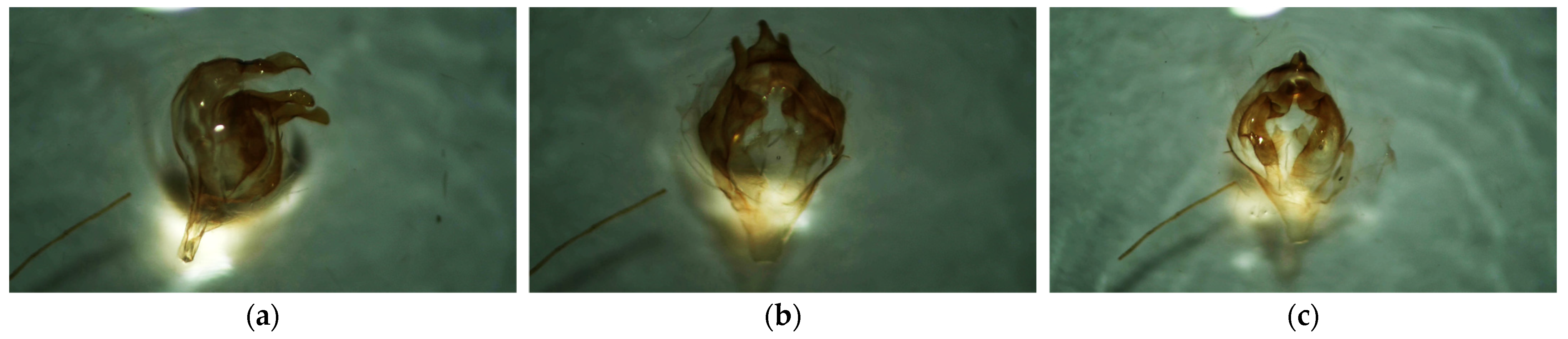
2.3. Identification of Collected Specimens
2.4. Temperature and Precipitation Dynamics in Eurasia over the Last Century
3. Results
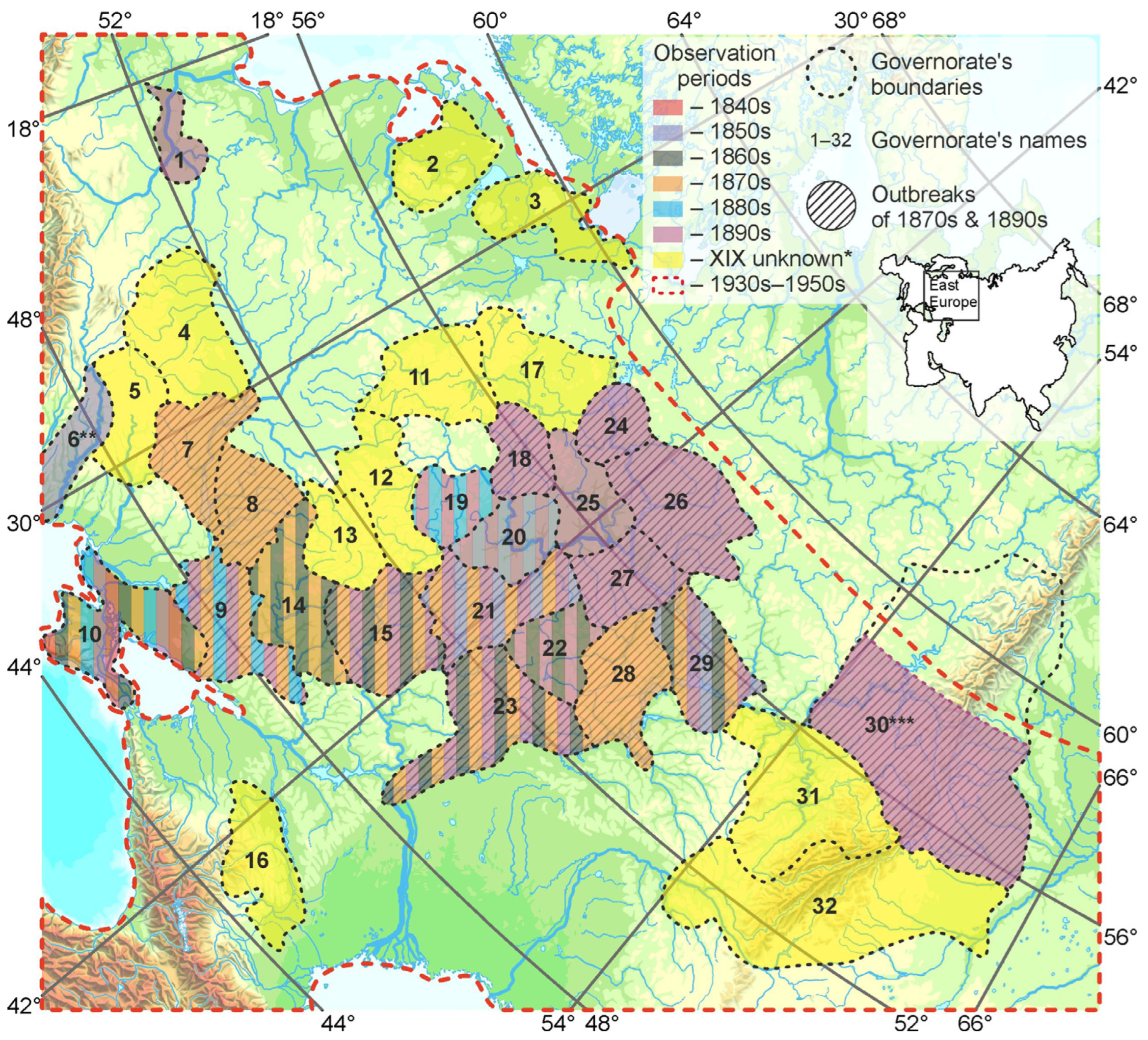
3.1. Historical Records of L. dispar Observations
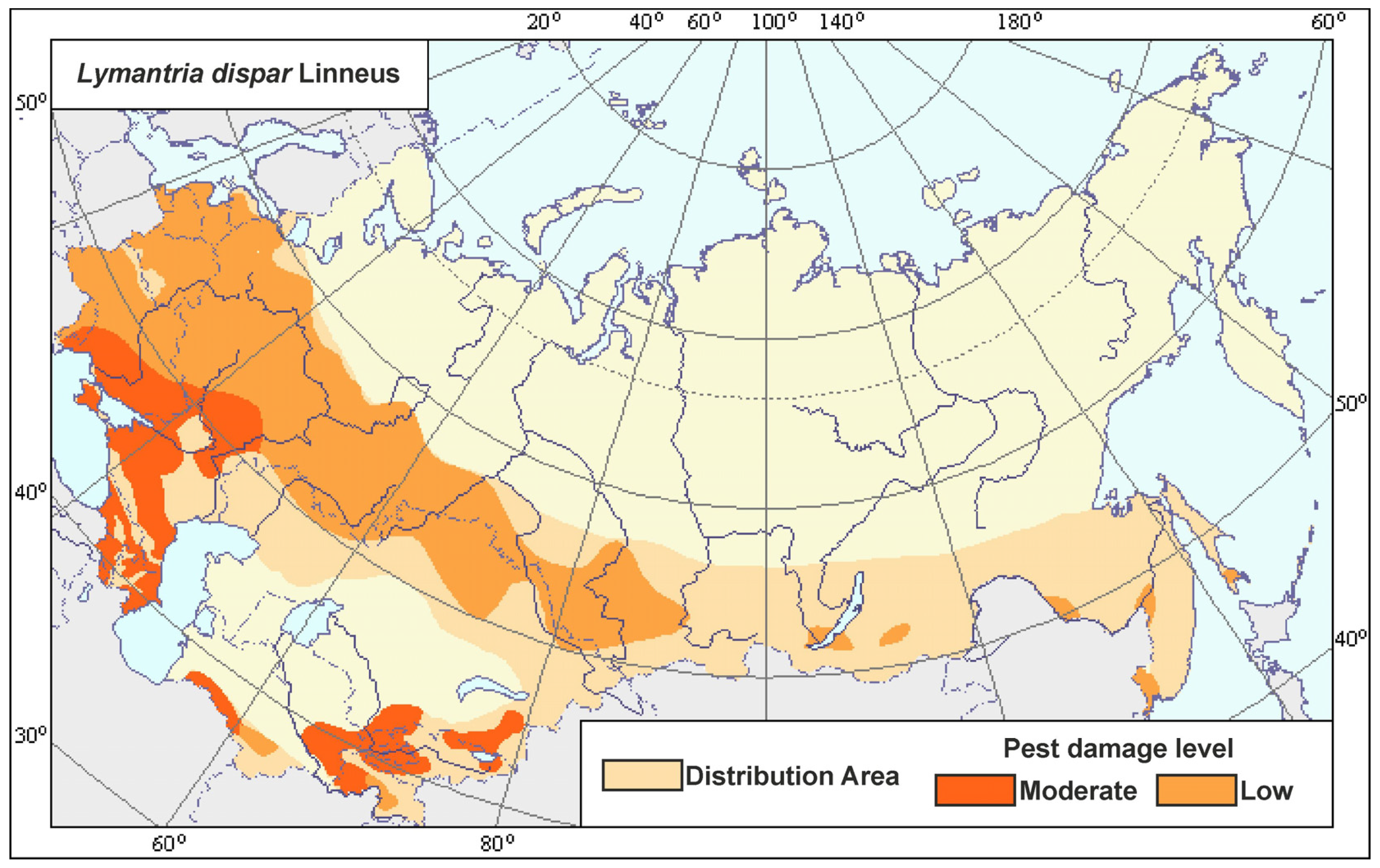
3.2. Current Monitoring of the Northern Range Border of L. dispar in Europe
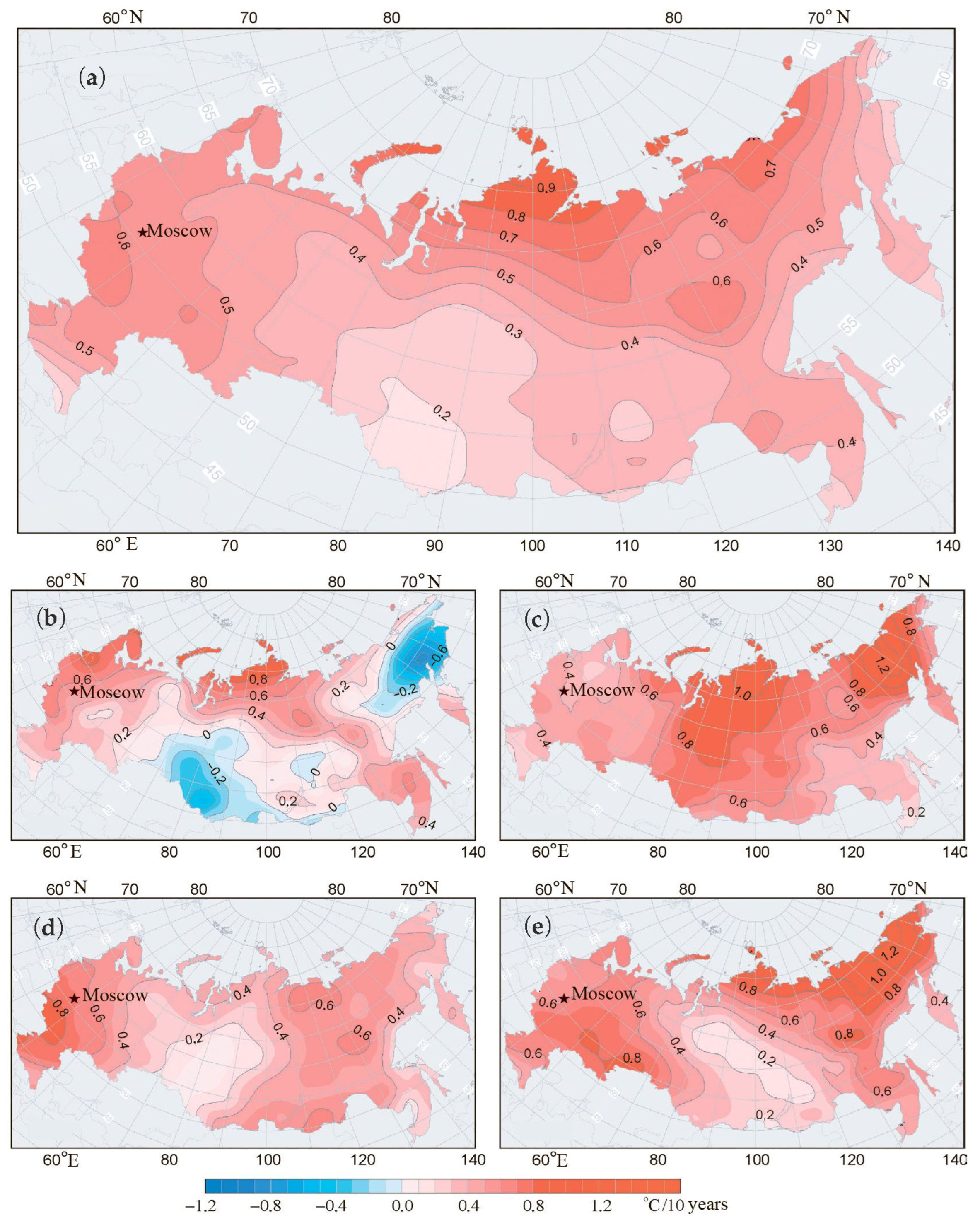
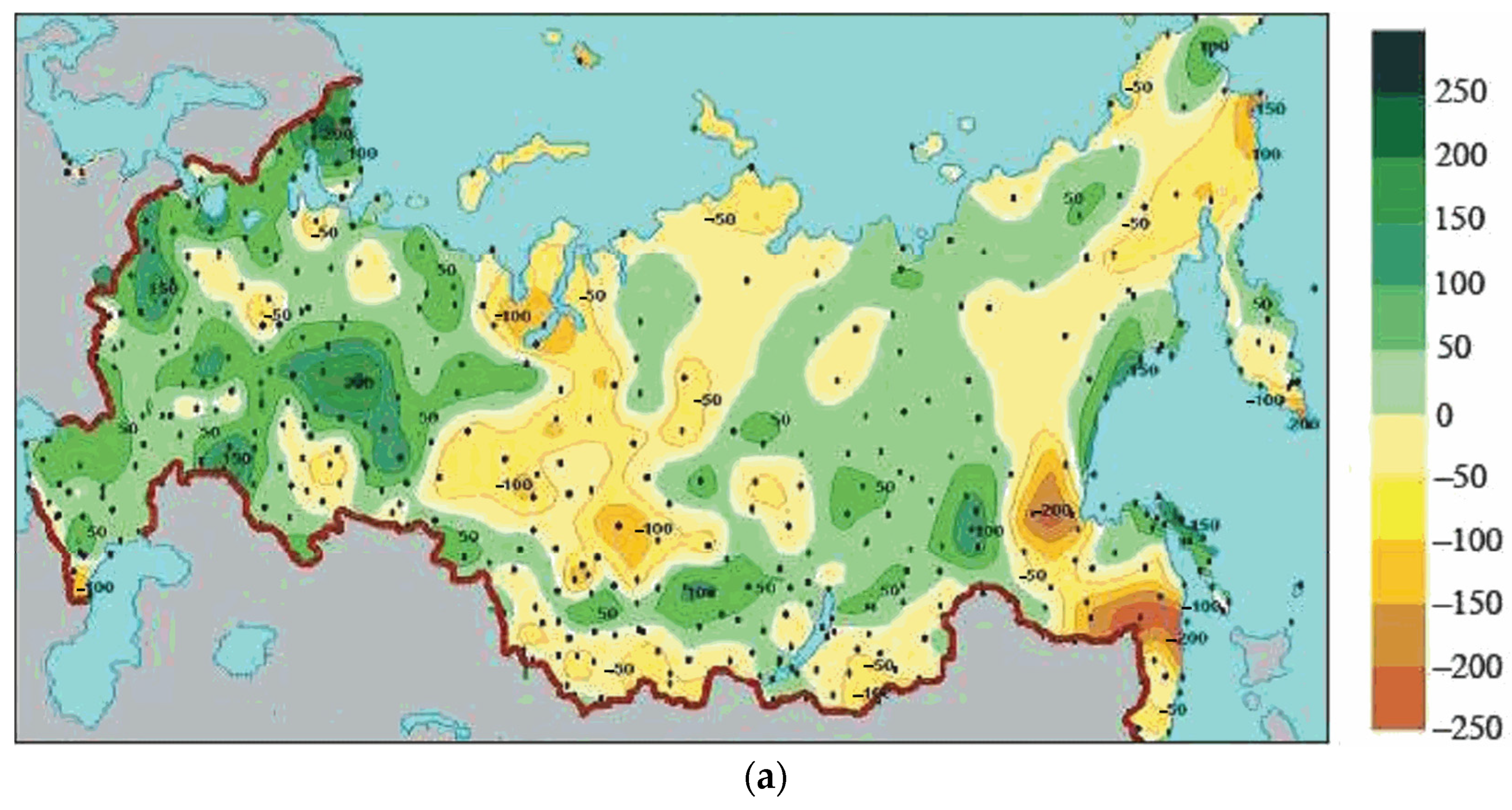
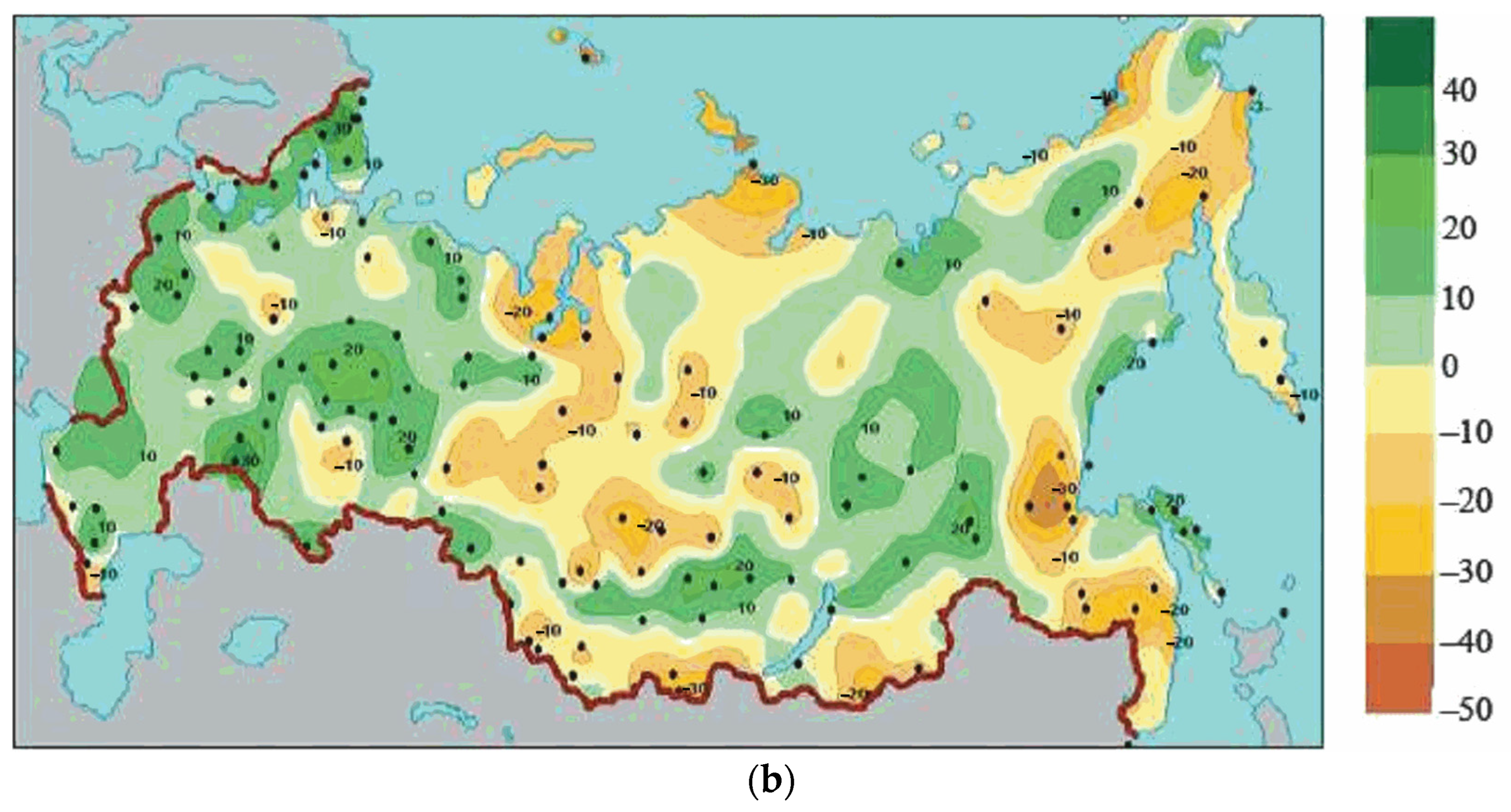
3.3. Comparison of Climate Dynamics in Northern Europe and Northern Asia
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simler-Williamson, A.B.; Rizzo, D.M.; Cobb, R.C. Interacting effects of global change on forest pest and pathogen dynamics. Ann. Rev. Ecol. Evol. Syst. 2019, 50, 381–403. [Google Scholar] [CrossRef]
- Allison, J.D.; Paine, T.D.; Slippers, B.; Wingfield, M.J. (Eds.) Forest Entomology and Pathology; Entomology; Springer: Cham, Switzerland, 2023; Volume 1. [Google Scholar] [CrossRef]
- Guégan, J.-F.; de Thoisy, B.; Gallego, M.G.; Jactel, H. World forests, global change, and emerging pests and pathogens. Curr. Opin. Environ. Sustain. 2023, 61, 101266. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Harding, S.; Bärring, L.; Ravn, H.P. Impact of climate change on the population dynamics of Ips typographus in southern Sweden. Agric. For. Meteorol. 2007, 146, 70–81. [Google Scholar] [CrossRef]
- Öhrn, P. The Spruce Bark Beetle Ips typographus in a Changing Climate—Effects of Weather Conditions on the Biology of Ips typographus. Introductory Research Essay; Swedish Unicersity of Agricultural Sciences: Uppsala, Sweden, 2012; Volume 18, p. 27. [Google Scholar]
- Selikhovkin, A.V.; Gninenko, Y.I. Mass reproduction outbreaks of the phyllophagous insects in forests of the Northwest of the European Part of Russia. For. Sci. 2023, 2, 102–115. (In Russian) [Google Scholar] [CrossRef]
- Mandelshtam, M.Y.; Selikhovkin, A.V. Bark and ambrosia beetles (Coleoptera, Curculionidae: Scolytinae) of Northwest Russia: History of the study, composition and genesis of the fauna. Entomol. Rev. 2020, 100, 800–826. (In Russian) [Google Scholar] [CrossRef]
- Kharuk, V.I.; Im, S.T.; Ranson, K.J.; Yagunov, M.N. Climate-induced northerly expansion of Siberian silkmoth range. Forests 2017, 8, 301. [Google Scholar] [CrossRef]
- Gninenko, Y.I. “Forgotten” conifer- and leaf-eating forest pests. In Readings in Memory of Andrei Ignatievich Ilyinsky; VNIILM: Pushkino, Russia, 2018; pp. 5–24. (In Russian) [Google Scholar]
- Bartalev, S.I.; Shvidenko, A.; Kheld, A. Natural forests disturbances. For. Russ. Clim. Change 2020, 11, 21–25. (In Russian) [Google Scholar]
- Ponomarev, V.I.; Klobukov, G.I.; Napalkova, V.V.; Akhanaev, Y.B.; Pavlushin, S.V.; Yakimova, M.E.; Subbotina, A.O.; Picq, S.; Cusson, M.; Martemyanov, V.V. Phenological features of the spongy moth, Lymantria dispar (L.) (Lepidoptera: Erebidae), in the northernmost portions of its Eurasian range. Insects 2023, 14, 276. [Google Scholar] [CrossRef]
- Turova, G.I. Gypsy Moth (Lymantria dispar L.) in the Forests of the Far East (Distribution, Biology, Economic Significance, Features of Supervision). Ph.D. Thesis, Krasnoyarsk, Russia, 1992. (In Russian). [Google Scholar]
- Nilssen, A.C.; Tenow, O.; Bylund, H. Waves and synchrony in Epirrita autumnata/Operophtera brumata outbreaks. II. Sunspot activity cannot explain cyclic outbreaks. J. Anim. Ecol. 2007, 76, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, J.U.; Hagen, S.B.; Ims, R.A.; Yoccoz, N.G. Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: Evidence of a recent outbreak range expansion. J. Anim. Ecol. 2008, 77, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.C.; Havill, N.P.; Griffin, B.P.; Jepsen, J.U.; Hagen, S.B.; Klemola, T.; Barrio, I.C.; Kjeldgaard, S.A.; Høye, T.T.; Murlis, J.; et al. Northern Fennoscandia via the British Isles: Evidence for a novel post-glacial recolonization route by winter moth (Operophtera brumata). Front. Biogeogr. 2021, 13, e49581. [Google Scholar] [CrossRef]
- Camarero, J.J.; Tardif, J.; Gazol, A.; Conciatori, F. Pine processionary moth outbreaks cause longer growth legacies than drought and are linked to the North Atlantic Oscillation. Sci. Total Environ. 2022, 819, 153041. [Google Scholar] [CrossRef]
- Kholodkovsky, N.A. Course of Theoretical and Applied Entomology, 4th ed.; Gosselkolkooplitizdat: Moscow, Russia; Leningrad, Russia, 1931. (In Russian) [Google Scholar]
- Rimskiy-Korsakov, M.N.; Gusev, V.I.; Poluboyarinov, I.I.; Shiperovich, V.Y.; Yatsentkovskiy, A.V. Forest Entomology, 3rd ed.; Goslesbumizdat: Moscow-Leningrad, Russia, 1949. (In Russian) [Google Scholar]
- Coulson, R.N.; Witter, J.A. Forest Entomology: Ecology and Management; Wiley & Sons Ltd.: Hoboken, NJ, USA, 1984; ISBN 978-0-471-02573-3. [Google Scholar]
- Maslov, A.D.; Vedernikov, N.M.; Andreeva, G.I. Forest Protection from Pests and Diseases, 2nd ed.; Agropromiztat: Moscow, Russia, 1988. (In Russian) [Google Scholar]
- Liebhold, A.M.; Halverson, J.A.; Elmes, G.A. Gypsy moth invasion in North America: A quantitative analysis. J. Biogeogr. 1992, 19, 513–520. [Google Scholar] [CrossRef]
- Kulagin, N.M. Gypsy Moth: An Outline of Life Pattern and Important Control Measures, 2nd ed.; Typo-Lithography of the Governorate Zemstvo Council: Vladimir, Russia, 1898. (In Russian) [Google Scholar]
- Ponomarev, V.I.; Klobukov, G.I.; Napalkova, V.V.; Tyurin, M.V.; Martemyanov, V.V. Influence of biotic and abiotic factors on the duration of development of the spongy moth Lymantria dispar (L.) (Lepidoptera: Erebidae) in the West Siberian population of different latitudinal origin. Contemp. Probl. Ecol. 2023, 16, 166–172. [Google Scholar] [CrossRef]
- Kulagin, N.M. Entomology. Harmful Insects and Measures to Combat Them; Richter: Kharkiv, Ukraine, 1906. (In Russian) [Google Scholar]
- Tindeman, G. Notes on some harmful insects in the forests of Kazan governorate. Russ. For. J. 1877, 7, 24–39. (In Russian) [Google Scholar]
- Romanyuk, I. On the appearance of the gypsy moth caterpillar in the forests of Volsky district, Saratov governorate, in 1978. Russ. For. J. 1879, 9, 63. [Google Scholar]
- Silantyev, S.S. Performance of the weevil Pissodes notatus L. and the gypsy moth Ocneria dispar L. in Zasurk Bungalow Penza district. Russ. For. J. 1892, 25, 148–149. (In Russian) [Google Scholar]
- Shugurov, A.M. Studies on the ecology of the gypsy moth (Porthetria dispar L.) in Russian forests. Russ. For. J. 1907, 8, 1205–1221. (In Russian) [Google Scholar]
- Sergeev, M.I. On the development of the gypsy moth and its parasites. For. Bull. 1910, 41, 448–452. (In Russian) [Google Scholar]
- Parkhomenko, V.Y. The Gypsy Moth (Porthetria dispar L.) in Forests of the Crimea; VUAN: Kiev, Ukraine, 1935. (In Ukrainian) [Google Scholar]
- Gypsy moth near Moscow. Russ. For. J. 1879, 26, 974–975.
- Sobolev, A.N. History of reproduction of gypsy moth in Tula governorate in 1892–96. Russ. For. J. 1879, 27, 194–215. (In Russian) [Google Scholar]
- Averkiev, I.S. The Study of the gypsy moth in the forests of Central Volga provinces. Lesn. Khozyaistvo 1939, 11, 50–52. (In Russian) [Google Scholar]
- Danilov, M.D. Composition of vegetation and root systems of plants in burnt areas and clearings in pine plantations of the Moldavian ASSR. Ed. Vol. PLTI 1940, 2, 103–109. (In Russian) [Google Scholar]
- Ilyinsky, A.I. Gypsy Moth and Measures to Control It; Goslesbumizdat: Moscow, Russia; Saint Petersburg, Russia, 1959. (In Russian) [Google Scholar]
- Matvievskiy, A.S. Evaluation of Accuracy of the Methods of Sampling Gypsy Moth Ocneria dispar L. Egg Masses and Their Improvement. Ph.D. Thesis, Leningrad, Russia, 1954. (In Russian). [Google Scholar]
- Chugunin, Y.V. Gypsy Moth; Gosselhozizdat: Moscow, Russia, 1958. (In Russian) [Google Scholar]
- Kellus, O.G. Geographic occurrence of the gypsy moth and its outbreak areas in the USSR. Pl. Prot. Bul. 1941, 1, 45–50. (In Russian) [Google Scholar]
- Bleker, H.F. New data on the fauna of Lepidoptera in the Petrograd governorate. Proc. Russ. Entomol. Soc. 1897, 30, 464–480. (In Russian) [Google Scholar]
- Yakobson, G.G. Report on entomological work in the summer of 1918 in the southwestern corner of the Leningrad governorate. Yearb. Zool. Mus. 1928, 28, 572–574. (In Russian) [Google Scholar]
- Unitrap. Available online: https://internationalpheromones.com/product/unitrap (accessed on 5 April 2025).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Butterflies—Pests of Agriculture and Forestry of the Far East: A Determinant; Far East Branch of the Academy of Sciences of the Soviet Union: Vladivostok, Russia, 1988. (In Russian)
- Lee, K.S.; Kang, T.H.; Jeong, J.W.; Ryu, D.P.; Lee, H.S. Taxonomic review of the genus Lymantria (Lepidoptera: Erebidae: Lymantriinae) in Korea. Entomol. Res. 2015, 45, 225–234. [Google Scholar] [CrossRef]
- Alekseev, G.V.; Ananicheva, M.D.; Anisimov, O.A.; Ashik, I.M.; Bardin, M.Y.; Bogdanova, E.G.; Bulygina, O.N.; Georgievsky, V.Y.; Gruza, G.V.; Danilov, A.I.; et al. Second Roshydromet Assessment Report on Climate Change and Its Consequences in Russian Federation; Kattsov, V.M., Semenov, S.M., Eds.; Roshydromet: Moscow, Russia, 2014. [Google Scholar]
- Lyamtsev, N.I. Gypsy moth outbreaks in European Russia. For. Bul. 2014, 6, 78–85. (In Russian) [Google Scholar]
- Grichanov, I.Y.; Ovsyannikova, E.I. Pests of Agricultural Crops. Ocneria dispar (L.)—Gypsy Moth or Spongy Moth. Agroecological Atlas of Russia and Adjacent Countries: Economically Significant Plants, Pests and Weeds. Available online: https://agroatlas.ru/ru/content/pests/Ocneria_dispar/map (accessed on 5 April 2025).
- Matov, A.Y.; Mironov, V.G. To the fauna of Noctuoidea (Lepidoptera) of Novgorod Province. Eversmannia 2016, 47–48, 81–95. (In Russian) [Google Scholar]
- Selikhovkin, A.V. Quantitative assessment of the effects of dendrophagous insects on forest health. Izv. St. Peterbg. Lesoteh. Akad. 2009, 187, 285–296. (In Russian) [Google Scholar]
- Selikhovkin, A.V.; Baryshnikova, S.V.; Denisova, N.V.; Timofeeva, Y.A. Species composition and population dynamics of dominant dendrophagous moths (Lepidoptera) in St. Petersburg and its environs. Entomol. Rev. 2018, 98, 963–978. (In Russian) [Google Scholar] [CrossRef]
- Fedorova, V.G. Insects of Novgorod Region, 2nd ed.; NovSU: Veliky Novgorod, Russia, 2006. (In Russian) [Google Scholar]
- Derzhavets, Y.A.; Ivanov, A.I.; Mironov, V.G.; Mischenko, O.A.; Prasolov, V.N.; Sinev, S.Y. Fauna of Lepidoptera of the USSR. List of Macrolepidoptera of the Leningrad Region. Ed. Vol. SUES 1986, 67, 186–270. (In Russian) [Google Scholar]
- Balashova, N.B.; Zavarzin, A.A. (Eds.) Biodiversity of the Leningrad Oblast; Saint-Petersburg University: Saint-Petersburg, Russia, 1999. (In Russian) [Google Scholar]
- Pavlovsky, E.N. (Ed.) Forest Pest’s Handbook; The Academy of Science of the Soviet Union: Moscow, Russia, 1955. (In Russian) [Google Scholar]
- Sinev, S.Y. (Ed.) Catalogue of the Lepidoptera of Russia, 2nd ed.; Zoological Institute RAS: St. Petersburg, Russia, 2019; pp. 294–295. (In Russian) [Google Scholar]
- Tatarinov, A.G.; Sedykh, K.F.; Dolgin, M.M. Fauna of the European North-East of Russia. Heterocera; Nauka: Saint Petersburg, Russia, 2003; Volume 7. (In Russian) [Google Scholar]
- GBIF Global Biodiversity Information Facility. Available online: https://www.gbif.org/species/1820406 (accessed on 5 April 2025).
- Grayson, K.L.; Johnson, D.M. Novel insights on population and range edge dynamics using an unparalleled spatiotemporal record of species invasion. J. Anim. Ecol. 2018, 87, 581–593. [Google Scholar] [CrossRef]
- Dyk, B.D.; Denisova, N.V.; Baryshnikova, S.V.; Selikhovkin, A.V. Actual changes in the species composition and the population density of phyllophagous insects in St. Petersburg. Izv. St. Peterbg. Lesoteh. Akad. 2020, 230, 73–99. (In Russian) [Google Scholar] [CrossRef]
- Lvovsky, A.L. Butterflies and moths (Insecta, Lepidoptera) within the borders of St. Petersburg. Izv. Kharkov Entomol. Soc. 1994, 2, 4–48. (In Russian) [Google Scholar]
- Régnière, J.; Nealis, V.; Porter, K. Climate suitability and management of the gypsy moth invasion into Canada. Biol. Invasions 2009, 11, 135–148. [Google Scholar] [CrossRef]
- Mikkola, K. The migratory habit of Lymantria dispar (Lep., Lymantriidae) adults of continental Eurasia in the light of a flight to Finland. Acta Entomol. Fenn. 1971, 28, 107–120. [Google Scholar]
- Mikkola, K. Direction of insect migrations in relation to the wind. In Insect Flight: Dispersal and Migration; Danthanarayana, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 128–144. [Google Scholar]
- Vorontsov, A.I. Results of the study of the gypsy moth. In Insects–Pests of the Forests of Bashkiria; Garifullin, F.S., Ed.; Biology Institute of the Academy of Science of the Soviet Union: Ufa, Russia, 1977; pp. 3–25. (In Russian) [Google Scholar]
- Pogue, M.G.; Schaefer, P.W. A Review of Selected Species of Lymantria Hübner [1819] (Lepidoptera: Noctuidae: Lymantriinae) from Subtropical and Temperate Regions of Asia, Including the Descriptions of Three New Species, Some Potentially Invasive to North America; FHTET: Washington, DC, USA, 2007.
- Iwaizumi, R.; Arakawa, K.; Koshio, C. Nocturnal flight activities of the female Asian gypsy moth, Lymantria dispar (Linnaeus) (Lepidoptera: Lymantriidae). Appl. Entomol. Zool. 2010, 45, 121–128. [Google Scholar] [CrossRef]
- Walter, J.A.; Liebhold, A.M. Recent developments in Lymantria dispar spread. In Slow the Spread: A 20-Year Reflection on the National Lymantria dispar Integrated Pest Management Program; Coleman, T.W., Liebhold, A.M., Eds.; Department of Agriculture, Forest Service, Northern Research Station: Madison, WI, USA, 2023; Chapter 4; pp. 70–85. [Google Scholar] [CrossRef]
- Martemyanov, V.; Bykov, R.; Demenkova, M.; Gninenko, Y.; Romancev, S.; Bolonin, I.; Mazunin, I.; Belousova, I.; Akhanaev, Y.; Pavlushin, S.; et al. Genetic evidence of broad spreading of Lymantria dispar in the West Siberian Plain. PLoS ONE 2019, 14, e0220954. [Google Scholar] [CrossRef]
- Madrid, F.J.; Stewart, R.K. Ecological significance of cold hardiness and winter mortality of eggs of the gypsy moth Lymantria dispar L., in Quebec. Environ. Entomol. 1981, 10, 586–589. [Google Scholar] [CrossRef]
- Fält-Nardmann, J.J.J.; Ruohomäki, K.; Tikkanen, O.-P.; Neuvonen, S. Cold hardiness of Lymantria monacha and L. dispar (Lepidoptera: Erebidae) eggs to extreme winter temperatures: Implications for predicting climate change impacts. Ecol. Entomol. 2018, 43, 422–430. [Google Scholar] [CrossRef]
- Ananko, G.G.; Kolosov, A.V. Asian gypsy moth (Lymantria dispar L.) populations: Tolerance of eggs to extreme winter temperatures. J. Therm. Biol. 2021, 102, 103123. [Google Scholar] [CrossRef] [PubMed]
- Ananko, G.G.; Kolosov, A.V.; Martemyanov, V.V. Rock microhabitats provide suitable thermal conditions for overwintering insects: A case study of the spongy moth (Lymantria dispar L.) population in the Altai mountains. Insects 2022, 13, 712. [Google Scholar] [CrossRef]
- Keena, M.A.; Richards, J.Y. Comparison of survival and development of gypsy moth Lymantria dispar L. (Lepidoptera: Erebidae) populations from different geographic areas on North American conifers. Insects 2020, 11, 260. [Google Scholar] [CrossRef]
- Soukhovolsky, V.G.; Ivanova, Y.D.; Kovalev, A.V. The development of outbreaks of forest insects on different spatial scale. Lesovedenie 2023, 2, 174–189. (In Russian) [Google Scholar] [CrossRef]
- Subbotina, A.; Chernyak, E.; Soukhovolsky, V.; Morozov, S.; Martemyanov, V. Latitudinal variation in constitutive chemical defense compounds in two host plants of Lymantria dispar (Lymantriidae): Betula pendula (Betulaceae) and Larix sibirica (Pinaceae). For. Ecol. Manag. 2025, 590, 122811. [Google Scholar] [CrossRef]
- Brodbeck, B.; Strong, D. Amino acid nutrition of herbivorous insects and stress to host plants. In Insect Outbreaks: Ecological and Evolutionary Perspectives; Barbosa, P., Schultz, J., Eds.; Academic Press: New York, NY, USA, 1987; pp. 347–364. [Google Scholar]
- White, T.C.R. An index to measure weather-induced stress of trees associated with outbreaks of psyllids in Australia. Ecology 1969, 50, 905–909. [Google Scholar] [CrossRef]
- Gely, C.; Laurance, S.G.; Stork, N.E. How do herbivorous insects respond to drought stress in trees? Biol. Rev. 2020, 95, 434–448. [Google Scholar] [CrossRef]
- Pilotto, F.; Kühn, I.; Adrian, R.; Alber, R.; Alignier, A.; Andrews, C.; Bäck, J.; Barbaro, L.; Beaumont, D.; Beenaerts, N.; et al. Meta-analysis of multidecadal biodiversity trends in Europe. Nat. Commun. 2020, 11, 3486. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Heinen, R.; Gols, R.; Thakur, M.P. Climate change-mediated temperature extremes and insects: From outbreaks to breakdowns. Glob. Change Biol. 2020, 26, 6685–6701. [Google Scholar] [CrossRef] [PubMed]
- Fält-Nardmann, J.J.J.; Tikkanen, O.P.; Ruohomäki, K.; Otto, L.F.; Leinonen, R.; Pöyry, J.; Saikkonen, K.; Neuvonen, S. The recent northward expansion of Lymantria monacha in relation to realised changes in temperatures of different seasons. For. Ecol. Manag. 2018, 427, 96–105. [Google Scholar] [CrossRef]
- Picq, S.; Wu, Y.; Martemyanov, V.V.; Pouliot, E.; Pfister, S.E.; Hamelin, R.; Cusson, M. Range-wide population genomics of the spongy moth, Lymantria dispar (Erebidae): Implications for biosurveillance, subspecies classification and phylogeography of a destructive moth. Evol. Appl. 2023, 16, 638–656. [Google Scholar] [CrossRef]
- Titkina, S.N.; Popov, I.O.; Semenov, S.M.; Yasjukevich, V.V. Changes in distribution in Russia and neighboring countries of gipsy moth and nun moth (Lymantria dispar L. and Lymantria monacha L., Lymantriidae, Lepidoptera) due to observed climate change and projected ones for XXI century. Probl. Ecol. Monit. Model. Ecosyst. 2013, 25, 275–394. (In Russian) [Google Scholar]
- Selikhovkin, A.V.; Merkuriev, S.A.; Gninenko, Y.I. Outbreaks of Pine Sawflies in the Northwest of the European part of Russia and climate warming. Biol. Bull. 2025, 52, 100. [Google Scholar] [CrossRef]

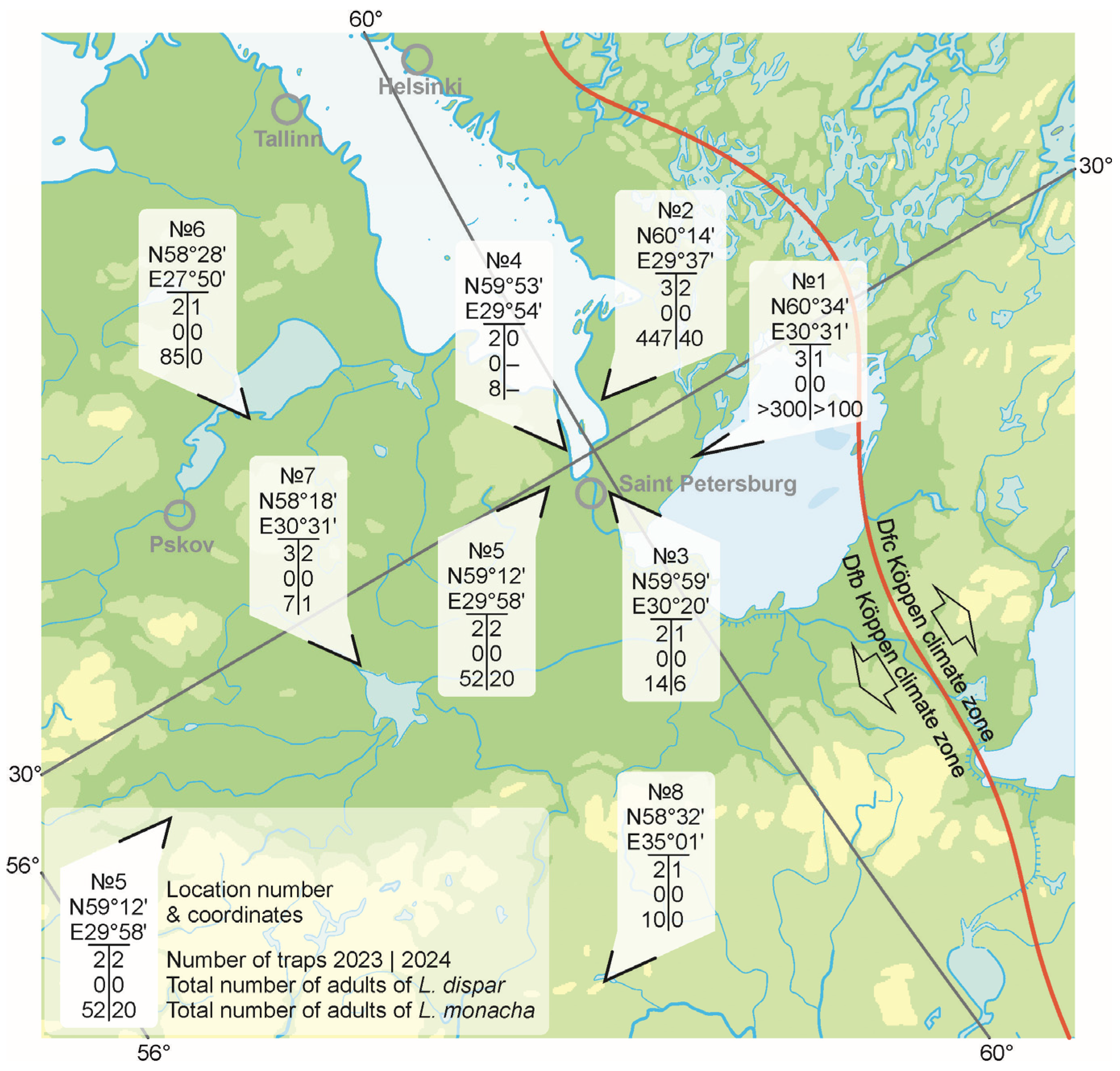
| Locality | No * | Coordinates | Locality Description |
|---|---|---|---|
| Leningrad oblast | 1 | 60°34′51.9″ N 30°31′38.0″ E | Pine–bilberry/lichen forest, 110–140 years, medium density; mixed plantation adjacent to the site: birch, willow, alder |
| 2 | 60°14′28.3″ N 29°37′47.1″ E | Garden: apple, plum, currant | |
| 3 | 59°59′39.1″ N 30°20′16.8″ E | Arboretum: mixed deciduous plantations, including fruit trees, and various tree species | |
| 4 | 59°53′04.8″ N 29°54′29.0″ E | Old apple orchard | |
| 5 | 59°12′25.4″ N 29°58′47.1″ E | Mixed forest: birch, alder, spruce/Garden: apple trees, plums, cherry | |
| Pskov oblast | 6 | 58°28′48.8″ N 27°50′17.2″ E | Pine forest, 70–100 years/Garden: apple trees, birches |
| 7 | 58°18′08.3″ N 30°31′16.7″ E | Garden: apple trees, plums, cherries | |
| Novgorod oblast | 8 | 58°32′04.4″ N 35°01′10.0″ E | Mixed forest: alder, birch, pine |
| Climate Parameter Change | Part of Russian Federation on Reference Latitude Line 60° N | ||
|---|---|---|---|
| European | Asian | Asian/European | |
| Temperature, °C per decade | 0.4 | 0.8 | 2 |
| Precipitation, mm | +0–50 | −50–100 | 2 |
| Precipitation, % | +0–10 | −10–20 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selikhovkin, A.; Mamaev, N.; Sherbakova, L.; Sinev, S.; Broshkov, A.; Alekseev, A.; Martemyanov, V. Range Dynamics of Spongy Moth (Lymantria dispar L.) in Northern European Russia over the Past Two Centuries. Insects 2025, 16, 1189. https://doi.org/10.3390/insects16121189
Selikhovkin A, Mamaev N, Sherbakova L, Sinev S, Broshkov A, Alekseev A, Martemyanov V. Range Dynamics of Spongy Moth (Lymantria dispar L.) in Northern European Russia over the Past Two Centuries. Insects. 2025; 16(12):1189. https://doi.org/10.3390/insects16121189
Chicago/Turabian StyleSelikhovkin, Andrey, Nikita Mamaev, Ludmila Sherbakova, Sergey Sinev, Andrey Broshkov, Aleksandr Alekseev, and Vyacheslav Martemyanov. 2025. "Range Dynamics of Spongy Moth (Lymantria dispar L.) in Northern European Russia over the Past Two Centuries" Insects 16, no. 12: 1189. https://doi.org/10.3390/insects16121189
APA StyleSelikhovkin, A., Mamaev, N., Sherbakova, L., Sinev, S., Broshkov, A., Alekseev, A., & Martemyanov, V. (2025). Range Dynamics of Spongy Moth (Lymantria dispar L.) in Northern European Russia over the Past Two Centuries. Insects, 16(12), 1189. https://doi.org/10.3390/insects16121189







