Amazonian Discovery Sheds Light on the Evolution of Caenocentron Schmid, 1982 (Trichoptera: Xiphocentronidae): Phylogenetic Placement and Description of a New Species †
Simple Summary
Abstract
1. Introduction
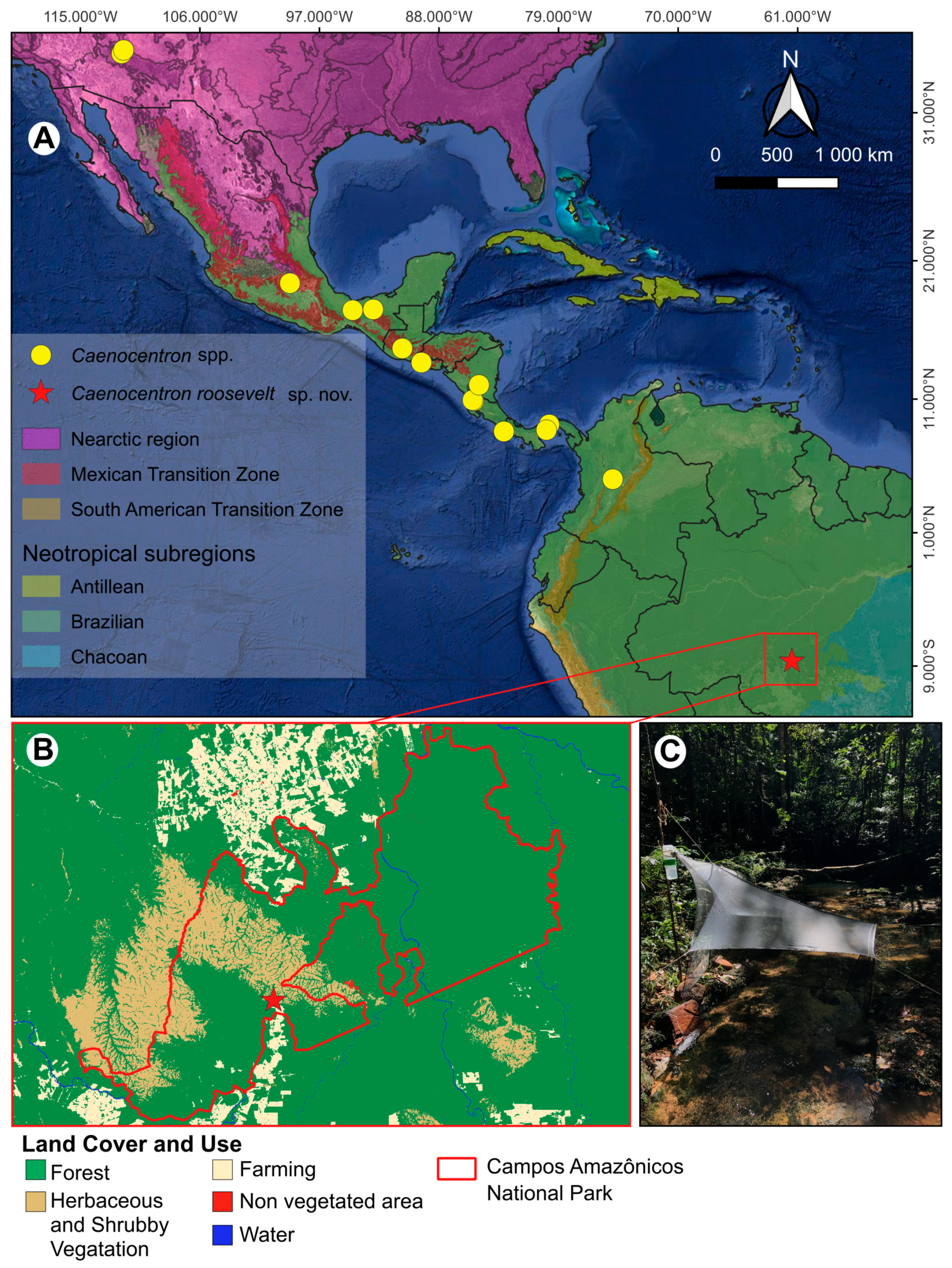
2. Materials and Methods
2.1. Study Area, Specimen Collection, Preparation, and Observation
2.2. Illustrations and Map
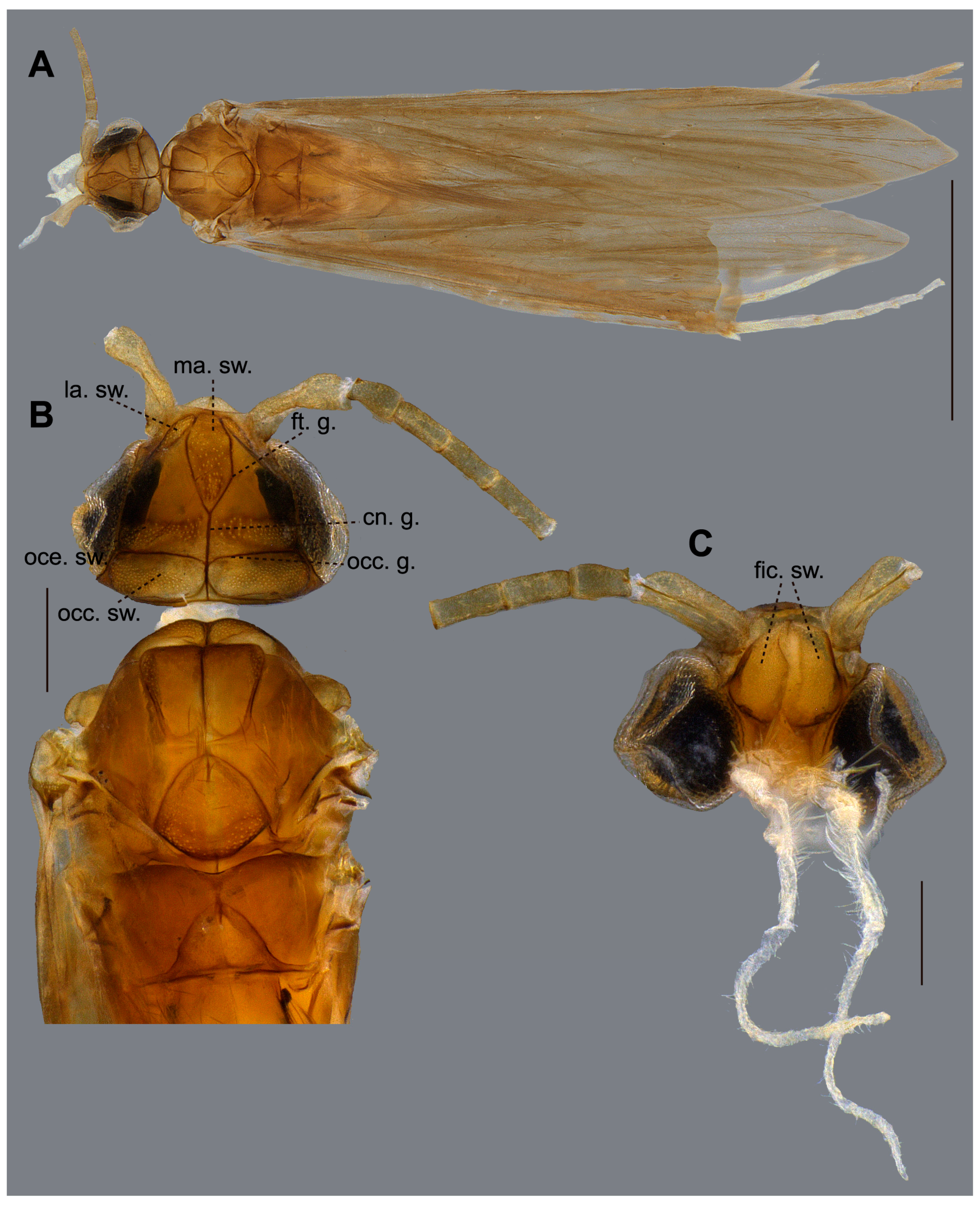
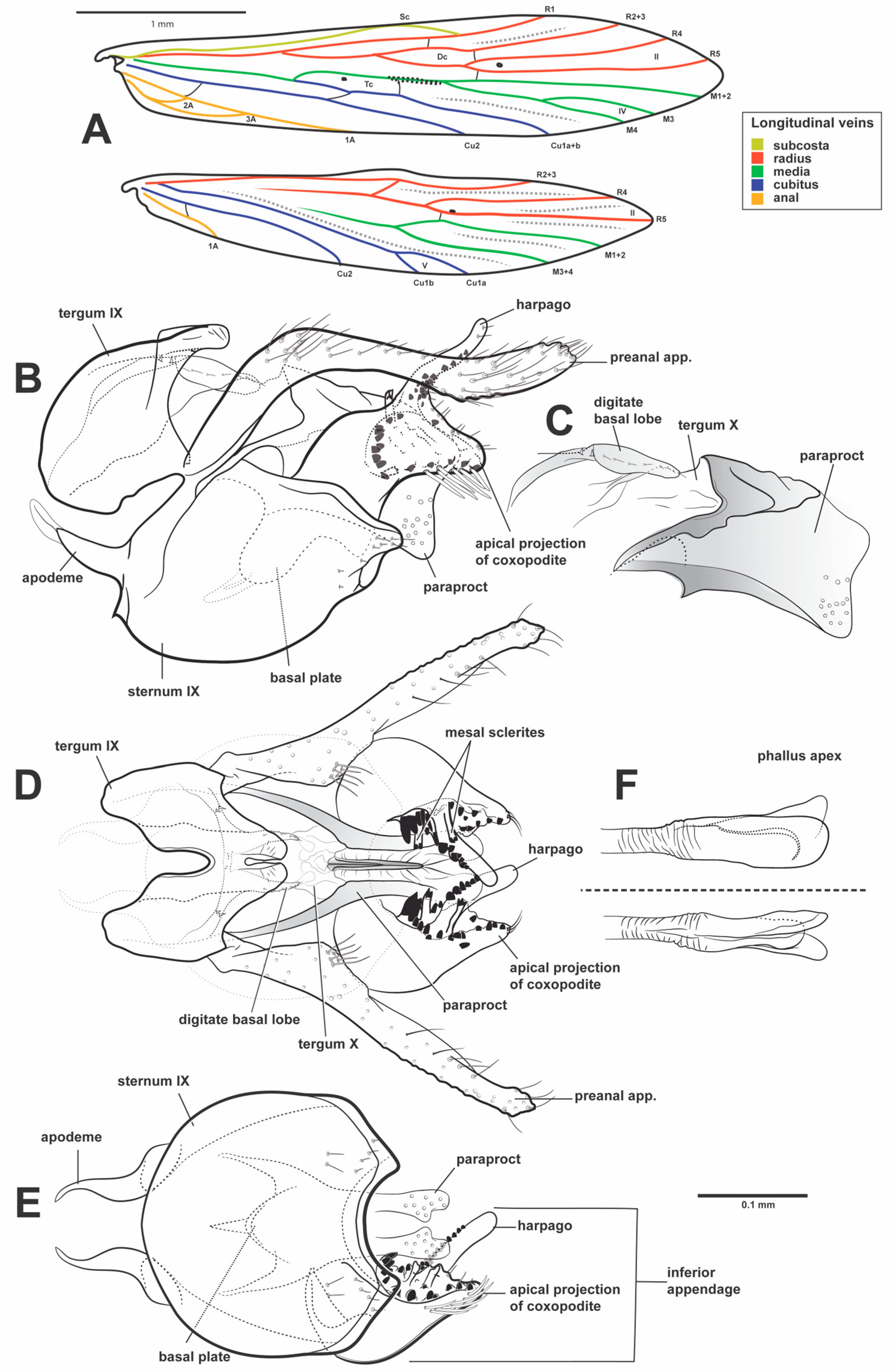
2.3. Morphological Terminology and Description
2.4. Phylogenetic Analysis
3. Results
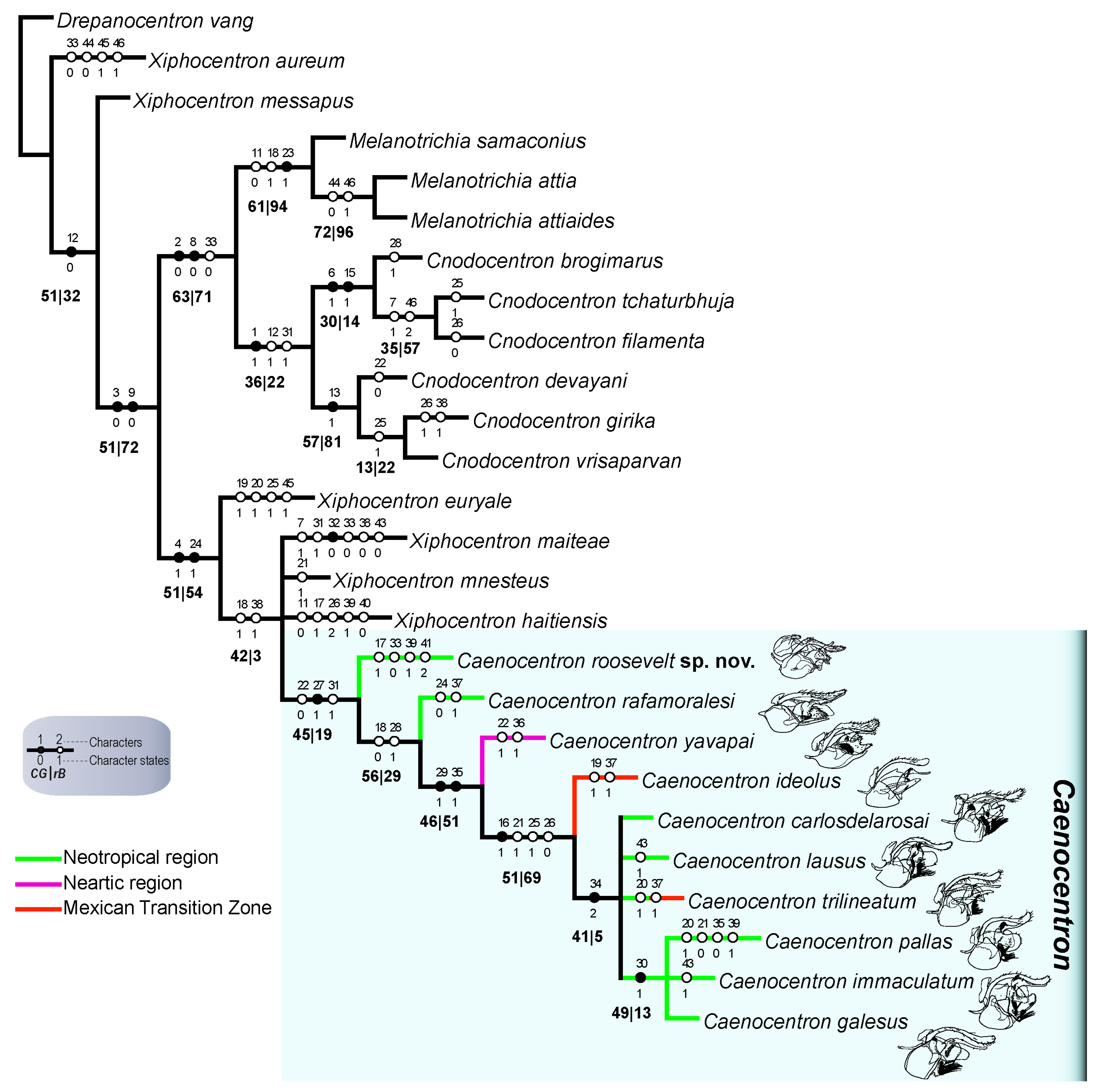
3.1. Phylogenetic Placement
3.2. Taxonomy
3.2.1. Species Description
3.2.2. Identification Key to Adult Male of Caenocentron (Modified from Vilarino et al. [11])
- 1
- Sternum IX with apical margin produced into elongate processes ……………..……. 2
- -
- Sternum IX with apical margin not produced, concave ……….……..….….………… 3
- 2
- Apical projection of sternum IX forming two narrow, elongate processes ………………………………………………………………………………... C. rafamoralesi
- -
- Apical projection of sternum IX forming a broad, deltoid plate with two short apical processes ………………………………………………..…………....................... C. yavapai
- 3
- Coxopodite apical margin projection narrow, acute …………………….….…………. 4
- -
- Coxopodite apical margin projection broad …………………………………..……….. 5
- 4
- Sternum IX in lateral view truncate apically; coxopodite in ventral view with ventral projection bearing long, dense setae along most of the inner margin, including mesal region; each coxopodite with one short subapical spine on inner face … C. trilineatum
- -
- Sternum IX in lateral view deltoid apically; coxopodite in ventral view with ventral projection bearing lateral setal brushes, mesal region glabrous; each coxopodite with two short spines on inner face …………………...……..………………………. C. ideolus
- 5
- Coxopodite apical margin with a short spine or multiple long spine-like setae...….. 6
- -
- Coxopodite apical margin lacking spines ………………………………...……………. 9
- 6
- Coxopodites broadly fused at base in ventral view; inner margin with brush of long setae; apical margin bearing a short spine ………………………………….………...… 7
- -
- Coxopodites separate at base in ventral view; inner margin without setal brush; apical margin with line of several long spine-like setae …………….. C. roosevelt sp. nov.
- 7
- Coxopodite inner margin (ventral view) with a pair of long sublateral stout spines; basal third of harpago strongly enlarged …….………………….……... C. immaculatum
- -
- Coxopodite inner margin (ventral view) lacking stout sublateral spines; basal third of harpago slightly or not enlarged ……………………………………….……………. 8
- 8
- Harpago base with linear setal brush; coxopodite apical margin projection broad to apex, bearing short ventroapical spine …………………….…………………… C. lausus
- -
- Harpago base lacking setal brush; coxopodite apical margin projection tapering to rounded apex, bearing a subapical short spine ………………………. C. carlosdelarosai
- 9
- Inferior appendage with lobe bearing brush of setae near harpago base; coxopodite median region with nearly indiscernible small setules; paraproct dorsal margin without process, ventroapical margin rounded …………...…………………..……... C. pallas
- -
- Inferior appendage without brush of setae near harpago base; coxopodite median region with transverse patch of setae; paraproct dorsal margin with short, acute process, ventroapical margin acute…………………………………………………. C. galesus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cn. g | coronal groove |
| fic. sw. | frontal wart |
| ft. g. | frontal grooves |
| la. sw. | lateroantennal wart |
| ma. sw. | medioantennal wart |
| occ. g. | occipital groove |
| occ. sw. | occipital wart |
| oce. sw. | ocellar wart |
References
- Vilarino, A.; Bispo, P.C. New Records and Two New Species of Xiphocentron Brauer 1870 (Trichoptera, Xiphocentronidae) from Southern Atlantic Forest, Brazil. Zootaxa 2020, 4851, 386–400. [Google Scholar] [CrossRef]
- Flint, O.S. New Species of Trichoptera from the Antilles. Fla. Entomol. 1968, 51, 151. [Google Scholar] [CrossRef]
- Schmid, F. La Famille des Xiphocentronides (Trichoptera: Annulipalpia). Mem. Entomol. Soc. Can. 1982, 114, 3–127. [Google Scholar] [CrossRef]
- Sturm, H. Die Terrestrischen Puppengehäuse von Xiphocentron sturmi Ross (Xiphocentronidae, Trichoptera). Zool. Jahrb. 1960, 87, 387–394. [Google Scholar]
- Muñoz-Quesada, F.; Holzenthal, R.W. A New Species of Xiphocentron (Antillotrichia) from Costa Rica with Semiterrestrial Immature Stages (Trichoptera: Xiphocentronidae). In Proceedings of the 8th International Symposium on Trichoptera, Minneapolis, MN, USA, 9–15 July 1995; pp. 355–363. [Google Scholar]
- Pes, A.M.; Hamada, N.; Nessimian, J.L.; Soares, C.C. Two New Species of Xiphocentronidae (Trichoptera) and Their Bionomics in Central Amazonia, Brazil. Zootaxa 2013, 3636, 561–574. [Google Scholar] [CrossRef]
- Wichard, W. Fossil Trichoptera Embedded in Mid-Cretaceous Burmese Amber. Contrib. Entomol. 2023, 73, 167–179. [Google Scholar] [CrossRef]
- Holzenthal, R.W.; Calor, A.R. Catalog of the Neotropical Trichoptera (Caddisflies). ZooKeys 2017, 654, 1–566. [Google Scholar] [CrossRef]
- Vilarino, A.; Holzenthal, R.W. Systematic Revision of the Caddisfly Genus Machairocentron Schmid (Trichoptera: Psychomyioidea: Xiphocentronidae). Insect Syst. Evol. 2020, 52, 375–411. [Google Scholar] [CrossRef]
- Bueno-Soria, J.; Vilarino, A.; Barba-Alvarez, R.; Ballesteros-Barrera, C. Three New Species of Xiphocentron Brauer, 1870 (Trichoptera, Xiphocentronidae) from Mexico. ZooKeys 2022, 1111, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Vilarino, A.; Dias, E.S.; Bispo, P.D.C. Phylogeny Indicates Polyphyly in Cnodocentron (Trichoptera: Xiphocentronidae): Biogeography and Revision of New World Species (Caenocentron). Zool. J. Linn. Soc. 2022, 194, 1341–1373. [Google Scholar] [CrossRef]
- Vilarino, A.; Salles, F.F.; Bispo, P.C. Xiphocentronidae (Trichoptera: Psychomyioidea) from the Andean Foothills: First Species of Machairocentron and Xiphocentron Described in the Peruvian Amazon. Eur. J. Taxon. 2023, 860, 62–80. [Google Scholar] [CrossRef]
- Vilarino, A.; Sganga, J.V.; Bispo, P.C. The Utility of Forewing Geometric Morphometrics for Species Discrimination in the Caddisfly Genus Xiphocentron (Trichoptera: Xiphocentronidae), with the Description of Six New Species. Zool. Anz. 2024, 309, 37–54. [Google Scholar] [CrossRef]
- Coates, A.G.; Collins, L.S.; Aubry, M.-P.; Berggren, W.A. The Geology of the Darien, Panama, and the Late Miocene-Pliocene Collision of the Panama Arc with Northwestern South America. Geol. Soc. Am. Bull. 2004, 116, 1327–1344. [Google Scholar] [CrossRef]
- Leigh, E.G.; O’Dea, A.; Vermeij, G.J. Historical Biogeography of the I Sthmus of P Anama. Biol. Rev. 2014, 89, 148–172. [Google Scholar] [CrossRef] [PubMed]
- MMA. Plano de Manejo Parque Nacional Dos Campos Amazônicos; Ministério Do Meio Ambiente: Brasília, Brazil, 2011; p. 346.
- Gressitt, J.L.; Gressitt, M.K. An Improved Malaise Trap. Pac. Insects 1962, 4, 87–90. [Google Scholar]
- Blahnik, R.J.; Holzenthal, R.W. Collection and Curation of Trichoptera, with an Emphasis on Pinned Material. Nectopsyche Neotrop. Trichoptera Newsl. 2004, 1, 8–20. [Google Scholar]
- Desiderio, G.R.; Santana, V.; Pereira, E.S.; Pes, A.M.; Hamada, N. On the Identity of Smicridea (Smicridea) aequalis Banks, 1920 (Trichoptera: Hydropsychidae): Morphology of Adults and Immature Stages, Bionomics, Distribution, and Male Color Dimorphism. Neotrop. Entomol. 2021, 50, 430–443. [Google Scholar] [CrossRef]
- One Earth Oneearth.Org. 2023. Available online: https://www.oneearth.org/ (accessed on 22 June 2025).
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. BioScience 2001, 51, 933. [Google Scholar] [CrossRef]
- MapBiomas BIOMAS. ESTADOS E MUNICÍPIOS (COLEÇÃO 10)—COBERTURA—Dados de Área (Hectares) Por Classe de Cobertura e Uso Da Terra Para o Cruzamento Entre Cada Bioma, Estado e Município Brasileiro Para o Período de 1985 a 2024. Available online: https://brasil.mapbiomas.org/estatisticas/ (accessed on 8 July 2025).
- Souza, C.M.; Shimbo, J.Z.; Rosa, M.R.; Parente, L.L.; Alencar, A.; Rudorff, B.F.T.; Hasenack, H.; Matsumoto, M.; Ferreira, L.G.; Souza-Filho, P.W.M.; et al. Reconstructing Three Decades of Land Use and Land Cover Changes in Brazilian Biomes with Landsat Archive and Earth Engine. Remote Sens. 2020, 12, 2735. [Google Scholar] [CrossRef]
- Olàh, J.; Johanson, K.A. Trinominal Terminology for Cephalic Setose Warts in Trichoptera (Insecta). Braueria 2007, 34, 43–50. [Google Scholar]
- Nielsen, A. A Comparative Study of the Genital Segments and Their Appendages in Male Trichoptera. Biol. Skr. 1957, 8, 1–159. [Google Scholar]
- Mosely, M.E.; Kimmins, D.E.; British Museum (Natural History). The Trichoptera (Caddis-Flies) of Australia and New Zealand; British Museum: London, UK, 1953. [Google Scholar]
- Desidério, G.R.; Vilarino, A.; Santos, L.S.; Pires, M.A.R.; Pes, A.M.; Matos, T.; Dias-Silva, K.; Hamada, N. The Net-Tube Caddisflies (Trichoptera: Psychomyioidea: Xiphocentronidae) from the Brazilian Amazon: Discovery of New Species and Reports of New Distributional Records. Biologia 2024, 79, 2771–2785. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis; Mesquite Project: Los Angeles, CA, USA, 2021. [Google Scholar]
- Goloboff, P.A.; Morales, M.E. TNT Version 1.6, with a Graphical Interface for MACOS and Linux, Including New Routines in Parallel. Cladistics 2023, 39, 144–153. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Farris, J.; Nixon, K. Tree Analysis Using New Technology. 2003. Available online: http://www.zmuc.dk/public/phylogeny/tnt (accessed on 13 July 2025).
- Goloboff, P.A.; Farris, J.S. Methods for Quick Consensus Estimation. Cladistics 2001, 17, S26–S34. [Google Scholar] [CrossRef]
- Nixon, K.C. WinClada, version 1.89; Nixon: Ithaka, NY, USA, 2002. [Google Scholar]
- Iturralde-Vinent, M.A.; MacPhee, R.D.E. Paleogeography of the Caribbean Region: Implications for Cenozoic Biogeography. Bull. Am. Mus. Nat. Hist. 1999, 238, 1–95. [Google Scholar]
- Iturralde Vinent, M.A.; MacPhee, R.D.E. New Evidence for Late Eocene-Early Oligocene Uplift of Aves Ridge and Paleogeography of GAARlandia. Geol. Acta 2023, 21, 1–10. [Google Scholar] [CrossRef]
- Flint, O.S. The Trichoptera of Surinam. Stud. Fauna Suriname Guyanas 1974, 14, 1–151. [Google Scholar]
- Desidério, G.R.; Barcelos-Silva, P.; Hamada, N. New Species, Records, and Immature Stages of Chimarra Stephens (Trichoptera: Philopotamidae) from Campos Amazônicos National Park, Northern Brazil. Zootaxa 2018, 4500, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.L.; Resende, A.F.; Barlow, J.; França, F.M.; Moura, M.R.; Maciel, R.; Alves-Martins, F.; Shutt, J.; Nunes, C.A.; Elias, F.; et al. Pervasive Gaps in Amazonian Ecological Research. Curr. Biol. 2023, 33, 3495–3504.e4. [Google Scholar] [CrossRef]
- SNUC Sistema Nacional de Unidades de Conservação Da Natureza. Lei N° 9.985, de 18 de Julho de 2000. 2000. Available online: https://www.planalto.gov.br/ccivil_03/leis/l9985.htm (accessed on 23 May 2024).
- Soares-Filho, B.; Moutinho, P.; Nepstad, D.; Anderson, A.; Rodrigues, H.; Garcia, R.; Dietzsch, L.; Merry, F.; Bowman, M.; Hissa, L.; et al. Role of Brazilian Amazon Protected Areas in Climate Change Mitigation. Proc. Natl. Acad. Sci. USA 2010, 107, 10821–10826. [Google Scholar] [CrossRef]
- Soares-Filho, B.; Rajão, R.; Merry, F.; Rodrigues, H.; Davis, J.; Lima, L.; Macedo, M.; Coe, M.; Carneiro, A.; Santiago, L. Brazil’s Market for Trading Forest Certificates. PLoS ONE 2016, 11, e0152311. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desidério, G.R.; Vilarino, A.; Santos, L.d.S.d.; Bispo, P.C.; Hamada, N. Amazonian Discovery Sheds Light on the Evolution of Caenocentron Schmid, 1982 (Trichoptera: Xiphocentronidae): Phylogenetic Placement and Description of a New Species. Insects 2025, 16, 1188. https://doi.org/10.3390/insects16121188
Desidério GR, Vilarino A, Santos LdSd, Bispo PC, Hamada N. Amazonian Discovery Sheds Light on the Evolution of Caenocentron Schmid, 1982 (Trichoptera: Xiphocentronidae): Phylogenetic Placement and Description of a New Species. Insects. 2025; 16(12):1188. https://doi.org/10.3390/insects16121188
Chicago/Turabian StyleDesidério, Gleison R., Albane Vilarino, Laissa da Silva dos Santos, Pitágoras C. Bispo, and Neusa Hamada. 2025. "Amazonian Discovery Sheds Light on the Evolution of Caenocentron Schmid, 1982 (Trichoptera: Xiphocentronidae): Phylogenetic Placement and Description of a New Species" Insects 16, no. 12: 1188. https://doi.org/10.3390/insects16121188
APA StyleDesidério, G. R., Vilarino, A., Santos, L. d. S. d., Bispo, P. C., & Hamada, N. (2025). Amazonian Discovery Sheds Light on the Evolution of Caenocentron Schmid, 1982 (Trichoptera: Xiphocentronidae): Phylogenetic Placement and Description of a New Species. Insects, 16(12), 1188. https://doi.org/10.3390/insects16121188






