Integrated Management Strategies for Wood Infested by Hylurgus ligniperda F. (Coleoptera: Curculionidae: Scolytinae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Samples and Infested Wood
2.2. Toxicity Testing of Fumigants Against RHB
2.2.1. Susceptibility of Different Developmental Stages to Fumigants
2.2.2. Assessment of Fumigant Penetration Ability in Wood and Wood Stacks
2.3. Fumigation Treatment of RHB in Infested Wood
2.3.1. Effect of Temperature on Fumigation Efficacy
2.3.2. Effect of Wood Moisture Content on Fumigation Efficacy
2.3.3. High- and Low-Temperature Treatment of RHB-Infested Wood
2.4. Treatment of Bark and Wood Chips During Downgrading of Infested Wood
2.5. Chemicals and Apparatus
2.6. Mortality Assessment of Test RHB
2.7. Statistical Analysis
3. Results
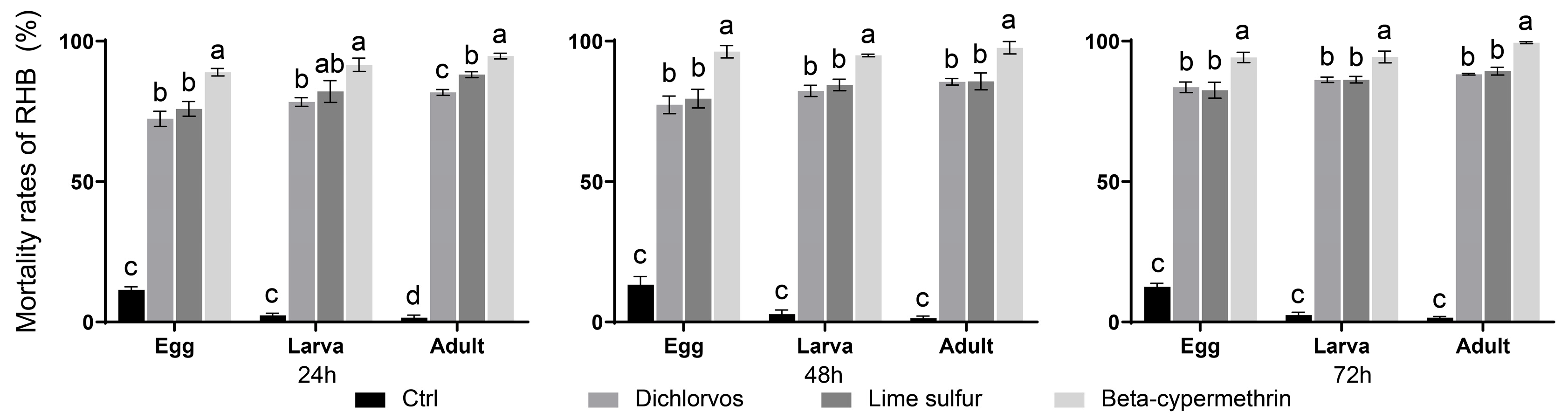
3.1. Fumigation Toxicity of AP and SF Against Various Developmental Stages of RHB
3.2. Penetration of AP and SF into P. thunbergii Wood
3.3. Influence of Fumigation Conditions on AP and SF Efficacy Against RHB in Infested Wood
3.4. Heating and Freezing Treatment Conditions for RHB in Infested Wood
3.5. Management Strategies for Bark and Chip Byproducts from Processing RHB-Infested Wood
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, Z.; Sun, J.; Don, O.; Zhang, Z. The red turpentine beetle, Dendroctonus valens LeConte (Scolytidae): An exotic invasive pest of pine in China. Biodivers. Conserv. 2005, 14, 1735–1760. [Google Scholar] [CrossRef]
- Santini, A.; Faccoli, M. Dutch elm disease and elm bark beetles: A century of association. Iforest-BiogeoSci. For. 2014, 8, 126–134. [Google Scholar] [CrossRef]
- Lin, W.; Park, S.; Jiang, Z.-R.; Ji, Y.-C.; Ernstsons, A.S.; Li, J.-J.; Li, Y.; Hulcr, J. Native or Invasive? The Red-Haired Pine Bark Beetle Hylurgus ligniperda (Fabricius) (Curculionidae: Scolytinae) in East Asia. Forests 2021, 12, 950. [Google Scholar] [CrossRef]
- Faccoli, M.; Gallego, D.; Branco, M.; Brockerhoff, E.G.; Corley, J.; Coyle, D.R.; Hurley, B.P.; Jactel, H.; Lakatos, F.; Lantschner, V.; et al. A first worldwide multispecies survey of invasive Mediterranean pine bark beetles (Coleoptera: Curculionidae, Scolytinae). Biol. Invasions 2020, 22, 1785–1799. [Google Scholar] [CrossRef]
- Mausel, D.L.; Gara, R.I.; Lanfranco, D.; Ruiz, C.; Ide, S.; Azat, R. The introduced bark beetles Hylurgus ligniperda and Hylastes ater (Coleoptera: Scolytidae) in Chile: Seasonal flight and effect of Pinus radiata log placement on colonization. Can. J. For. Res. 2007, 37, 156–169. [Google Scholar] [CrossRef]
- Chase, K.D.; Kelly, D.; Liebhold, A.M.; Bader, M.K.-F.; Brockerhoff, E.G. Long-distance dispersal of non-native pine bark beetles from host resources. Ecol. Entomol. 2017, 42, 173–183. [Google Scholar] [CrossRef]
- Clare, G.; George, E.M. Life cycle and mass-rearing of Hylurgus ligniperda using a novel egg-collection method. New Zealand Plant Prot. 2016, 69, 143–152. [Google Scholar] [CrossRef]
- Park, S.; Jung, J.; Han, T. A new species and five newly recorded species of Scolytinae (Coloptera: Curculionidae) from Korea. Entomol. Res. Bull. 2017, 33, 131–137. [Google Scholar]
- Ren, L.L.; Tao, J.; Wu, H.W.; Zong, S.X.; Wang, C.; Hua, D.; Shi, J.; Liu, Y.Z.; Luo, Y.Q. The first discovery and infective characteristics of a major invasive pest Hylurgus ligniperda (Coleoptera: Scolytidae) in China. Sci. Silvae Sin. 2021, 57, 140–150. [Google Scholar] [CrossRef]
- Hoebeke, E. Hylurgus ligniperda: A new exotic pine bark beetle in the United States. Newsl. Mich. Entomol. Soc. 2001, 46, 1–2. [Google Scholar]
- Jankowiak, R.; Bilański, P. Ophiostomatoid fungi associated with root-feeding bark beetles on Scots pine in Poland. For. Pathol. 2013, 43, 422–428. [Google Scholar] [CrossRef]
- de Errasti, A.; Pildain, M.B.; Rajchenberg, M. Ophiostomatoid fungi isolated from three different pine species in Argentinian Patagonia. For. Pathol. 2018, 48, e12393. [Google Scholar] [CrossRef]
- Lu, M.; Hulcr, J.; Sun, J. The role of symbiotic microbes in insect invasions. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 487–505. [Google Scholar] [CrossRef]
- Fabre, J.-P.; Carle, P. Contribution à l’étude biologique d’Hylurgus Ligniperda F. (Coleoptera Scolytidae) dans le Sud-est de la France. Ann. Des Sci. For. 1975, 32, 55–71. [Google Scholar] [CrossRef]
- Page, A.B.P.; Lubatti, O.F. Fumigation of insects. Annu. Rev. Entomol. 1963, 8, 239–264. [Google Scholar] [CrossRef]
- Mellouki, A.; Talukdar, R.K.; Schmoltner, A.-M.; Gierczak, T.; Mills, M.J.; Solomon, S.; Ravishankara, A.R. Atmospheric lifetimes and ozone depletion potentials of methyl bromide (CH3Br) and dibromomethane (CH2Br2). Geophys. Res. Lett. 1992, 19, 2059–2062. [Google Scholar] [CrossRef]
- Najar-Rodriguez, A.J.; Hall, M.K.D.; Adlam, A.R.; Afsar, S.; Esfandi, K.; Wilks, C.; Noakes, E.; Clare, G.K.; Barrington, A.; Brash, D.W.; et al. Efficacy of quarantine treatments using reduced methyl bromide concentrations to disinfest Pinus radiata logs from New Zealand. J. Stored Prod. Res. 2020, 89, 101718. [Google Scholar] [CrossRef]
- Porter, I.; Banks, J.; Mattner, S.; Fraser, P. Global phaseout of methyl bromide under the montreal protocol: Implications for bioprotection, biosecurity and the ozone layer. In Recent Developments in Management of Plant Diseases; Gisi, U., Chet, I., Gullino, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 293–309. [Google Scholar] [CrossRef]
- Sankarganesh, E.; Girish, A.G.; Alice, R.P.; Mariadoss, A. Influence of relative humidity on phosphine concentration during aluminium phosphide (ALP) fumigation in pigeon pea. Int. J. Chem. Stud. 2020, 8, 2082–2084. [Google Scholar] [CrossRef]
- Najar-Rodriguez, A.J.; Afsar, S.; Esfandi, K.; Hall, M.K.D.; Adlam, A.R.; Wilks, C.; Noakes, E.; Richards, K. Laboratory toxicity and large-scale commercial validation of the efficacy of ethanedinitrile, a potential alternative fumigant to methyl bromide, to disinfest New Zealand Pinus radiata export logs. J. Stored Prod. Res. 2020, 88, 101671. [Google Scholar] [CrossRef]
- Abrams, A.E.; Kawagoe, J.C.; Najar-Rodriguez, A.; Walse, S.S. Sulfuryl fluoride fumigation to control brown marmorated stinkbug (Hempitera: Pentatomidae). Postharvest Biol. Technol. 2020, 163, 111111. [Google Scholar] [CrossRef]
- Lee, B.-H.; Yang, J.-O.; Beckett, S.; Ren, Y. Preliminary trials of the ethanedinitrile fumigation of logs for eradication of Bursaphelenchus xylophilus and its vector insect Monochamus alternatus. Pest Manag. Sci. 2017, 73, 1446–1452. [Google Scholar] [CrossRef]
- Spence, D.J.; Smith, J.A.; Ploetz, R.; Hulcr, J.; Stelinski, L.L. Effect of chipping on emergence of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) and recovery of the laurel wilt pathogen from infested wood chips. J. Econ. Entomol. 2013, 106, 2093–2100. [Google Scholar] [CrossRef]
- Pawson, S.M.; Bader, M.K.F.; Brockerhoff, E.G.; Heffernan, W.J.B.; Kerr, J.L.; O’Connor, B. Quantifying the thermal tolerance of wood borers and bark beetles for the development of Joule heating as a novel phytosanitary treatment of pine logs. J. Pest Sci. 2019, 92, 157–171. [Google Scholar] [CrossRef]
- Eliopoulos, P.A.; Prasodimou, G.Z.; Pouliou, A.V. Time–mortality relationships of larvae and adults of grain beetles exposed to extreme cold. Crop Prot. 2011, 30, 1097–1102. [Google Scholar] [CrossRef]
- Estes, P.M. The effects of time and temperature on methyl bromide fumigation of adults of Sitophilus granarius and Tribolium confusum. J. Econ. Entomol. 1965, 58, 611–614. [Google Scholar] [CrossRef]
- Nayak, M.K.; Collins, P.J. Influence of concentration, temperature and humidity on the toxicity of phosphine to the strongly phosphine-resistant psocid Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae). Pest Manag. Sci. 2008, 64, 971–976. [Google Scholar] [CrossRef]
- Park, C.G.; Son, J.K.; Lee, B.H.; Cho, J.H.; Ren, Y. Comparison of ethanedinitrile (C2N2) and metam sodium for control of Bursaphelenchus xylophilus (Nematoda: Aphelenchidae) and Monochamus alternatus (Coleoptera: Cerambycidae) in naturally infested logs at low temperatures. J. Econ. Entomol. 2014, 107, 2055–2060. [Google Scholar] [CrossRef]
- Zhao, L.; Ramaswamy, H.; Wang, S. Thermal-death kinetics of the bark beetle (Dendroctonus armandi; Coleoptera: Scolytidae). Scand. J. For. Res. 2018, 33, 735–740. [Google Scholar] [CrossRef]
- Noseworthy, M.K.; Humble, L.M.; Souque, T.J.; John, E.P.; Roberts, J.; Lloyd, C.R.; Allen, E.A. Determination of specific lethal heat treatment parameters for pests associated with wood products using the Humble water bath. J. Pest Sci. 2023, 96, 1187–1197. [Google Scholar] [CrossRef]
- Hopf-Biziks, A.; Schröder, T.; Schütz, S. Long-term survival and non-vector spread of the pinewood nematode, Bursaphelenchus xylophilus, via wood chips. For. Pathol. 2017, 47, e12340. [Google Scholar] [CrossRef]
- Gu, Y.; Ge, S.; Li, J.; Ren, L.; Wang, C.; Luo, Y. Composition and diversity of the endobacteria and ectobacteria of the invasive bark beetle Hylurgus ligniperda (Fabricius) (Curculionidae: Scolytinae) in newly colonized areas. Insects 2024, 15, 12. [Google Scholar] [CrossRef]
- Romo, C.M.; Bader, M.K.; Pawson, S.M. Comparative growth and survival of Hylurgus ligniperda (Coleoptera: Scolytinae) and Arhopalus ferus (Coleoptera: Cerambycidae) reared on artificial or natural diet at 15 or 25 °C. J. Econ. Entomol. 2016, 109, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Throne, J.; Weaver, D.; Chew, V.; Baker, J. Probit Analysis of Correlated Data: Multiple observations over time at one concentration. J. Econ. Entomol. 1995, 88, 1510–1512. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Schläfli, L.; Cornejo, C.; Kappeler, J.; Orbach, J.; Tiefenbacher, A.; Kupper, Q.; Avtzis, D.; Branco, M.; Carnegie, A.J.; et al. Worldwide spread of Hylurgus ligniperda (Coleoptera: Scolytinae), and the potential role of bridgehead invasions. bioRxiv 2025. [Google Scholar] [CrossRef]
- Pranamornkith, T.; Hall, M.; Adlam, A.; Page, B.; Connolly, P.; Somerfield, K.; Brash, D. Relative methyl bromide tolerances of Arhopalus ferus (Mulsant), Hylurgus ligniperda (F.) and Hylastes ater (Paykull) adults. New Zealand Plant Prot. 2014, 67, 80–85. [Google Scholar] [CrossRef][Green Version]
- Brockerhoff, E.; Bain, J.; Kimberley, M.; Knizek, M. Interception frequency of exotic bark and ambrosia beetles (Coleoptera: Scolytinae) and relationship with establishment in New Zealand and worldwide. Can. J. For. Res. 2006, 36, 289–298. [Google Scholar] [CrossRef]
- Ide, S.; Valenzuela, J.; Estay, S.; Jaksic, F.; Castro, S. Presión de ingreso de insectos forestales exóticos a Chile desde 1996. En libro invasiones biológicas en chile: Causas globales e impactos locales. In Invasiones Biológicas en Chile: Causas Globales e Impactos Locales; Pontificia Universidad Católica de Chile: Santiago, Chile, 2014; pp. 437–457. [Google Scholar]
- Turner, R.M.; Liebhold, A.M.; Nahrung, H.F.; Phillips, C.B.; Yamanaka, T.; Brockerhoff, E.G. The known unknowns in international border interceptions of non-native insects. Biol. Invasions 2024, 27, 50. [Google Scholar] [CrossRef]
- Yan, D.; Liu, J.; Wang, X.; Fang, W.; Li, Y.; Cao, A.; Wang, Q. A review on the mechanisms of fumigant action. New Plant Prot. 2025, 2, e27. [Google Scholar] [CrossRef]
- Jeon, J.C.; Kim, H.K.; Koo, H.N.; Kim, B.S.; Yang, J.O.; Kim, G.H. Synergistic Effect of cold treatment combined with ethyl formate fumigation against Drosophila suzukii (Diptera: Drosophilidae). Insects 2022, 13, 664. [Google Scholar] [CrossRef]
- Ramadan, G.R.M.; Mosallam, E.M.; Phillips, T.W. Methyl benzoate and its derivative, acetophenone, as fumigants to control stored product insects. J. Stored Prod. Res. 2024, 105, 102248. [Google Scholar] [CrossRef]
- Jagadeesan, R.; Singarayan, V.T.; Nayak, M.K. A co-fumigation strategy utilizing reduced rates of phosphine (PH3) and sulfuryl fluoride (SF) to control strongly resistant rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae). Pest Manag. Sci. 2021, 77, 4009–4015. [Google Scholar] [CrossRef]
- Su, N.-Y.; Scheffrahn, R.H. Efficacy of sulfuryl fluoride against four beetle pests of museums (Coleoptera: Dermestidae, Anobiidae). J. Econ. Entomol. 2014, 83, 879–882. [Google Scholar] [CrossRef]
- Eisenback, J.D.; Chen, Z.; White, M. Evaluating vacuum and steam heat to eliminate pinewood nematodes in naturally infested whole pine logs. J. Nematol. 2024, 56, 20240038. [Google Scholar] [CrossRef]
- Zou, H.; Li, L.; Zhang, J.; Li, B.; Xiao, Y.; Ren, Y.; Huang, J.; Chen, W.; Liu, T. Low-temperature phosphine fumigation is effective against Drosophila suzukii in sweet cherry. Insects 2025, 16, 635. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.S.; Jeong, J.S.; Choi, D.S.; Park, J.; Kim, I. Phytosanitary cold treatment of spotted-wing Drosophila, Drosophila suzukii (Diptera: Drosophilidae) in ‘campbell early’ grape. J. Econ. Entomol. 2018, 111, 1638–1643. [Google Scholar] [CrossRef]
- King, A.M.; MacRae, T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.S.; Hammad, L.A.; Montooth, K.L. Thermal adaptation of cellular membranes in natural populations of Drosophila melanogaster. Funct. Ecol. 2014, 28, 886–894. [Google Scholar] [CrossRef]
- Cheng, L.; Pei, J.; Chen, X.; Shi, F.; Bao, Z.; Hou, Q.; Zhi, L.; Zong, S.; Tao, J. Cold tolerance and metabolism of red-haired pine bark beetle Hylurgus ligniperda (Coleoptera: Curculionidae) during the overwintering period. J. Econ. Entomol. 2024, 117, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Hýsek, Š.; Löwe, R.; Turčáni, M. What happens to wood after a tree is attacked by a bark beetle? Forests 2021, 12, 1163. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, J.; Zhang, H.; Xing, Y.; Chi, D.; Yu, J. Breaking the beetle barrier: Innovative strategies for controlling Hylurgus ligniperda in China. Ecotoxicol. Environ. Saf. 2025, 299, 118433. [Google Scholar] [CrossRef]
| Fumigant | Stage | LCT50 a (mg·h·L−1) (95% CL b) | LCT99 (mg·h·L−1) (95% CL) | χ2 e | df | Slope ± SE | p-Value |
|---|---|---|---|---|---|---|---|
| AP c | Egg | 118.7 (111.0–127.0) | 808.0 (681.1–958.6) | 57.0 | 8 | 1.2 ± 0.1 | <0.001 |
| Larva | 82.9 (76.1–90.2) | 623.8 (498.9–780.0) | 37.6 | 5 | 1.2 ± 0.1 | <0.001 | |
| Pupa | 105.0 (97.7–112.9) | 732.5 (610.3–879.1) | 51.6 | 7 | 1.2 ± 0.1 | <0.001 | |
| Adult | 57.4 (52.0–63.5) | 368.4 (300.5–451.7) | 16.7 | 4 | 1.3 ± 0.1 | <0.001 | |
| SF d | Egg | 41.8 (39.1–44.7) | 204.7 (175.7–238.5) | 38.9 | 6 | 1.5 ± 0.1 | <0.001 |
| Larva | 26.9 (24.8–29.2) | 132.0 (112.2–155.2) | 29.6 | 4 | 1.5 ± 0.1 | <0.001 | |
| Pupa | 35.6 (33.2–38.3) | 168.1 (143.8–196.4) | 41.1 | 5 | 1.5 ± 0.1 | <0.001 | |
| Adult | 22.2 (20.2–24.4) | 132.4 (108.3–161.8) | 26.4 | 3 | 1.3 ± 0.1 | <0.001 |
| Fumigant | Condition | LCT50 (mg·h·L−1) (95% CL) | LCT99 (mg·h·L−1) (95%CL) | χ2 | df | Slope ± SE | p-Value | Penetration Rate (%) |
|---|---|---|---|---|---|---|---|---|
| AP | Open | 97.8 (93.5–102.2) | 279.4 (255.3–305.7) | 8.4 | 8 | 2.2 ± 0.1 | =0.401 | 29.5 |
| Sealed | 215.6 (204.9–226.8) | 948.7 (836.2–1076.2) | 41.9 | 7 | 1.6 ± 0.1 | <0.001 | ||
| SF | Open | 25.3 (19.9–32.3) | 81.4 (63.8–103.9) | 0.3 | 3 | 2.0 ± 0.4 | =0.969 | 12.6 |
| Sealed | 160.8 (152.5–169.5) | 644.8 (574.1–724.3) | 12.7 | 8 | 1.7 ± 0.1 | =0.121 |
| Fumigant | Depth | LCT50 (mg·h·L−1) (95% CL) | LCT99 (mg·h·L−1) (95%CL) | χ2 | df | Slope ± SE | p-Value |
|---|---|---|---|---|---|---|---|
| AP | 0.5 | 59.0 (54.6–63.8) | 423.9 (342.8–524.1) | 29.0 | 7 | 1.2 ± 0.1 | <0.001 |
| 1 | 59.5 (54.4–65.1) | 603.5 (469.8–775.3) | 21.4 | 7 | 1.0 ± 0.1 | =0.003 | |
| 1.5 | 60.2 (54.9–66.1) | 683.6 (537.3–869.7) | 16.1 | 8 | 1.0 ± 0.1 | =0.041 | |
| SF | 0.5 | 22.9 (21.0–25.0) | 130.5 (106.5–160.1) | 18.5 | 5 | 1.3 ± 0.1 | =0.002 |
| 1 | 24.1 (22.2–26.2) | 125.5 (103.4–152.4) | 12.7 | 5 | 1.4 ± 0.1 | =0.026 | |
| 1.5 | 27.8 (25.5–30.4) | 177.0 (143.5–218.2) | 5.9 | 5 | 1.3 ± 0.1 | =0.318 |
| Fumigant | Temp. (℃) | LCT50 (mg·h·L−1) (95% CL) | LCT99 (mg·h·L−1) (95%CL) | χ2 | df | Slope ± SE | p-Value |
|---|---|---|---|---|---|---|---|
| AP | 11.5–15.4 | 889.3 (842.6–938.7) | 8884.2 (7665.2–10,297.0) | 114.4 | 18 | 1.0 ± 0.03 | <0.001 |
| 18.5–25.5 | 658.6 (620.7–698.8) | 6421.7 (5584.2–7384.7) | 178.9 | 18 | 1.0 ± 0.04 | <0.001 | |
| 26.5–29.5 | 451.6 (414.0–492.5) | 7373.1 (6136.3–8859.1) | 200.9 | 16 | 0.8 ± 0.03 | <0.001 | |
| SF | 11.5–15.4 | 674.4 (635.3–715.8) | 5146.5 (4502.7–5882.3) | 110.5 | 15 | 1.1 ± 0.04 | <0.001 |
| 18.5–25.5 | 619.6 (583.2–658.3) | 5101.0 (4394.8–5920.8) | 99.6 | 14 | 1.1 ± 0.1 | <0.001 | |
| 26.5–29.5 | 623.3 (584.9–664.3) | 5795.5 (4945.2–6791.9) | 60.6 | 15 | 1.0 ± 0.04 | <0.001 |
| Fumigant | Moisture Content (%) | LCT50 (mg·h·L−1) (95% CL) | LCT99 (mg·h·L−1) (95%CL) | χ2 | df | Slope ± SE | p-Value |
|---|---|---|---|---|---|---|---|
| AP | 21.3–25.5 | 721.3 (329.8–826.0) | 6264.6 (4712.1–8270.2) | 38.9 | 5 | 1.1 ± 0.1 | <0.001 |
| 31.6–33.6 | 803.1 (706.4–913.0) | 7278.1 (5401.1–9807.3) | 49.6 | 5 | 1.1 ± 0.1 | <0.001 | |
| 45.8–48.8 | 1055.0 (950.8–1170.6) | 8649.2 (6634.1–11,276.4) | 61.2 | 6 | 1.1 ± 0.1 | <0.001 | |
| SF | 21.3–25.5 | 456.9 (392.9–531.4) | 6740.3 (4609.8–9855.4) | 51.4 | 6 | 0.9 ± 0.1 | <0.001 |
| 31.6–33.6 | 532.1 (465.4–608.2) | 7499.1 (5487.7–10,247.7) | 45.9 | 8 | 0.9 ± 0.1 | <0.001 | |
| 45.8–48.8 | 778.4 (705.3–859.0) | 8510.9 (6522.0–11,106.3) | 25.0 | 9 | 1.0 ± 0.1 | <0.001 |
| Thermal Treatment | Temp. (°C) | LET50 a (h) (95% CL) | LET99 (h) (95% CL) | χ2 | df | Slope ± SE | p-Value |
|---|---|---|---|---|---|---|---|
| Heat | 45 | 7.0 (6.1–8.0) | 333.8 (233.4–477.3) | 36.3 | 6 | 0.6 ± 0.04 | <0.001 |
| 50 | 5.0 (4.5–5.5) | 37.5 (33.4–42.2) | 71.2 | 6 | 1.2 ± 0.1 | <0.001 | |
| 55 | 2.2 (1.8–2.8) | 16.9 (14.5–19.2) | 24.9 | 6 | 1.2 ± 0.1 | <0.001 | |
| 60 | 1.9 (1.7–2.0) | 7.0 (6.0–8.1) | 0.86 | 3 | 1.8 ± 0.1 | =0.835 | |
| 65 | 1.1 (0.9–1.3) | 6.3 (5.3–7.4) | 24.0 | 4 | 1.3 ± 0.1 | <0.001 | |
| Cold | −15 | 383.5 (188.6–780.0) | 69,417.9 (9553.0–504,435.8) | 8.3 | 6 | 0.4 ± 0.1 | =0.216 |
| −20 | 32.9 (30.8–35.3) | 501.3 (364.3–689.8) | 15.9 | 6 | 0.9 ± 0.04 | =0.014 | |
| −25 | 17.2 (16.3–18.1) | 206.5 (168.9–252.4) | 18.3 | 6 | 0.9 ± 0.04 | =0.005 | |
| −30 | 8.6 (8.2–9.0) | 32.1 (29.6–34.8) | 133.1 | 4 | 1.8 ± 0.1 | <0.001 | |
| −35 | 5.4 (5.1–5.7) | 26.4 (24.2–28.8) | 104.3 | 6 | 1.5 ± 0.1 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Chen, X.; Xie, D.; Yang, Q.; Niu, F.; Chi, D.; Yu, J. Integrated Management Strategies for Wood Infested by Hylurgus ligniperda F. (Coleoptera: Curculionidae: Scolytinae). Insects 2025, 16, 1154. https://doi.org/10.3390/insects16111154
Chen H, Chen X, Xie D, Yang Q, Niu F, Chi D, Yu J. Integrated Management Strategies for Wood Infested by Hylurgus ligniperda F. (Coleoptera: Curculionidae: Scolytinae). Insects. 2025; 16(11):1154. https://doi.org/10.3390/insects16111154
Chicago/Turabian StyleChen, Huanwen, Xiaowei Chen, Dan Xie, Qingshan Yang, Fang Niu, Defu Chi, and Jia Yu. 2025. "Integrated Management Strategies for Wood Infested by Hylurgus ligniperda F. (Coleoptera: Curculionidae: Scolytinae)" Insects 16, no. 11: 1154. https://doi.org/10.3390/insects16111154
APA StyleChen, H., Chen, X., Xie, D., Yang, Q., Niu, F., Chi, D., & Yu, J. (2025). Integrated Management Strategies for Wood Infested by Hylurgus ligniperda F. (Coleoptera: Curculionidae: Scolytinae). Insects, 16(11), 1154. https://doi.org/10.3390/insects16111154






