Insecticidal Activity of Eupatorium fortunei Essential Oil Against Schizaphis graminum and Its Effects on Detoxification Enzymes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Culture and Reagents
2.2. Contact Killing Activity of EFEO and Pyrethrin Against S. graminum
2.3. Fumigation Activity of EFEO Against S. graminum
2.4. Contact Killing Activity of the Main Compounds of EFEO Against S. graminum
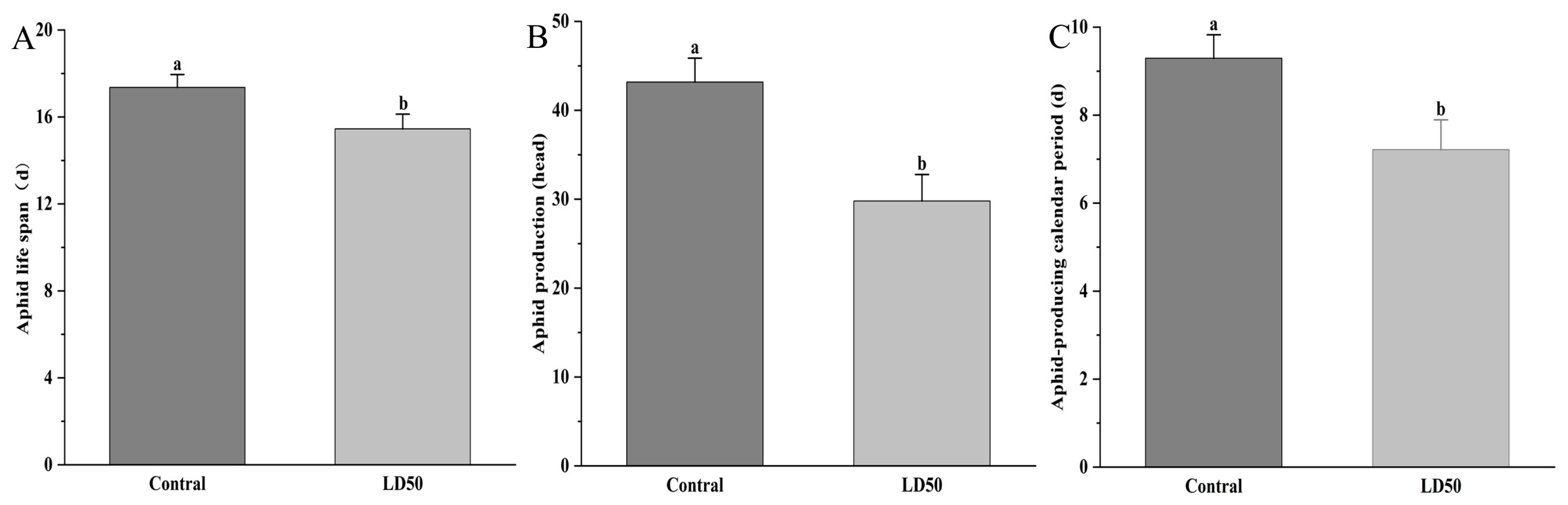
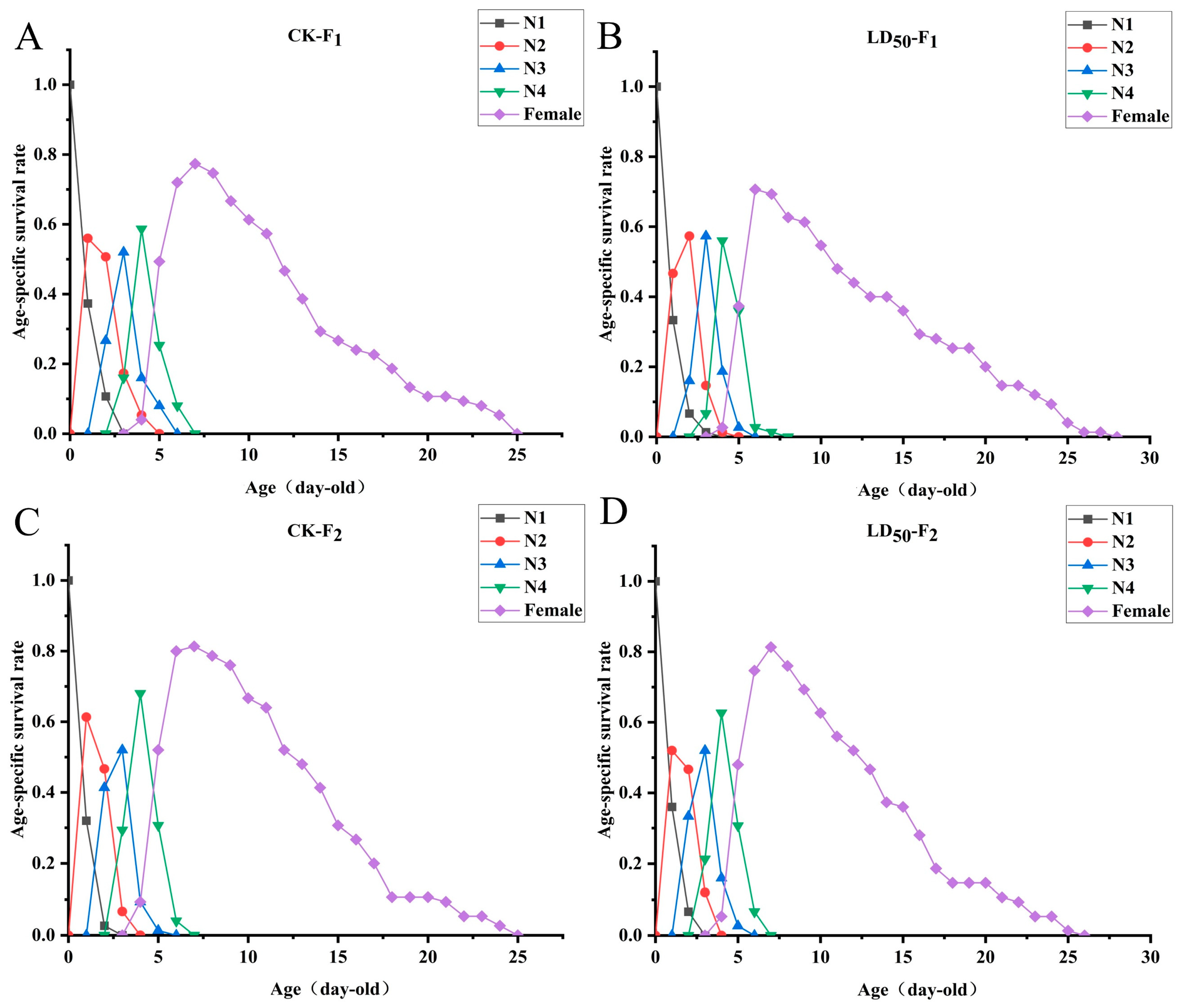
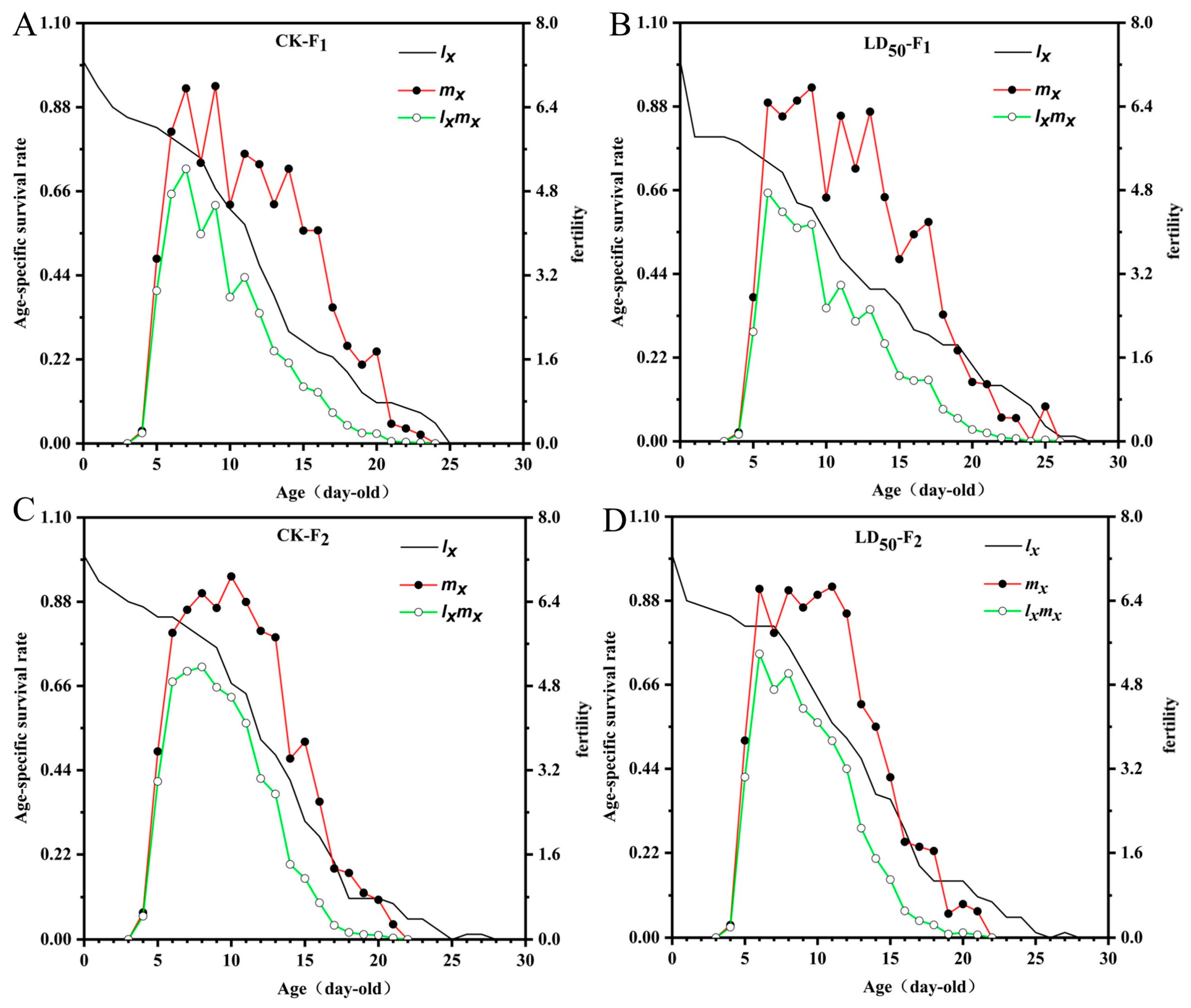
2.5. Sublethal Effects of EFEO (LD50) on the Population of S. graminum
2.6. EFEO LD50 on the Enzyme Activity of S. graminum
2.7. Safety Evaluation of EFEO
2.8. Preparation and Physicochemical Property Characterization of EFEO Nanoemulsion
2.9. Data Analysis and Statistics
3. Results
3.1. Contact Killing Effect of EFEO Against S. graminum
3.2. Contact Killing Effect of Pyrethrin Against S. graminum
3.3. Fumigation Effect of EFEO Against S. graminum
3.4. Contact Killing Effect of Main Compounds in EFEO Against S. graminum
3.5. Inhibitory Effect of EFEO LD50 on the Population of S. graminum
3.6. EFEO LD50 Impact on the Enzyme Activity of S. graminum
3.7. Safety Verification of EFEO
3.8. The Control Efficiency of EFEO Nanoemulsion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, D.H.; Duan, W.B.; Wang, H.; Zhang, K.; Guo, J.L.; Yuan, L.L.; Wang, L.K.; Wu, S.L. Assessment of the effects of lethal and sublethal exposure to dinotefuran on the wheat aphid Rhopalosiphum padi (Linnaeus). Ecotoxicology 2019, 28, 825–833. [Google Scholar] [CrossRef]
- Gong, P.P.; Li, X.A.; Wang, C.; Li, X.R.; Zhang, Y.H.; Li, J.H.; Zhu, X. Research advances in pyrethroid insecticide resistance in wheat aphids. Plant Prot. 2021, 47, 8–14. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.A.; Liu, E.L.; Gao, H.F.; Zhang, Y.H.; Li, J.H.; Zhu, X. Research status of wheat aphid resistance to insecticides in China. J. Environ. Entomol. 2022, 44, 626–635. [Google Scholar] [CrossRef]
- Thornton, J. Implementing green chemistry. An environmental policy for sustainability. Pure Appl. Chem. 2001, 73, 1231–1236. [Google Scholar] [CrossRef]
- Mahmood, A.; Malik, R.N.; Li, J.; Zhang, G. Human health risk assessment and dietary intake of organochlorine pesticides through air, soil and food crops (wheat and rice) along two tributaries of river Chenab, Pakistan. Food Chem. Toxicol. 2014, 71, 17–25. [Google Scholar] [CrossRef]
- Xue, B.; Tang, Q.Z.; Jin, M.Q.; Zhou, S.S.; Zhang, H.S. Residues and enantiomeric profiling of organochlorine pesticides in sediments from Xinghua Bay, southern East China Sea. J. Environ. Sci. Health B 2014, 49, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.H.; Li, Y.M.; He, L.Y.; Liu, W.G.; Yi, X.; Yu, F.; Wu, M.J.; Sun, N. Research progress on chemical constituents and bioactivities of Peilan. Chin. Arch. Tradit. Chin. Med. 2024, 42, 40–46. [Google Scholar] [CrossRef]
- Huang, J.L.; Bai, W.D.; Liu, G.L.; Li, X.L.; Wang, J.Y.; Wang, H. Progress in chemical composition, efficacy, and delivery systems of plant essential oils. Food Mach. 2025, 41, 197–205. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhao, N.N.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Zhou, L.G.; Deng, Z.W. Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem. 2011, 59, 9910–9915. [Google Scholar] [CrossRef]
- Liu, C.; Hong, Y.Y.; Wang, F.; He, J.; Zhang, R.; Xiao, L.F. Evaluation of the indoor bioactivity of 26 essential oils against the adults of Poratrioza sinica. Ningxia J. Agri. For. Sci. Technol. 2020, 61, 20–23. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G.; Sprawka, I.; Sytykiewicz, H. Aphicidal activity of selected Asteraceae essential oils and their effect on enzyme activities of the green peach aphid, Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2018, 145, 84–92. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Sytykiewicz, H.; Chrzanowski, G. The effect of essential oils from Asteraceae plants on behavior and selected physiological parameters of the bird cherry-oat aphid. Molecules 2024, 29, 1673. [Google Scholar] [CrossRef]
- Akbari, S.; Aramideh, S. Fumigant toxicity and sublethal effects of Achilla millefolium L. and Mentha pulegium L. essential oils on life table parameters of Aphis gossypii Glover. Iran. J. Med. Aromat. Plants 2023, 39, 188–202. [Google Scholar] [CrossRef]
- De Elguea-Culebras, G.O.; Sánchez-Vioque, R.; Berruga, M.I.; Herraiz-Penalver, D.; González-Coloma, A.; Andrés, M.F.; Santana-Méridas, O. Biocidal potential and chemical composition of industrial essential oils from Hyssopus officinalis, Lavandula × intermedia var. Super, and Santolina chamaecyparissus. Chem. Biodivers. 2018, 15, e1700313. [Google Scholar] [CrossRef]
- Tabanca, N.; Bernier, U.R.; Tsikolia, M.; Becnel, J.J.; Sampson, B.; Werle, C.; Demirci, B.; Başer, K.H.C.; Blythe, E.K.; Pounders, C.; et al. Eupatorium capillifolium essential oil: Chemical composition, antifungal activity, and insecticidal activity. Nat. Prod. Commun. 2010, 5, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.C.; Cecati, F.M.; Ardanáz, C.E.; Donadel, O.J.; Tonn, C.E.; Sosa, M.E. Assessment of the insecticidal potential of the Eupatorium buniifolium essential oil against Triatoma infestans (Hemiptera: Reduviidae). A chiral recognition approach. Neotrop. Entomol. 2018, 14, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.E.; Lancelle, H.G.; Tonn, C.E.; Andres, M.F.; Gonzalez-Coloma, A. Insecticidal and nematicidal essential oils from Argentinean Eupatorium and Baccharis spp. Biochem. Syst. Ecol. 2012, 43, 132–138. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, Q.Y.; Li, D.W.; Zhang, Z.M.; You, C.X. Antagonistic storage potential of Tagetes minuta, Eupatorium fortune and Ocimum basilicum oils with volatile secondary metabolites against Tribolium castaneum and Lasioderma serricorne. Ind. Crops Prod. 2022, 187, 115502. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.Q.; Feng, J.T.; Wu, H.; Han, L.R. Review on research and development of botanical pesticides. Chin. J. Biol. Control 2015, 31, 685–698. [Google Scholar] [CrossRef]
- Kiran, S.R.; Reddy, A.S.; Devi, P.S.; Reddy, K.J. Insecticidal, antifeedant and oviposition deterrent effects of the essential oil and individual compounds from leaves of Chloroxylon swietenia DC. Pest Manag. Sci. 2006, 62, 1116–1121. [Google Scholar] [CrossRef]
- Wang, C.; Tang, M.; Wei, Q.; Li, Q. Insecticidal activity of Cercidiphyllum japonicum oil against 2 kinds of aphids. J. Sichuan Norm. Univ. 2018, 41, 393–398. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.L.; Zhang, R.; Huang, W.G.; Wang, F.; Quan, M.R. Control of three main pests of wolfberry (Lycium barbarum) by the essential oil of Artemisia mongolica. Chin. J. Biol. Control 2022, 38, 1400–1409. [Google Scholar] [CrossRef]
- Nathan, S.S.; Hisham, A.; Jayakumar, G. Larvicidal and growth inhibition of the malaria vector Anopheles stephensi by triterpenes from Dysoxylum malabaricum and Dysoxylum beddomei. Fitoterapia 2008, 79, 106–111. [Google Scholar] [CrossRef]
- Martin, F.; Garzo, E.; Guirao, P.; Pascual-Villalobos, M.J.; Fereres, A.; Moreno, A. Persistence of nanoemulsions of bioactive volatiles and their impact on aphid feeding behaviour. J. Pest Sci. 2024, 97, 2115–2129. [Google Scholar] [CrossRef]
- Mondal, P.C.; Salim, R.; Kumar, V.; Kaushik, P.; Shakil, N.A.; Rana, V.S. Aphidicidal activity of nano-emulsions of spearmint oil and carvone against Rhopalosiphum maidis and Sitobion avenae. Sci. Rep. 2024, 14, 24226. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; Essawy, M.; Quraytam, A.; Abdallah, F.; Mostafa, H.; Shoueir, K.; Fouad, H.; Hassan, F.A.S.; Hafez, Y. Toxicity of essential oils nanoemulsion against Aphis craccivora and their inhibitory activity on insect enzymes. Processes 2021, 9, 624. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Ma, K.S.; Liu, J.J.; Lu, L.Y.; Chen, X.L.; Zhang, S.P.; Gao, X.W. Differential expression of genes in greenbug (Schizaphis graminum Rondani) treated by imidacloprid and RNA interference. Pest Manag. Sci. 2019, 75, 1726–1733. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.J.; Zuo, J.F.; Zhang, X.H.; Peng, X.; Wang, K.; Chen, M.H. Characterization and fitness cost of bifenthrin resistance in Rhopalosiphum padi (Hemiptera: Aphididae). J. Econ. Entomol. 2023, 116, 1795–1803. [Google Scholar] [CrossRef]
- Ji, X.; Jiang, Y.T.; Guo, T.X.; Zhang, P.; Li, X.A.; Kong, F.B.; Zhang, B.Z. Sublethal effects of imidacloprid on the fitness of two species of wheat aphids, Schizaphis graminum (R.) and Rhopalosiphum padi (L.). PLoS ONE 2023, 18, e0294877. [Google Scholar] [CrossRef]
- Chen, R.X.; Xu, T.; Hao, D.J.; Teale, S.A. Cuticular hydrocarbon recognition in the mating behavior of two Pissodes species. Insects 2019, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Liu, X.; Tang, H.C.; Li, M.T.; Gao, P.; Peng, X.; Chen, M.H. UGT2B13 and UGT2C1 are involved in lambda-cyhalothrin resistance in Rhopalosiphum padi. Pestic. Biochem. Physiol. 2023, 194, 105528. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Urvashi; Gupta, H.; Anmol; Sharma, U.; Reddy, S.G.E. Chemical composition and insecticidal potential of essential oil from Murraya koenigii (L.) obtained by natural deep eutectic solvents. Neotrop. Entomol. 2024, 53, 1318–1331. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Contact, fumigant, and cytotoxic activities of thyme and lemongrass essential oils against larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni. J. Pest Sci. 2016, 89, 183–193. [Google Scholar] [CrossRef]
- Lami, F.; Burgio, G.; Magagnoli, S.; Depalo, L.; Lanzoni, A.; Frassineti, E.; Marotti, I.; Alpi, M.; Mercatante, D.; Rodriguez-Estrada, M.T.; et al. The effects of natural insecticides on the green peach aphid Myzus persicae (Sulzer) and its natural enemies Propylea quatuordecimpunctata (L.) and Aphidius colemani viereck. Insects 2024, 15, 556. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, Z.H.; Li, J.K.; Xu, Y.H.; Zhou, F.; Zhang, F.L.; Li, D.M.; Zhou, L.; Liu, R.Q. The low-lethal concentrations of rotenone and pyrethrins suppress the population growth of Rhopalosiphum padi. Sci. Rep. 2024, 14, 16570. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Xue, M.; Zhang, Q.C.; Zhou, F.Y.; Wei, J.Q. Toxicity of β-caryophyllene from Vitex negundo to Aphis gossypii and its action mechanism. Acta Entomol. Sin. 2010, 53, 396–404. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Shen, D.R.; He, C.; Zhang, R.; Yuan, S.Y.; Tian, X.J.; Zhang, H.R. Toxicity effect of four botanical insecticides against grape thrips. Acta Agric. Boreali-Occident. Sin. 2020, 29, 1751–1757. [Google Scholar] [CrossRef]
- Wang, Z.H.; Fan, J.M.; Chen, J.C.; Gong, Y.J.; Wei, S.J. Sublethal effects of sulfoxaflor on the growth and reproduction ofthe green peach aphid Myzus persicae. Sci. Agric. Sin. 2017, 50, 496–503. [Google Scholar] [CrossRef]
- Tang, Q.L.; Ma, K.S.; Chi, H.; Hou, Y.M.; Gao, X.W. Transgenerational hormetic effects of sublethal dose of flupyradifurone on the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). PLoS ONE 2019, 14, e0208058. [Google Scholar] [CrossRef]
- Yu, H.L.; Li, X.A.; Lan, C.; Li, Q.C.; Sun, Y.L.; Tian, X.J.; Zhu, S.G.; Ren, J.H.; Yan, Z.; Li, W.X.; et al. A sublethal concentration of chitosan oligosaccharide suppresses the population growth of the wheat aphid, Rhopalosiphum padi (Linnaeus). J. Appl. Entomol. 2023, 147, 622–629. [Google Scholar] [CrossRef]
- Moores, G.D.; Gao, X.W.; Denholm, I.; Devonshire, A.L. Characterisation of insensitive acetylcholinesterase in insecticide resistant cotton aphids, Aphis gossypii Glover (Homoptera: Aphididae). Pestic. Biochem. Physiol. 1996, 63, 102–110. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.Z.; Chang, J.; Li, H.P. Toxicity and the effects of four pyrethroid insceticides on the activity of ATPase and GSTs in Aphis sp. Chin. J. Pestic. Sci. 2015, 17, 235–240. [Google Scholar] [CrossRef]
- Chen, X.K.; Xia, X.M.; Wang, H.Y.; Qiao, K.; Wang, K.Y. Cross-resistance to clothianidin and acetamiprid in the imidacloprid-resistant strain of Aphis gossypii (Hemiptera: Aphididae) and the related enzyme mechanisms. Acta Entomol. Sin. 2013, 56, 1143–1151. [Google Scholar] [CrossRef]
- Ren, X.X.; Wang, G.; Zuo, Y.M.; Wang, K.Y.; Wang, J. The toxicity and effects of sublethal doses on detoxifying enzymes of clothianidin to Myzus persicae. Acta Entomol. Sin. 2011, 54, 299–305. [Google Scholar] [CrossRef]
- You, C.X.; Zhang, W.J.; Guo, S.S.; Wang, C.F.; Yang, K.; Liang, J.Y.; Wang, Y.; Geng, Z.F.; Du, S.S.; Deng, Z.W. Chemical composition of essential oils extracted from six Murraya species and their repellent activity against Tribolium castaneum. Ind. Crop. Prod. 2015, 76, 681–687. [Google Scholar] [CrossRef]
- Li, W.Q.; Jiang, C.H.; Chu, S.S.; Zuo, M.X.; Liu, Z.L. Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules 2010, 15, 5831–5839. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, H.; Anmol; Aggarwal, G.; Bhattacharyya, K.; Sharma, U.; Reddy, S.G.E. Cyperus rotundus L.: Invasive weed plant with insecticidal potential against Aphis craccivora Koch and Planococcus lilacinus (Cockerell). Pestic. Biochem. Physiol. 2024, 198, 105720. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Shen, Z.R. Selective toxicity of four insecticides on green peach aphid and predator multicolored Asian ladybird and the coordination evaluation of biological &chemical control to insect pest. Chin. J. Pestic. Sci. 2002, 4, 34–38. [Google Scholar]
- Dong, J.T.; Li, H.X.; Wang, G.Q.; Li, H.M.; Zhao, Y.L.; Li, X.A.; Xu, L.; Zhou, F.; Liu, R.Q. Construction of pyrethrin nanopesticides based on zeolite Imidazole-8 framework via one-pot method for effectively wheat aphid control. Ind. Crops Prod. 2025, 224, 120424. [Google Scholar] [CrossRef]
- Choupanian, M.; Omar, D.; Basri, M.; Asib, N. Preparation and characterization of neem oil nanoemulsion formulations against Sitophilus oryzae and Tribolium castaneum adults. J. Pestic. Sci. 2017, 42, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Ma, J.Y.; Chen, R.; Lu, J.Z. Botanical pesticides matrine nano preparation and the preventive effects of aphid preliminary exploration. Anhui Agric. Sci. Bull. 2011, 17, 116–117. [Google Scholar] [CrossRef]

| Time (h) | Corrected Mortality Rate (%) | ||||
|---|---|---|---|---|---|
| Concentration (μg/head) | |||||
| 4.60 | 7.80 | 11.00 | 14.20 | 17.40 | |
| 4 | 32.33 ± 2.33 d | 48.67 ± 2.96 c | 56.67 ± 2.03 c | 72.33 ± 2.33 b | 90.00 ± 1.73 a |
| 8 | 30.30 ± 1.58 d | 46.58 ± 2.45 c | 56.67 ± 2.03 c | 69.25 ± 3.02 b | 86.76 ± 2.03 a |
| 24 | 28.67 ± 2.96 d | 49.00 ± 4.16 c | 55.56 ± 2.03 bc | 69.00 ± 5.86 ab | 80.00 ± 1.73 a |
| Time (h) | Toxicity Regression Curves | Slope | R2 | LD50 (μg/head) | 95% CI (μg/head) | χ2 | p | df |
|---|---|---|---|---|---|---|---|---|
| 4 | y = 2.56x − 2.33 | 2.56 ± 0.28 | 0.98 | 8.09 | 4.99–10.62 | 8.53 | 0.036 | 3 |
| 8 | y = 2.49x − 2.27 | 2.49 ± 0.28 | 0.99 | 8.13 | 5.48–10.36 | 6.58 | 0.086 | 3 |
| 24 | y = 2.26x − 2.18 | 2.26 ± 0.29 | 0.98 | 9.23 | 8.13–10.33 | 1.08 | 0.781 | 3 |
| Time (h) | Corrected Mortality Rate (%) | ||||
|---|---|---|---|---|---|
| Concentration (μg/head) | |||||
| 0.0035 | 0.0089 | 0.0222 | 0.0555 | 0.1387 | |
| 4 | 9.57 ± 0.08 d | 13.04 ± 1.42 d | 20.87 ± 1.67 c | 28.70 ± 2.25 b | 60.00 ± 3.33 a |
| 8 | 13.16 ± 1.68 d | 18.42 ± 2.21 d | 29.82 ± 1.43 c | 53.51 ± 2.21 b | 80.70 ± 3.04 a |
| 24 | 20.18 ± 1.68 d | 24.56 ± 3.04 d | 50.00 ± 4.15 c | 66.67 ± 3.36 b | 90.35 ± 1.68 a |
| Time (h) | Toxicity Regression Curves | Slope | R2 | LD50 (μg/head) | 95% CI (μg/head) | χ2 | p | df |
|---|---|---|---|---|---|---|---|---|
| 4 | y = 1.02x + 0.94 | 1.02 ± 0.13 | 0.99 | 0.12 | 0.06–1.30 | 7.22 | 0.067 | 3 |
| 8 | y = 1.31x + 1.82 | 1.31 ± 0.13 | 0.98 | 0.04 | 0.02–0.08 | 6.59 | 0.092 | 3 |
| 24 | y = 1.35x + 2.24 | 1.35 ± 0.12 | 0.98 | 0.02 | 0.01–0.04 | 6.64 | 0.092 | 3 |
| Time (h) | Toxicity Regression Curves | Slope | R2 | LD50 (μg/head) | 95% CI (μg/head) | χ2 | p | df |
|---|---|---|---|---|---|---|---|---|
| 24 | y = 2.88x − 2.852 | 2.88 ± 0.34 | 0.99 | 9.779 | 5.922–10.653 | 4.89 | 0.180 | 3 |
| Treatment | Concentration (μg/head) | Corrected Mortality Rate (%) | ||
|---|---|---|---|---|
| 4 h | 8 h | 24 h | ||
| l-Caryophyllene | 4.5 | 30.25 ± 2.12 c | 24.37 ± 4.23 c | 26.89 ± 4.42 c |
| 7.7 | 42.86 ± 6.29 c | 42.02 ± 7.05 b | 42.86 ± 7.64 b | |
| 10.8 | 59.66 ± 1.37 b | 59.66 ± 1.37 a | 59.66 ± 1.37 a | |
| 14 | 63.03 ± 3.07 b | 62.18 ± 2.87 a | 61.34 ± 2.91 a | |
| 17.1 | 78.99 ± 7.05 a | 72.27 ± 7.69 a | 70.59 ± 12.69 a | |
| Lily aldehyde | 1.3 | 17.95 ± 4.19 d | 15.38 ± 3.52 d | 14.66 ± 3.55 d |
| 2.9 | 40.17 ± 2.96 c | 38.46 ± 4.63 c | 32.76 ± 2.99 c | |
| 4.6 | 64.10 ± 4.30 b | 41.00 ± 5.31 b | 62.07 ± 5.08 b | |
| 6.2 | 69.23 ± 1.97 b | 68.38 ± 1.64 b | 62.93 ± 1.65 b | |
| 7.8 | 83.76 ± 1.64 a | 81.20 ± 2.21 a | 76.72 ± 3.26 a | |
| α-Terpineol | 1.4 | 11.76 ± 0.84 e | 10.92 ± 1.68 e | 10.08 ± 1.61 d |
| 4.7 | 36.97 ± 5.02 d | 38.66 ± 5.88 d | 38.66 ± 6.91 c | |
| 8 | 63.03 ± 4.34 c | 56.30 ± 7.88 c | 50.42 ± 7.56 c | |
| 11.2 | 73.95 ± 2.97 b | 73.11 ± 3.07 b | 69.75 ± 4.12 b | |
| 14.5 | 89.92 ± 2.38 a | 90.76 ± 1.61 a | 85.71 ± 0.84 a | |
| Cineole | 4.7 | 18.80 ± 2.15 c | 18.10 ± 2.17 c | 19.83 ± 1.65 c |
| 8 | 23.93 ± 6.45 bc | 25.86 ± 7.78 bc | 31.03 ± 9.01 bc | |
| 11.2 | 35.90 ± 4.27 b | 36.21 ± 4.98 b | 40.52 ± 3.82 b | |
| 14.5 | 59.83 ± 1.64 a | 56.03 ± 2.17 a | 60.34 ± 2.99 a | |
| 17.8 | 67.52 ± 7.58 a | 67.24 ± 8.27 a | 65.52 ± 7.84 a | |
| Treatment | Time (h) | Toxicity Regression Curves | Slope | R2 | LD50 (μg/head) | 95% CI (μg/head) | χ2 | p | df |
|---|---|---|---|---|---|---|---|---|---|
| l-Caryophyllene | 4 | y = 2.14x − 1.98 | 2.14 ± 0.27 | 0.99 | 8.49 | 7.45–9.51 | 3.67 | 0.299 | 3 |
| 8 | y = 2.18x − 2.11 | 2.18 ± 0.27 | 0.98 | 9.27 | 8.23–10.36 | 1.27 | 0.737 | 3 | |
| 24 | y = 1.97x − 1.90 | 1.97 ± 0.27 | 0.98 | 9.16 | 8.01–10.35 | 1.28 | 0.734 | 3 | |
| Lily aldehyde | 4 | y = 2.37x − 1.26 | 2.37 ± 0.23 | 0.98 | 3.40 | 3.01–3.79 | 2.71 | 0.438 | 3 |
| 8 | y = 2.42x − 1.34 | 2.42 ± 0.23 | 0.99 | 3.57 | 3.18–3.97 | 2.03 | 0.567 | 3 | |
| 24 | y = 2.31x − 1.38 | 2.31 ± 0.24 | 0.98 | 3.96 | 3.52–4.44 | 4.20 | 0.240 | 3 | |
| α-Terpineol | 4 | y = 2.33x − 1.72 | 2.33 ± 0.19 | 0.98 | 5.50 | 3.71–7.41 | 7.82 | 0.050 | 3 |
| 8 | y = 2.33x − 1.76 | 2.33 ± 0.20 | 0.99 | 5.71 | 3.57–8.11 | 10.34 | 0.016 | 3 | |
| 24 | y = 2.17x − 1.73 | 2.17 ± 0.20 | 0.99 | 6.27 | 4.22–8.75 | 8.14 | 0.043 | 3 | |
| Cineole | 4 | y = 2.54x − 2.82 | 2.54 ± 0.31 | 0.99 | 12.88 | 9.56–22.26 | 9.12 | 0.028 | 3 |
| 8 | y = 2.46x − 2.75 | 2.46 ± 0.31 | 0.98 | 13.12 | 10.34–19.44 | 5.84 | 0.120 | 3 | |
| 24 | y = 2.28x − 2.48 | 2.28 ± 0.30 | 0.99 | 12.31 | 11.02–14.00 | 3.12 | 0.373 | 3 |
| Time (h) | Corrected Mortality Rate (%) | ||||
|---|---|---|---|---|---|
| Concentration (μg/head) | |||||
| 2.90 | 5.70 | 11.50 | 23.00 | 45.90 | |
| 4 | 00.00 ± 0.00 d | 10.00 ± 4.10 cd | 20.00 ± 0.00 bc | 27.50 ± 4.80 b | 52.50 ± 4.80 a |
| 8 | 00.00 ± 0.00 d | 12.50 ± 2.50 cd | 20.00 ± 0.00 bc | 27.50 ± 4.80 b | 62.50 ± 8.50 a |
| 24 | 12.50 ± 2.50 c | 22.50 ± 2.50 bc | 25.00 ± 2.50 bc | 32.50 ± 4.80 b | 65.00 ± 9.60 a |
| 48 | 22.50 ± 4.80 c | 32.50 ± 4.80 bc | 32.50 ± 2.50 bc | 42.50 ± 8.50 b | 67.50 ± 7.50 a |
| Time (h) | Toxicity Regression Curves | Slope | R2 | LD50 (μg/head) | 95% CI (μg/head) | χ2 | p | df |
|---|---|---|---|---|---|---|---|---|
| 4 | y = 1.75x − 2.89 | 1.75 ± 0.30 | 0.97 | 44.80 | 32.06–80.17 | 2.26 | 0.520 | 3 |
| 8 | y = 1.88x − 2.96 | 1.88 ± 0.34 | 0.98 | 37.34 | 27.97–58.77 | 5.17 | 0.180 | 3 |
| 24 | y = 1.12x − 1.76 | 1.12 ± 0.24 | 0.98 | 36.71 | 23.68–83.77 | 4.99 | 0.292 | 3 |
| 48 | y = 0.86x − 1.22 | 0.86 ± 0.22 | 0.97 | 26.07 | 15.94–67.68 | 3.85 | 0.441 | 3 |
| Time (h) | Corrected Mortality Rate (%) | ||||
|---|---|---|---|---|---|
| Concentration (μg/head) | |||||
| 0.0444 | 0.0666 | 0.0887 | 0.1109 | 0.1331 | |
| 4 | 15.00 ± 2.90 e | 27.50 ± 2.50 d | 47.50 ± 2.50 c | 67.50 ± 2.50 b | 77.50 ± 2.50 a |
| 8 | 22.50 ± 4.80 d | 40.00 ± 4.10 c | 57.50 ± 4.80 b | 72.50 ± 2.50 a | 80.00 ± 4.10 a |
| 24 | 30.80 ± 2.60 c | 41.00 ± 4.90 c | 69.20 ± 4.20 b | 82.10 ± 2.60 a | 84.60 ± 3.00 a |
| 48 | 32.40 ± 2.70 d | 43.20 ± 2.70 c | 70.30 ± 2.70 b | 83.80 ± 3.10 a | 86.50 ± 2.70 a |
| Time (h) | Toxicity Regression Curves | Slope | R2 | LD50 (μg/head) | 95% CI (μg/head) | χ2 | p | df |
|---|---|---|---|---|---|---|---|---|
| 4 | y = 3.93x + 4.15 | 3.93 ± 0.61 | 0.98 | 0.09 | 0.08–0.10 | 0.91 | 0.822 | 3 |
| 8 | y = 3.42x + 3.82 | 3.42 ± 0.58 | 0.99 | 0.08 | 0.07–0.09 | 0.19 | 0.979 | 3 |
| 24 | y = 3.55x + 4.18 | 3.55 ± 0.61 | 0.97 | 0.07 | 0.06–0.08 | 1.97 | 0.580 | 3 |
| 48 | y = 3.62x + 4.31 | 3.62 ± 0.65 | 0.98 | 0.07 | 0.05–0.07 | 1.70 | 0.637 | 3 |
| Time (d) | Population Decline Rate (%) | ||
|---|---|---|---|
| Treatment | |||
| EFEO Nanoemulsion | 10% EFEO | CK | |
| 1 | 54.00 ± 6.11 a | 32.67 ± 6.57 b | 0.00 ± 0.00 c |
| 3 | 73.33 ± 4.80 a | 48.00 ± 4.16 b | 0.00 ± 0.00 c |
| 5 | 74.67 ± 4.67 a | 60.00 ± 2.31 b | 6.00 ± 2.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Lü, D.; Ge, X.; Zhang, Z.; Meng, F.; Chen, L.; Kuanysh, K.; Li, X.; Zhang, B.; Dani, S.; et al. Insecticidal Activity of Eupatorium fortunei Essential Oil Against Schizaphis graminum and Its Effects on Detoxification Enzymes. Insects 2025, 16, 1141. https://doi.org/10.3390/insects16111141
Wang G, Lü D, Ge X, Zhang Z, Meng F, Chen L, Kuanysh K, Li X, Zhang B, Dani S, et al. Insecticidal Activity of Eupatorium fortunei Essential Oil Against Schizaphis graminum and Its Effects on Detoxification Enzymes. Insects. 2025; 16(11):1141. https://doi.org/10.3390/insects16111141
Chicago/Turabian StyleWang, Guochang, Dongbiao Lü, Xing Ge, Ziyue Zhang, Fanning Meng, Liuping Chen, Kassen Kuanysh, Xinan Li, Baizhong Zhang, Sarsekova Dani, and et al. 2025. "Insecticidal Activity of Eupatorium fortunei Essential Oil Against Schizaphis graminum and Its Effects on Detoxification Enzymes" Insects 16, no. 11: 1141. https://doi.org/10.3390/insects16111141
APA StyleWang, G., Lü, D., Ge, X., Zhang, Z., Meng, F., Chen, L., Kuanysh, K., Li, X., Zhang, B., Dani, S., & Wang, H. (2025). Insecticidal Activity of Eupatorium fortunei Essential Oil Against Schizaphis graminum and Its Effects on Detoxification Enzymes. Insects, 16(11), 1141. https://doi.org/10.3390/insects16111141






