Efficiency Enhancement Technology of Dastarcus helophoroides (Coleoptera: Bothrideridae) for Controlling Monochamus alternatus (Coleoptera: Cerambycidae): Drilling Optimization and Biological Collaboration
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment Materials
2.2. Separate Release Treatment of Dastarcus helophoroides Eggs and Adults
2.3. Release Treatment of Dastarcus helophoroides Adults After Drilling Holes in Lure Logs
2.4. Release Treatment of Dastarcus helophoroides Adults Carrying Synergistic Species
2.5. Statistical Analysis
3. Results
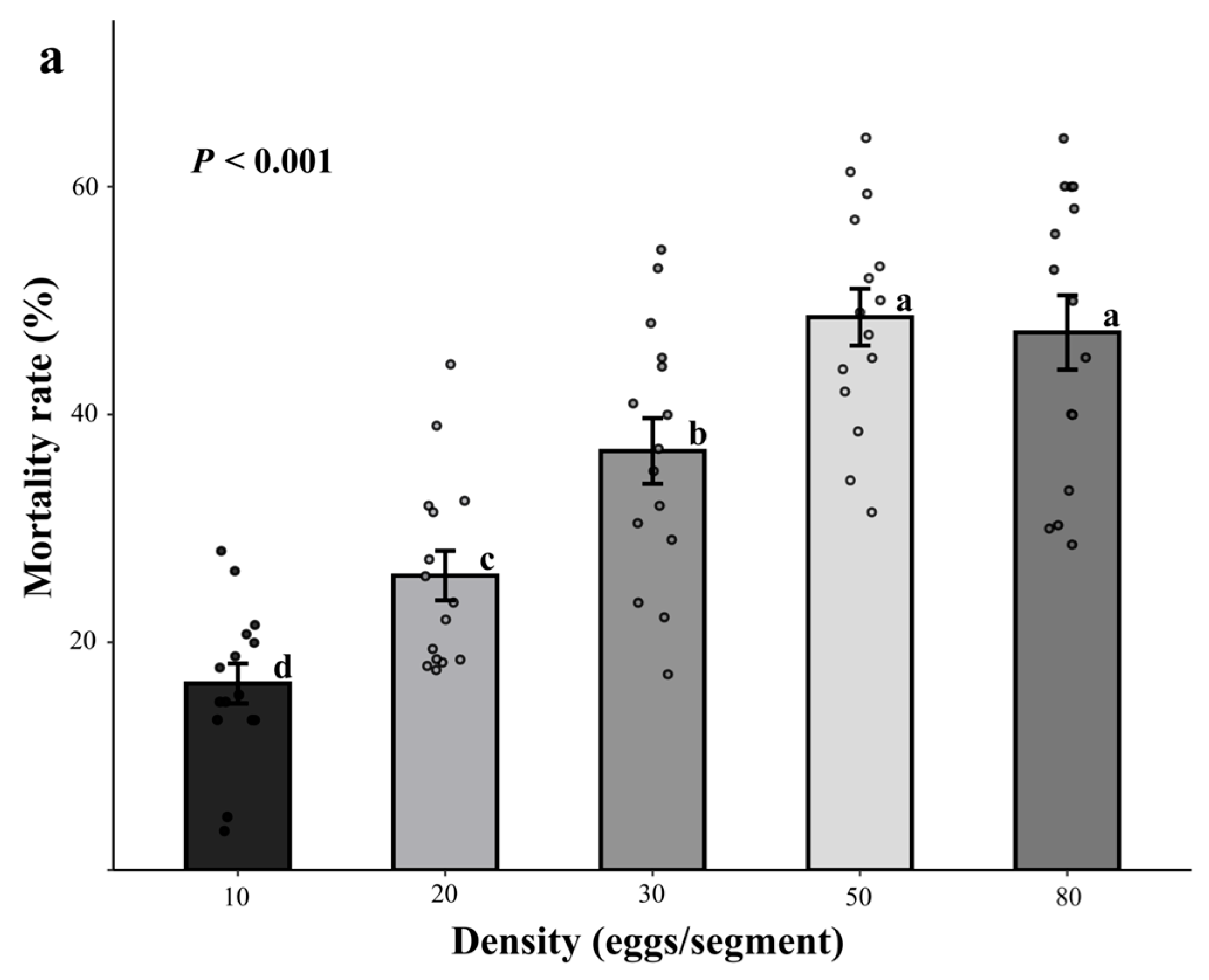
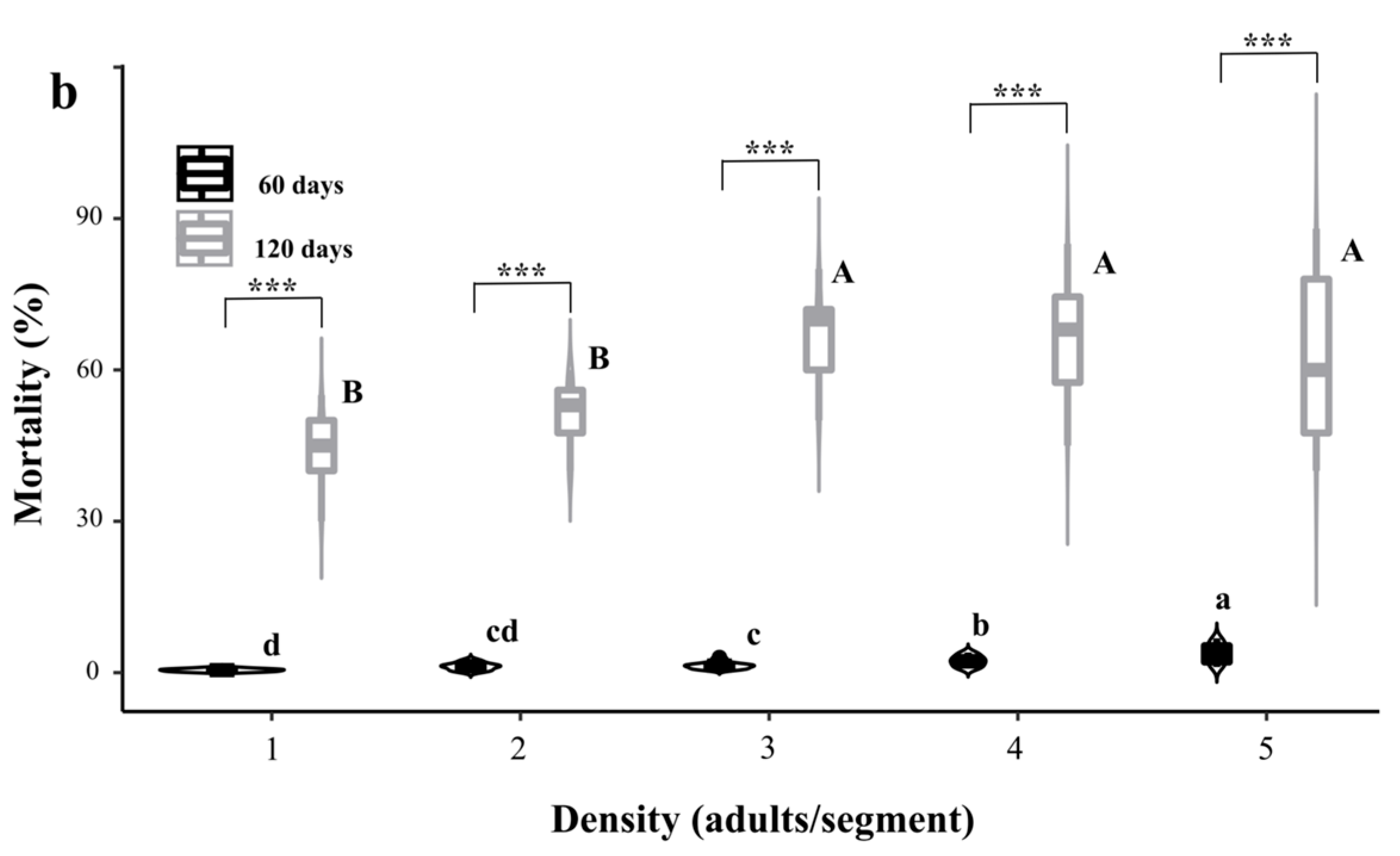
3.1. Control Efficacy of Releasing Dastarcus helophoroides Separately Under Different Developmental Stages and Densities
3.1.1. Effect of Releasing Eggs
3.1.2. Effect of Releasing Adults
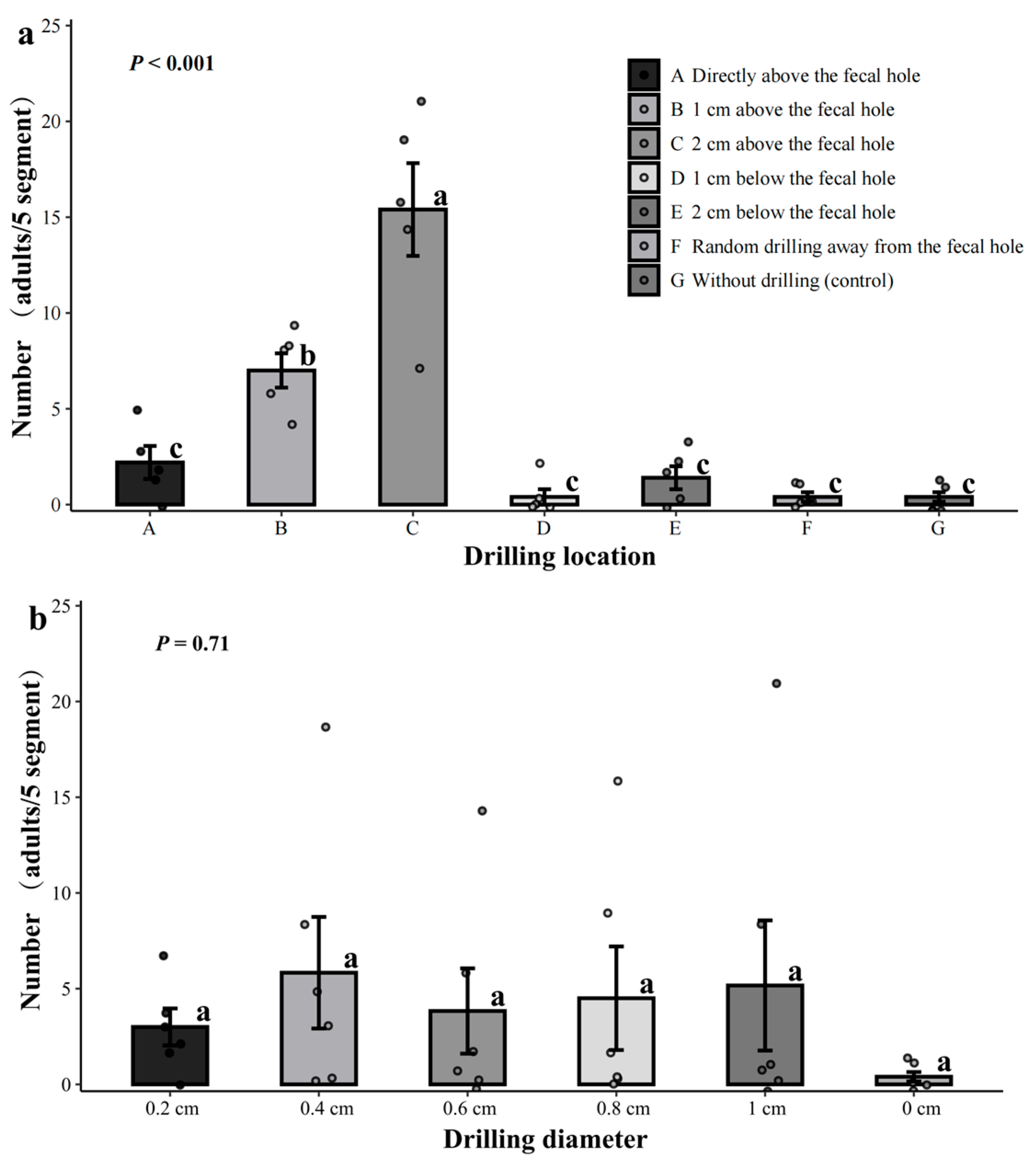
3.2. Control Efficacy of Drill Hole on Dastarcus helophoroides Searching and Parasitic Behavior
3.2.1. Effect of Lure Logs Drill Hole Location and Diameter
3.2.2. Effect of Lure Logs Drill Hole Number
3.3. Synergistic Control Efficacy of Dastarcus helophoroides
3.3.1. Effect of Carrying Pyemotes zhonghuajia
3.3.2. Effect of Carrying Beauveria bassiana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PWN | Pine wood nematode (Bursaphelenchus xylophilus) |
| PWD | Pine wilt disease |
References
- Yang, B.J.; Wang, L.F.; Zhao, W.X.; Xu, F.Y.; Zhang, P.; Li, Z.P. The Latent Infection of Bursaphelenchus xylophilus and a New Transmission Way of PWN by Monochamus alternatus. For. Res. 2002, 15, 251–255. (In Chinese) [Google Scholar]
- Yang, H.W.; Gao, S.K.; Wang, J.X.; Li, W.; Hou, Q.F.; Qiu, J.F. Three-Dimensional Reconstruction of Monochamus alternatus Galleries Using CT Scans. Insects 2022, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Fang, Y.; Tang, J.; Sun, J.H. Advances in research on Bursaphelenchus xylophilus and its key vector Monochamus spp. Entomol. Knowl. 2004, 41, 97–104. (In Chinese) [Google Scholar]
- Akbulut, S.; Stamps, W.T. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Xu, Q.W. Study on Critical Risky Issues in Managing Chinese Forest Invasive Species. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2025. (In Chinese). [Google Scholar]
- Zhang, J.J.; Zhang, R.Z.; Chen, J.Y. Species and dispersal ability of vector insects of Bursaphelenchus xylophilus. J. Zhejiang For. Coll. 2007, 24, 350–356. (In Chinese) [Google Scholar]
- Sun, Y.C. Pine wood nematode found in Sun Yat-sen Mausoleum. Nanjing. J. Jiangsu For. Sci. Technol. 1982, 9, 47. (In Chinese) [Google Scholar]
- Xu, Q.W.; Zhang, X.J.; Li, J.X.; Ren, J.R.; Ren, L.L.; Luo, Y.Q. Pine Wilt Disease in Northeast and Northwest China: A comprehensive risk review. Forests 2023, 14, 174. [Google Scholar] [CrossRef]
- Liu, F.; Su, H.J.; Ding, T.T.; Huang, J.X.; Liu, T.; Ding, N.; Fang, G.F. Refined assessment of economic loss from Pine Wilt Disease at the subcompartment scale. Forests 2023, 14, 139. [Google Scholar] [CrossRef]
- Hao, D.J.; Yang, J.X.; Dai, H.G. Research progress and prospect on chemical ecology of Monochamus alternatus. Chin. J. Ecol. 2008, 27, 1227–1233. (In Chinese) [Google Scholar]
- Zheng, Y.N.; Yang, Z.Q.; Wang, X.Y. Mechanism of chemical ecology of Bursaphelenchus xylophilus vectored by Monochamus alternatus. Plant Prot. 2014, 40, 12–15+29. (In Chinese) [Google Scholar]
- Shi, X.H.; Ma, T.; Lu, X.L.; Shen, J.; Sun, Z.H.; Wen, X.J.; Deng, P.X. Research progress in adult behavior and chemical ecology of Monochamus alternatus. For. Res. 2017, 30, 854–865. (In Chinese) [Google Scholar]
- Zhu, C.Q.; Wang, B.; Shen, J.; Mou, J.; Qin, W.Q.; Wen, X.J. Research progress on integrated controlling techniques against Monochamus alternatus. China Plant Prot. 2017, 37, 19–24. (In Chinese) [Google Scholar]
- Liu, X.; Song, J.; Li, X.S.; Xu, H.L.; Wang, Z.S. Current status of research on control methods of Monochamus alternatus Hope (Coleoptera: Cerambycidae). Int. Core J. Eng. 2008, 8, 21–29. [Google Scholar] [CrossRef]
- Dai, J.C.; Zhao, J.N.; Zhang, G.X.; Chen, X.Z.; Wu, Z.L.; Bao, L.F. Study on chemical control of Monochamus alternatus. For. Res. 1998, 12, 412–416. (In Chinese) [Google Scholar]
- Zhang, H.F.; Wei, Z.Y.; Wei, C.J.; Huang, H.X.; Cai, J.B.; Hong, Z.Y. Experiment against Monochamus alternatus with thiacloprid 1% micro-capsule granular in the forest. For. Pest Dis. 2010, 29, 35–37. (In Chinese) [Google Scholar]
- Suh, D.Y.; Jung, J.K.; Lee, S.K.; Seo, S.T. Effect of aerial spraying of thiacloprid on pine sawyer beetles (Monochamus alternatus) and honey bees (Apis mellifera) in pine forests. Entomol. Res. 2021, 51, 83–89. [Google Scholar] [CrossRef]
- Hao, D.J.; Fan, B.Q.; Tang, J.G.; Wang, Y.; Ma, F.L. Screening of attractant for Monochamus alternatus and its attractaction effects. J. Northeast For. Univ. 2009, 37, 86–87. (In Chinese) [Google Scholar]
- Hu, Q. Study on the Function Characteristics of Semiochemicals and the Evaluation of Trapping Efficacy of Monochamus alternatus. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2019. (In Chinese). [Google Scholar]
- Feng, Y.; Hu, S.Y.; Mao, Y.Y.; Ma, T. Research advance on prevention and control technology of Monochamus alternatus in China. Hunan For. Sci. Technol. 2022, 49, 81–86. (In Chinese) [Google Scholar]
- Zhang, Y.N. Investigation on Insect Natural Enemies of Monochamus alternatus Hope (Coleoptera: Cerambycidae) and Studies on Bio-Control of the Pest. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2006. (In Chinese). [Google Scholar]
- Zhang, Y.L.; Wang, X.Y.; Yang, Z.Q.; Wei, K.; Cao, L.M. Research progress on natural enemies and their application of the vector insects of Bursaphelenchus xylophilus. For. Pest Dis. 2022, 41, 21–29. (In Chinese) [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N. Recent advances in biological control of important native and invasive forest pests in China. Biol. Control 2014, 68, 117–128. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N.; Zhang, Y.L. Research advances of Chinese major forest pests by integrated management based on biological control. Chin. J. Biol. Control 2018, 34, 163–183. (In Chinese) [Google Scholar]
- Tang, Y.L.; Yang, Z.Q.; Wang, X.Y.; Tang, H.; Jiang, J.; Wei, K.; Lv, J. Biocontrol of oak longhorn beetle, Massicus raddei by releasing parasitoid Dastarcus helophoroides (Coleoptera: Bothrideridae) adults and eggs. Sci. Silvae Sin. 2012, 48, 186–191. (In Chinese) [Google Scholar]
- Li, X.J.; Dong, G.P.; Fang, J.M.; Liu, H.J.; Yang, L.; Guo, W.L. Host foraging behavior of Dastarcus helophoroides (Coleoptera: Bothrideridae) adults, a Coleopteran parasitoid of Monochamus alternatus (Coleoptera: Cerambycidae). J. Insect Behav. 2016, 29, 108–116. [Google Scholar] [CrossRef]
- Wen, X.S.; Liao, S.L.; Tang, Y.L.; Yang, Z.Q. Study on the efficacy of releasing Dastarcus helophoroides eggs against Monochamus alternatus in pine forests. Sci. Silvae Sin. 2017, 53, 133–138. (In Chinese) [Google Scholar]
- Yan, X.W. A Study on the Artificial Diet and Application Technology of Dastarcus helophoroides Fairmaire (Coleoptera: Bothrideridae). Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2014. (In Chinese). [Google Scholar]
- Liu, H.J.; Piao, C.G.; Wang, L.F.; Shin, S.C.; Chung, Y.J.; Shu, Q.L. Biocontrol of Monochamus alternatus by Beauveria bassiana and Scleroderma guani. Sci. Silvae Sin. 2007, 43, 64–68. (In Chinese) [Google Scholar]
- Deng, J.D.; Zhuang, W.X.; Liu, Y.J.; Song, L.W.; Zhang, L.W. Pathogenicity of white muscardine fungus Beauveria bassiana against Japanese pine sawyer beetle Monochamus alternatus and its compatibility with ectoparasitic beetle Dastarcus helophoroides. J. Plant Prot. 2021, 48, 602–609. (In Chinese) [Google Scholar]
- Gong, B.Y.; Liao, W.; Long, X.Y.; Xiang, G.Z.; Zeng, B.; Liu, H.; Deng, S.F.; Cao, S.; Xiao, F.L.; Yang, S.Z. Control effects of Dastarcus helophoroides against longhorn beetles in citrus orchard. China Plant Prot. 2023, 43, 61–65. (In Chinese) [Google Scholar]
- Yang, X.T. Study on the Mechanism of Regulating the Immune of Monochamus alternatus Larvae at the Immature Stages of Dastarcus helophoroides Based on Hypermetamorphosis. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2023. (In Chinese). [Google Scholar]
- Wang, L.N.; Cao, P.; Tang, Y.L.; Qian, Z.M.; Liu, L.L.; Wei, K.; Zhang, Y.L. Parasitism of prepupae of Monochamus alternatus (Coleoptera: Cerambycidae) by Dastarcus helophoroides (Coleoptera: Bothrideridae). Chin. J. Biol. Control 2025, 41, 674–679. (In Chinese) [Google Scholar]
- Zhang, Y.L.; Yang, Z.Q.; Zhang, Y.N.; Wang, X.Y.; Wu, C.J.; Ma, S.F.; Lu, Z.G. Biocontrol of the overwinter Monochamus alternatus with Dastarcus helophoroides. Sci. Silvae Sin. 2014, 50, 92–98. (In Chinese) [Google Scholar]
- Qu, Z.X.; Huang, Y.Q.; Zhao, H.; Chen, Z.Q.; Pan, T.; Hao, C.W.; Gao, S.K. Study on the control of Aromia bungii Fald by the combined release of adult and egg of Dastarcus helophoroides (Fairmaire). J. Shandong Agri. Univ. (Nat. Sci. Ed.) 2022, 53, 863–867. (In Chinese) [Google Scholar]
- Mi, F.; Wang, M.Q. Identification volatile compounds involved in host location by Dastarcus helophoroides. Chin. J. Biol. Control 2020, 36, 685–689. (In Chinese) [Google Scholar]
- Liu, Y.; Zhao, Z.P.; Zhang, M.; Yan, X.W. Review on host-searching behavior of Dastarcus helophoroides. Shandong For. Sci. Technol. 2021, 51, 94–97+93. (In Chinese) [Google Scholar]
- Wen, X.S.; Song, D.F.; Yang, Z.Q.; Wang, Z.H.; Shi, M.Q. Relationships between the emergence of Dastarcus helophoroides (Coleoptera: Bothrideridae) and the emergence of the host Monochamus alternatus (Coleoptera: Cerambycidae) in Pinus massoniana forests. Sci. Silvae Sin. 2020, 56, 193–200. (In Chinese) [Google Scholar]
- Guo, Z.; Wang, Q.Z.; Peng, G.D.; Zhong, H.H.; Zhang, J.T.; Luo, Z.D.; Chen, Y.S.; Liu, X.P. Effects of adult body size on reproductive fitness and offspring development in Dastarcus helophoroides (Fairmaire). Chin. J. Biol. Control 2024, 40, 33–43. (In Chinese) [Google Scholar]
- Cui, Y.S.; Liu, Y.P.; Song, Y.S.; Xu, F.Y.; Xu, K.Q. Control of Monochamus alternatus with natural enemies. For. Pest Dis. 2011, 30, 31–33. (In Chinese) [Google Scholar]
- Lu, J.F.; Zhao, B.; Ren, X.M.; Cai, J.Y.; Wang, X.Y. Influence of host-searching distance, inoculation proportion and interspecific interference on the parasitism of Dastarcus helophoroides. For. Pest Dis. 2025, 44, 48–51. (In Chinese) [Google Scholar]
- Guo, X.; Xu, Z.C.; Xiong, D.P. Study of utilizing Pyemotes zhonghuajia to control Semanotus bifasciatus beetles. Chin. Bull. Entomol. 2010, 47, 529–532. (In Chinese) [Google Scholar]
- Shimazu, M.; Kushida, T.; Tsuchiya, D.; Mitsuhashi, W. Microbial control of Monochamus alternatus Hope (Coleoptera: Cerambycidae) by implanting wheat-bran pellets with Beauveria bassiana in infested tree trunks. J. Japan For. Soc. 1992, 74, 325–330. [Google Scholar] [CrossRef]
- Kinuura, H.; Ohya, E.; Makihara, H.; Nagaki, A.; Fujioka, H. Infection rate of Monochamus alternatus Hope (Coleoptera: Cerambycidae) by an entomogenous fungus through mass-release of vector beetles Cryphalus fulvus Niijima (Coleoptera: Scolytidae) using an improved application device. J. Japan For. Soc. 1999, 81, 17–21. [Google Scholar] [CrossRef]
- Hu, Z.C.; Yang, Y.; Ma, L.J.; Sun, S.T. Exploration on the active infection of Monochamus alternatus by Scleroderma sichuanensis carrying Beauveria bassiana. J. Zhejiang. Sci. Technol. 2007, 27, 48–50. (In Chinese) [Google Scholar]
- Yang, Z.; Lu, Y.; Mao, G.; Zhao, Y.; Zhang, Q.H.; Sui, L.; Zhao, Y.; Li, Q.Y.; Zhang, Z.K. Use of Trichogramma phoretic Beauveria bassiana in control of Asian corn borer in field. Chin. J. Biol. Control 2020, 36, 52–57. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Q.Z. Influence of Exogenous Factors on the Behavior and Fitness of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae). Ph.D. Thesis, Jiangxi Agricultural University, Nanchang, China, 2023. (In Chinese). [Google Scholar]
- Chen, Y.S.; Huang, J.L.; Luo, Z.D.; Fan, H.Y. Conditions to rapidly increase Dastarcus helophoroides population in pine forest. Fujian J. Agri. Sci. 2025, 40, 307–315. (In Chinese) [Google Scholar]
- Hu, X.Y. Determing Hunger Tolerance and Related Trehalosein Dastarcus helophoroides Under Artificial Breeding Condition. Master’s Thesis, Northwest A&F University, Yangling, China, 2022. (In Chinese). [Google Scholar]
- Guo, J.L.; Zhang, C.Y.; Zhang, M.; Tang, G.H.; Zhang, Z.Q. Effects of different diets on the growth and development, reproduction and the adult intestinal bacterial community of Dastarcus helophoroides (Coleoptera: Bothrideridae). Acta Entomol. Sin. 2023, 66, 1467–1481. (In Chinese) [Google Scholar]
- Guo, Z. Mass-Rearing Technical of Dastarcus helophoroides (Fairmair)—Effects of Body Size, Sex Ratio and Adult Age. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2023. (In Chinese). [Google Scholar]
- Qiu, H.J. Effects of Aircraft Canopy Spraying on Insect Diversityand Natural Enemy Dastarcus helophoroides in Artificialforest of Pinus massoniana. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2021. (In Chinese). [Google Scholar]
- Yin, H.Y.; Li, X.J.; Dong, G.P.; Guo, W.L.; Wang, Q.T. Study on biological control technology and application based on unmanned aerial vehicle (UAV) delivery of natural enemy insect Dastarcus helophoroides. Anhui For. Sci. Technol. 2023, 49, 10–13. (In Chinese) [Google Scholar]
- Qiu, L.F.; Zhong, L.; Shao, J.L.; Che, S.C.; Li, G.; Wang, J.H.; Wei, J.R. Influence of environmental temperature and adult body size on themortality and fecundity of Dastarcus helophoroides. Chin. J. Appl. Entomol. 2021, 58, 959–965. (In Chinese) [Google Scholar]
| Number of Drilling Holes (Holes/m) | Total Number of Monochamus alternatus in Lure Logs | Total Number of Larvae and Adults of Dastarcus helophoroides | Total Number of Parasitized Monochamus alternatus | Total Predation of Monochamus alternatus | Mortality of Monochamus alternatus (%) |
|---|---|---|---|---|---|
| 1 | 157 | 56 | 41 | 4 | 28.7 ± 2.8 b |
| 2 | 162 | 67 | 54 | 4 | 35.8 ± 2.3 a |
| 3 | 154 | 53 | 50 | 6 | 36.4 ± 1.0 a |
| Release Density (Million Individuals/m) | Mortality of Monochamus alternatus (%) | |
|---|---|---|
| Pyemotes zhonghuajia Carried by Dastarcus helophoroides | Pyemotes zhonghuajia Separate Release | |
| 0.2 | 29.7 ± 1.8 aBC | 15.5 ± 1.4 bBC |
| 0.4 | 48.2 ± 2.7 aAB | 24.5 ± 2.7 bAB |
| 0.6 | 67.6 ± 1.1 aAB | 30.4 ± 1.0 bAB |
| Release Density (g/m) | Mortality of Monochamus alternatus (%) | |
|---|---|---|
| Beauveria bassiana Carried by Dastarcus helophoroides | Beauveria bassiana Separate Spray | |
| 1 | 12.0 ± 1.5 a | 5.2 ± 1.3 b |
| 2 | 11.1 ± 2.5 a | 7.1 ± 2.4 b |
| 3 | 15.2 ± 1.3 a | 8.1 ± 1.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, M.; Li, Z.; Li, X.; Peng, Y.; Zhou, W.; Zhao, Z.; Yan, X. Efficiency Enhancement Technology of Dastarcus helophoroides (Coleoptera: Bothrideridae) for Controlling Monochamus alternatus (Coleoptera: Cerambycidae): Drilling Optimization and Biological Collaboration. Insects 2025, 16, 1138. https://doi.org/10.3390/insects16111138
Li J, Zhang M, Li Z, Li X, Peng Y, Zhou W, Zhao Z, Yan X. Efficiency Enhancement Technology of Dastarcus helophoroides (Coleoptera: Bothrideridae) for Controlling Monochamus alternatus (Coleoptera: Cerambycidae): Drilling Optimization and Biological Collaboration. Insects. 2025; 16(11):1138. https://doi.org/10.3390/insects16111138
Chicago/Turabian StyleLi, Jiale, Min Zhang, Zhilan Li, Xiaohui Li, Yong Peng, Wenxiu Zhou, Zhengping Zhao, and Xuewu Yan. 2025. "Efficiency Enhancement Technology of Dastarcus helophoroides (Coleoptera: Bothrideridae) for Controlling Monochamus alternatus (Coleoptera: Cerambycidae): Drilling Optimization and Biological Collaboration" Insects 16, no. 11: 1138. https://doi.org/10.3390/insects16111138
APA StyleLi, J., Zhang, M., Li, Z., Li, X., Peng, Y., Zhou, W., Zhao, Z., & Yan, X. (2025). Efficiency Enhancement Technology of Dastarcus helophoroides (Coleoptera: Bothrideridae) for Controlling Monochamus alternatus (Coleoptera: Cerambycidae): Drilling Optimization and Biological Collaboration. Insects, 16(11), 1138. https://doi.org/10.3390/insects16111138





