Diversity and Influencing Factors of Endosymbiotic Bacteria in Tetranychus truncatus Sourced from Major Crops in Xinjiang
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Populations
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Data Analysis
3. Results
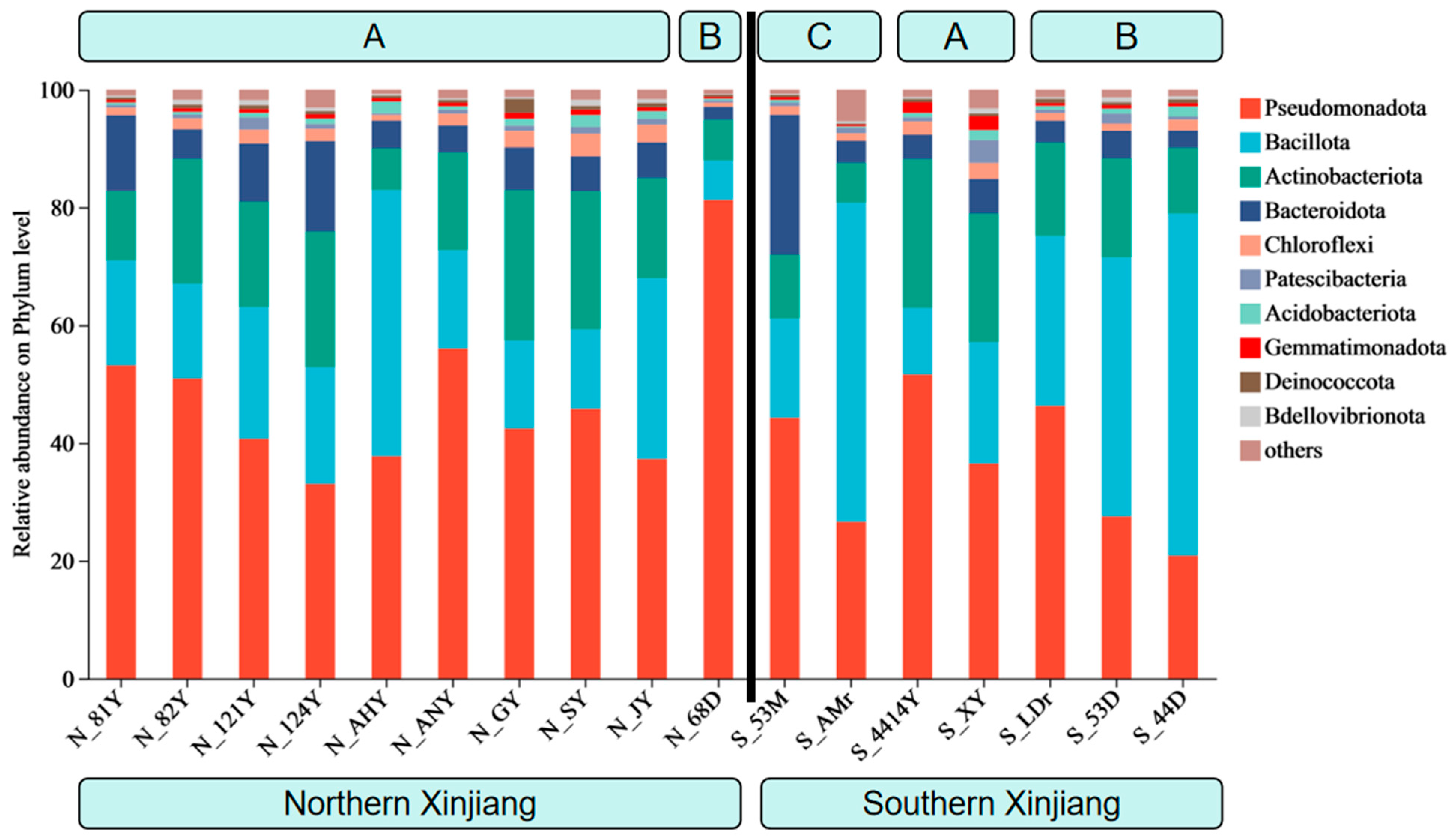
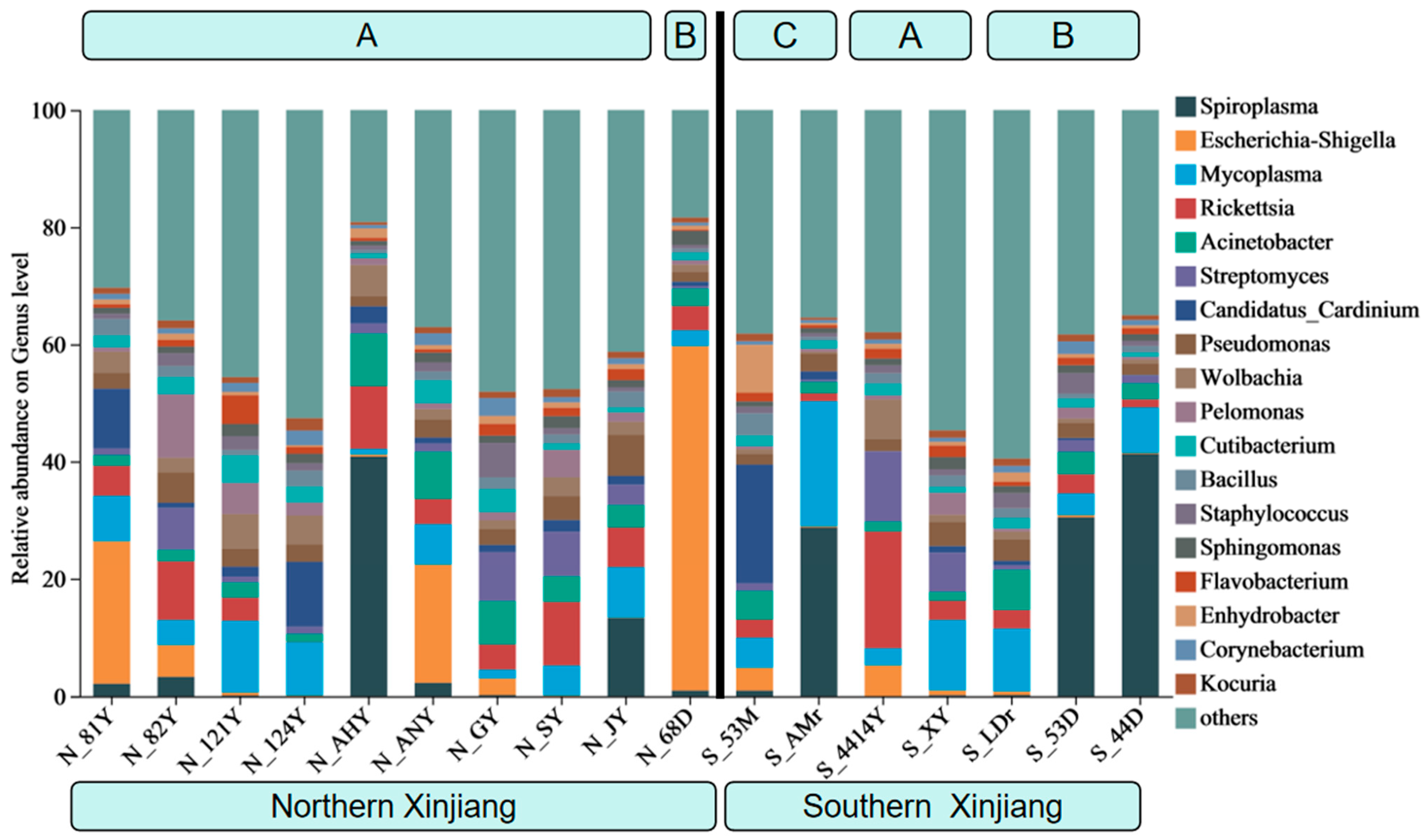
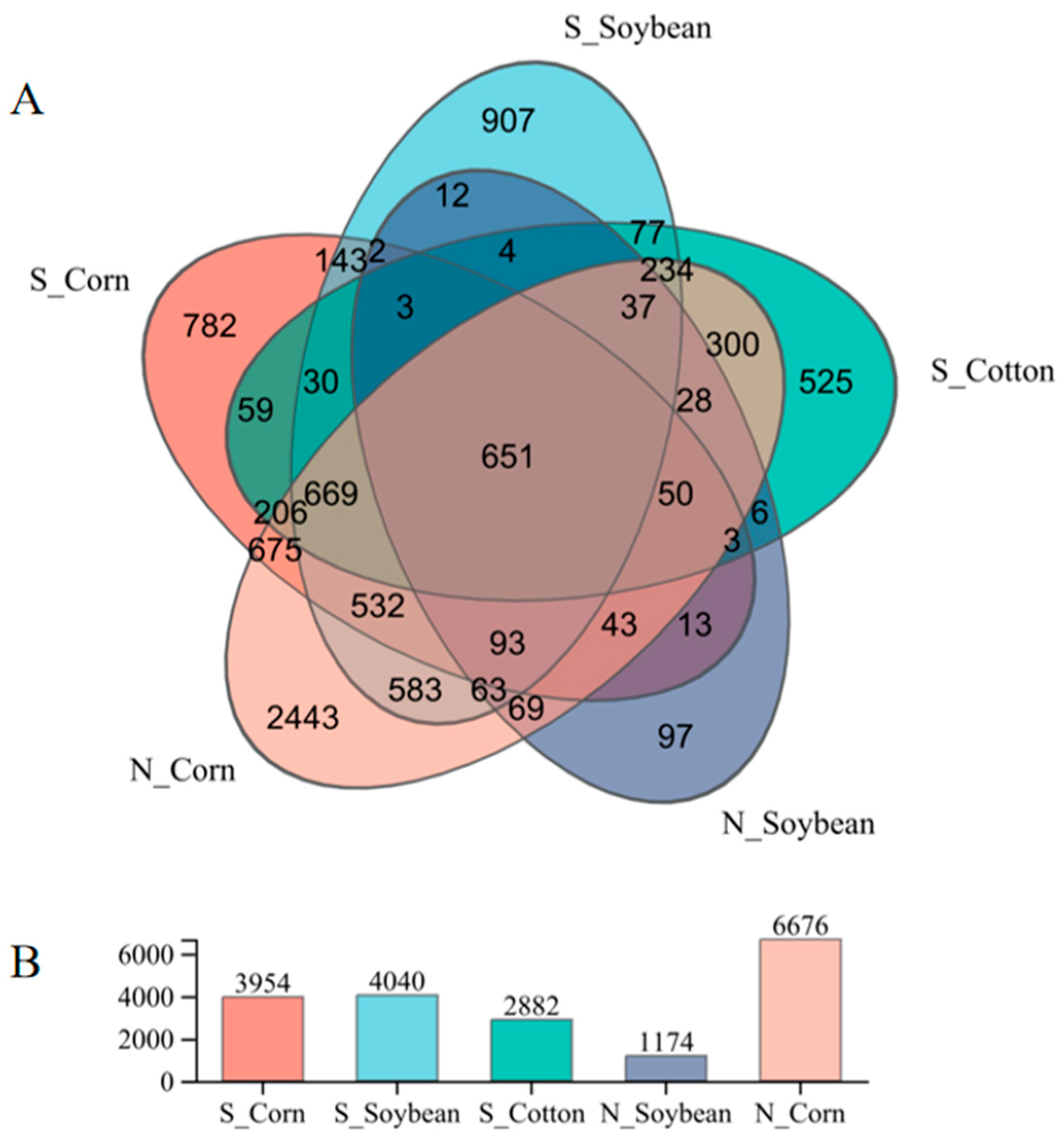
3.1. The Composition of Endosymbiotic Bacterial Communities in Different Populations of T. truncatus in Xinjiang
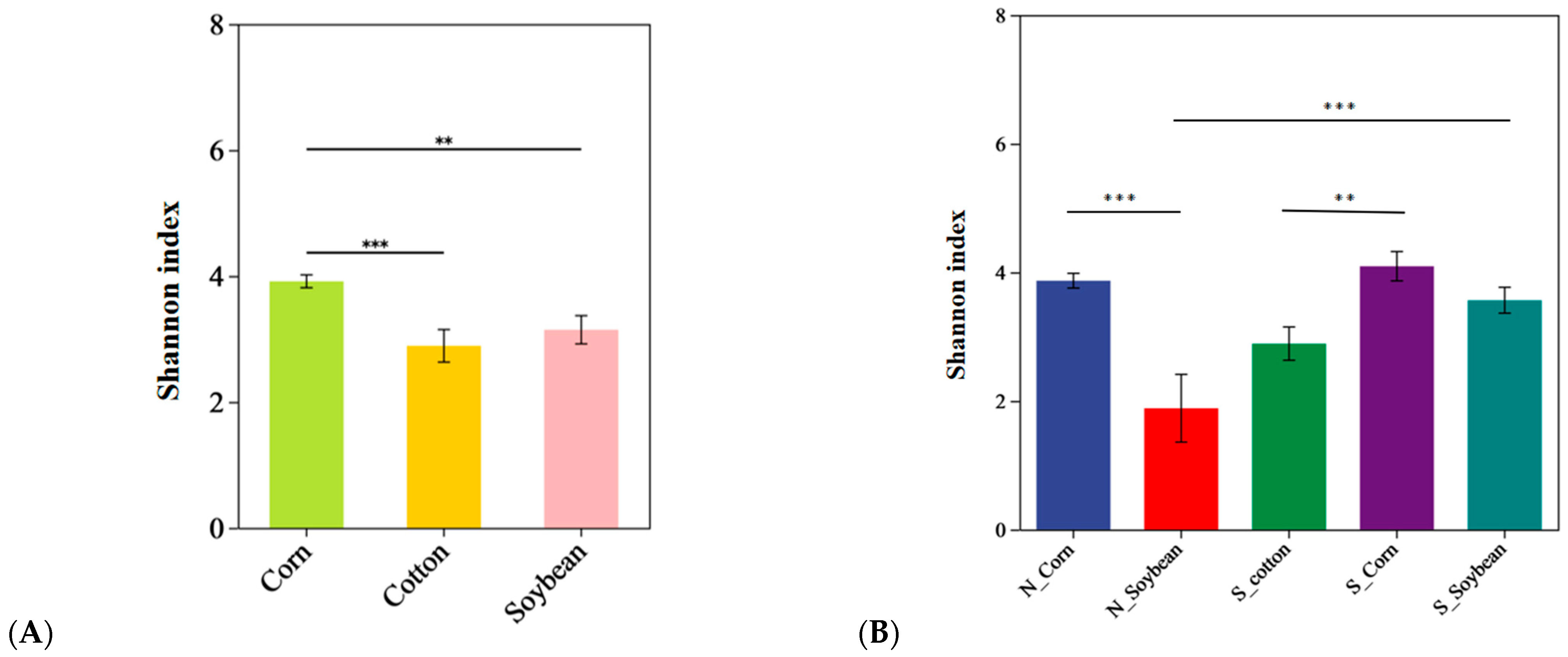
3.2. Analysis of the Alpha Diversity of Endosymbiotic Bacteria Communities in T. truncatus from Different Geographical Populations in Xinjiang
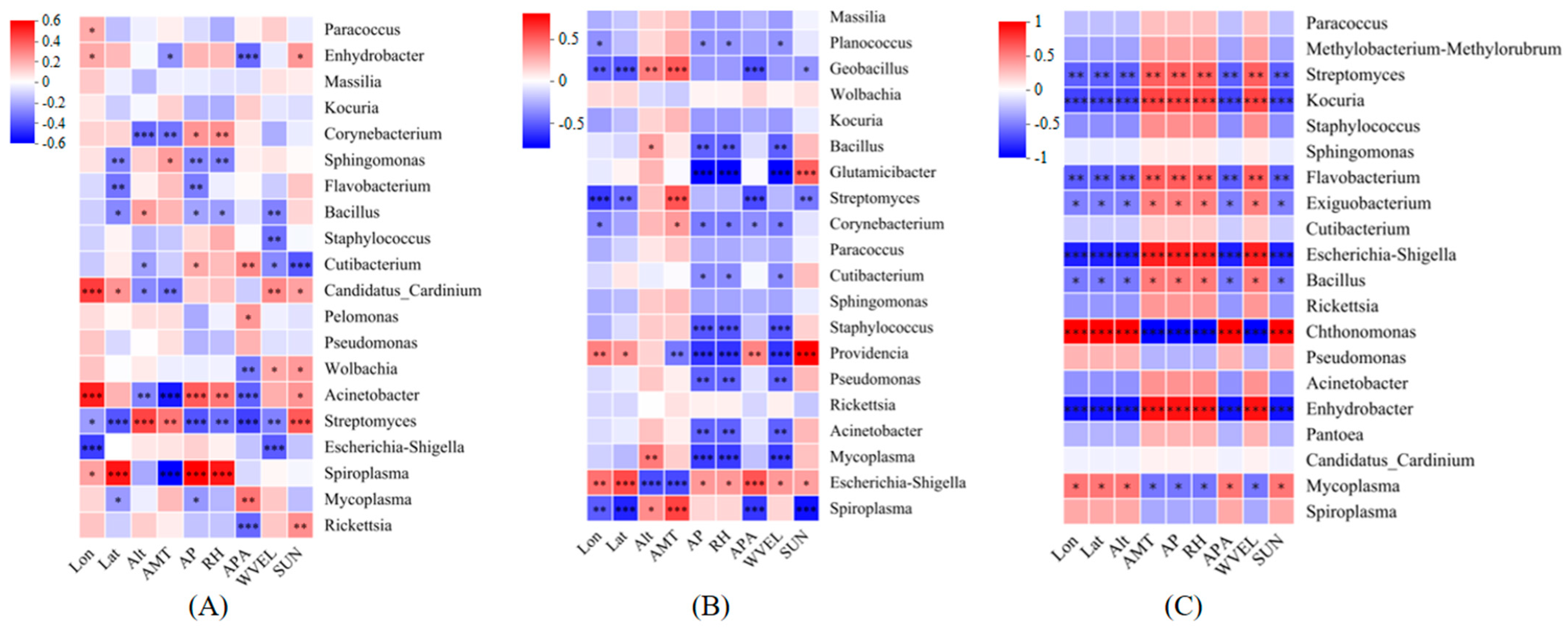
3.3. Correlation Between Endosymbiotic Bacteria of T. truncatus and Environmental Factors
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, X.T.; Cai, L.; Shen, Y.; Du, Y.Z. Diversity and evolution of the endosymbionts of Bemisia tabaci in China. PeerJ 2018, 6, e5516. [Google Scholar] [CrossRef]
- Baumann, P.; Baumann, L.; Lai, C.Y.; Rouhbakhsh, D.; Moran, N.A.; Clark, M.A. Genetics, physiology, and evolutionary relationships of the genus Buchnera: Intracellular symbionts of aphids. Annu. Rev. Microbiol. 1995, 49, 55–94. [Google Scholar] [CrossRef]
- Chen, D.Q.; Montllor, C.B.; Purcell, A.H. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid, Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol. Exp. Appl. 2000, 95, 315–323. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef]
- Russell, J.A.; Moreau, C.S.; Goldman-Huertas, B.; Fujiwara, M.; Lohman, D.J.; Pierce, N.E. Bacterial gut symbionts are tightly linked with the evolution of herbivory in ants. Proc. Natl. Acad. Sci. USA 2009, 106, 21236–21241. [Google Scholar] [CrossRef]
- Pers, D.; Hansen, A.K. The boom and bust of the aphid’s essential amino acid metabolism across nymphal development. G3 2021, 11, jkab115. [Google Scholar] [CrossRef]
- Turelli, M.; Katznelson, A.; Ginsberg, P.S. Why Wolbachia-induced cytoplasmic incompatibility is so common. Proc. Natl. Acad. Sci. USA 2022, 119, e2211637119. [Google Scholar] [CrossRef] [PubMed]
- Doremus, M.R.; Stouthamer, C.M.; Kelly, S.E.; Schmitz-Esser, S.; Hunter, M.S. Cardinium Localization During Its Parasitoid Wasp Host’s Development Provides Insights Into Cytoplasmic Incompatibility. Front. Microbiol. 2020, 11, 606399. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.J.; Kim, M.S.; Park, D.S.; Yoon, C.; Nam, Y.; Kim, Y.; Choi, J.; et al. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef]
- Toju, H.; Fukatsu, T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: Relevance of local climate and host plants. Mol. Ecol. 2011, 20, 853–868. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.H.C.; Godfray, H.C.J.; Ellers, J.; Henry, L.M. Host relatedness influences the composition of aphid microbiomes. Environ. Microbiol. Rep. 2019, 11, 808–816. [Google Scholar] [CrossRef]
- Adair, K.L.; Bost, A.; Bueno, E.; Kauisto, S.; Kortet, R.; Peters-Schulze, G.; Martison, V.G.; Douglas, A.E. Host determinants of among-species variation in microbiome composition in drosophilid flies. ISME J. 2020, 14, 217–229. [Google Scholar] [CrossRef]
- Hansen, A.K.; Moran, N.A. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol. Ecol. 2014, 23, 1473–1496. [Google Scholar] [CrossRef]
- Chung, S.H.; Scully, E.D.; Peiffer, M.; Geib, S.M.; Rosa, C.; Hoover, K.; Felton, G.W. Host plant species determines symbiotic bacterial community mediating suppression of plant defenses. Sci. Rep. 2017, 7, 39690. [Google Scholar] [CrossRef]
- Zélé, F.; Santos, J.L.; Godinho, D.P.; Magalhães, S. Wolbachia both aids and hampers the performance of spider mites on different host plants. FEMS Microbiol. Ecol. 2018, 94, fiy187. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, X.B.; Zhang, T.T.; Bai, S.; He, K.; Zhang, Y.; Francis, F.; Wang, Z. Effects of host plants on aphid feeding behavior, fitness, and Buchnera aphidicola titer. Insect Sci. 2024, 32, 927–942. [Google Scholar] [CrossRef]
- Medina, R.F.; Nachappa, P.; Tamborindeguy, C. Differences in bacterial diversity of host-associated populations of Phylloxera notabilis Pergande (Hemiptera: Phylloxeridae) in pecan and water hickory. J. Evol. Biol. 2011, 24, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.M.; Asplen, M.K.; Desneux, N.; Heimpel, G.E.; Hopper, K.R.; Linnen, C.R.; Oliver, K.M.; Wulff, J.A.; White, J.A. Worldwide populations of the aphid Aphis craccivora are infected with diverse facultative bacterial symbionts. Microb. Ecol. 2014, 67, 195–204. [Google Scholar] [CrossRef]
- Guidolin, A.S.; Cônsoli, F.L. Symbiont Diversity of Aphis (Toxoptera) citricidus (Hemiptera: Aphididae) as Influenced by Host Plants. Microb. Ecol. 2017, 73, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Zytynska, S.E.; Weisse, W.W. The natural occurrence of secondary bacterial symbionts in aphids. Ecol. Entomol. 2016, 41, 13–26. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Shibao, H.; Matsumoto, T.; Fukatsu, T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2002, 11, 2123–2135. [Google Scholar] [CrossRef]
- Hurst, G.D.; Johnson, A.P.; Schulenburg, J.H.; Fuyama, Y. Male-killing Wolbachia in Drosophila: A temperature-sensitive trait with a threshold bacterial density. Genetics 2000, 156, 699–709. [Google Scholar] [CrossRef]
- Ross, P.A.; Axford, J.K.; Yang, Q.; Staunton, K.M.; Ritchie, S.A.; Richardson, K.M.; Hoffmann, A.A. Heatwaves cause fluctuations in wMel Wolbachia densities and frequencies in Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0007958. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J. Analysis of Temperature and Precipitation Variation Characteristics in Northern and Southern Xinjiang from 1981 to 2020. J. Anhui Agric. Sci. 2022, 24, 214–219+240. [Google Scholar] [CrossRef]
- Weeks, A.R.; Stouthamer, R. Increased fecundity associated with infection by a cytophaga-like intracellular bacterium in the predatory mite, Metaseiulus occidentalis. Proc. Biol. Sci. 2004, 271 (Suppl. S4), S193–S195. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Jiao, X.D.; Xu, J.J.; Yang, S.; Duan, X.K.; Zhang, J.P. Population dynamics of Tetranychus dunhuangensis and Tetranychus truncatus and their selectivity preferences for different host plants. Chin. J. Appl. Entomol. 2024, 61, 559–568. [Google Scholar] [CrossRef]
- Hong, X. Agricultural Acarology; China Agriculture Press: Beijing, China, 2012. [Google Scholar]
- Bolland, H.R.; Gutierrez, J.; Flechtmann, C.H.W. World Catalogue of the Spider Mite Family (Acari: Tetranychidae), With References to Taxonomy, Synonymy, Host Plants and Distribution; Brill Academic Press: Leiden, The Netherlands, 1998; 392p. [Google Scholar]
- Yang, K.; Chen, H.; Bing, X.L.; Xia, X.; Zhu, Y.X.; Hong, X.Y. Wolbachia and Spiroplasma could influence bacterial communities of the spider mite Tetranychus truncatus. Exp. Appl. Acarol. 2021, 83, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Song, Y.L.; Hoffmann, A.A.; Jin, P.Y.; Huo, S.M.; Hong, X.Y. A change in the bacterial community of spider mites decreases fecundity on multiple host plants. Microbiologyopen 2019, 8, e00743. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Han, C.; Shi, C.; Liu, L.; Han, J.; Yang, Q.; Wang, Y.; Li, X.; Fu, W.; Gao, H.; Huang, H.; et al. Majorbio Cloud 2024: Update single-cell and multiomics workflows. Imeta 2024, 3, e217. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Harriet, A.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Yang, K.; Zhu, Y.X.; Hong, X.Y. Symbiont-conferred reproduction and fitness benefits can favour their host occurrence. Ecol. Evol. 2018, 8, 1626–1633. [Google Scholar] [CrossRef]
- Liu, H.H.; Chen, L.; Shao, H.B.; Gao, S.; Hong, X.Y.; Bing, X.L. Environmental Factors and the Symbiont Cardinium Influence the Bacterial Microbiome of Spider Mites Across the Landscape. Microb. Ecol. 2023, 87, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Chen, Y.T.; Yang, K.; Qiao, G.X.; Hong, X.Y. Screening of spider mites (Acari: Tetranychidae) for reproductive endosymbionts reveals links between co-infection and evolutionary history. Sci. Rep. 2016, 6, 27900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Song, Y.L.; Zhang, Y.K.; Hoffmann, A.A.; Zhou, J.C.; Sun, J.T.; Hong, X.Y. Incidence of Facultative Bacterial Endosymbionts in Spider Mites Associated with Local Environments and Host Plants. Appl. Environ. Microbiol. 2018, 84, e02546-17. [Google Scholar] [CrossRef]
- Brumin, M.; Lebedev, G.; Kontsedalov, S.; Ghanim, M. Levels of the endosymbiont Rickettsia in the whitefly Bemisia tabaci are influenced by the expression of vitellogenin. Insect Mol. Biol. 2020, 29, 241–255. [Google Scholar] [CrossRef]
- Douglas, A.E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef] [PubMed]
- Kudjordjie, E.N.; Sapkota, R.; Steffensen, S.K.; Fomsgaard, I.S.; Nicolaisen, M. Maize synthesized benzoxazinoids affect the host associated microbiome. Microbiome 2019, 7, 59. [Google Scholar] [CrossRef]
- Wang, X.; Howell, C.P.; Chen, F.; Yin, J.; Jiang, Y. Gossypol–A polyphenolic compound from cotton plant. Adv. Food Nutr. Res. 2009, 58, 215–263. [Google Scholar] [CrossRef]
- Abutheraa, R.; Hettiarachchy, N.; Kumar-Phillips, G.; Horax, R.; Chen, P.; Morawicki, R.; Kwon, Y.M. Antimicrobial Activities of Phenolic Extracts Derived from Seed Coats of Selected Soybean Varieties. J. Food Sci. 2017, 82, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, Z.; Zhou, J.; Liu, X.; Zhang, Y.; Chu, D. Ecological Factors Associated with the Distribution of Bemisia tabaci Cryptic Species and Their Facultative Endosymbionts. Insects 2023, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Alimu, A.; Gao, Y.; Liu, J.; Lu, Y. Geographic factors influence communities of symbiotic bacterial communities in Aphis gossypii across China’s major cotton regions. Front. Microbiol. 2025, 16, 1569543. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, N.N.; Thorshauge, P.M.; Kristensen, T.N.; de Jonge, N.; Bahrndorff, S.; Kjeldal, H.; Nielsen, J.L. Strong responses of Drosophila melanogaster microbiota to developmental temperature. Fly 2018, 12, 1–12. [Google Scholar] [CrossRef]
- Horváthová, T.; Babik, W.; Kozłowski, J.; Bauchinger, U. Vanishing benefits–The loss of actinobacterial symbionts at elevated temperatures. J. Therm. Biol. 2019, 82, 222–228. [Google Scholar] [CrossRef]
- Majeed, M.Z.; Sayed, S.; Bo, Z.; Raza, A.; Ma, C.S. Bacterial Symbionts Confer Thermal Tolerance to Cereal Aphids Rhopalosiphum padi and Sitobion avenae. Insects 2022, 13, 231. [Google Scholar] [CrossRef]

| Regional Distribution | Sampling Location | Host | Host Variety | Sample Size | Population Code | Lon (°E) | Lat (°N) | Alt (m) | AMT (°C) | AP (mm) | RH (%) | APA (hPa) | WVEL (m/s) | SUN (h) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Northern Xinjiang | Kekedala City | Soybean | Xindadou 26 | 12 | N_68D | 80.624 | 44.127 | 578 | 12.90 | 241.51 | 42.68 | 934.81 | 3.57 | 4797.40 |
| Shuanghe City | Corn | Xinyu 97 | 9 | N_81Y | 82.485 | 44.772 | 270 | 11.36 | 149.61 | 44.23 | 961.04 | 2.42 | 4776.80 | |
| Shuanghe City | Corn | Xinyu 97 | 12 | N_82Y | 82.592 | 44.782 | 636 | 11.36 | 149.61 | 44.23 | 961.04 | 2.42 | 4776.80 | |
| Shihezi City | Corn | Huamei 1 | 12 | N_121Y | 86.048 | 44.291 | 493 | 10.96 | 172.4 | 45.38 | 964.71 | 2.98 | 4776.00 | |
| Huyanghe City | Corn | Huaxi 703 | 6 | N_124Y | 84.874 | 44.796 | 284 | 10.38 | 177.76 | 45.24 | 948.90 | 3.11 | 4799.00 | |
| Habahe County, Altay Prefecture | Corn | Heyu 187 | 12 | N_AHY | 86.480 | 48.085 | 512 | 3.10 | 331.78 | 56.56 | 887.88 | 3.12 | 4812.80 | |
| Urumqi City | Corn | Bixiang 101 | 12 | N_ANY | 87.505 | 43.982 | 566 | 10.96 | 225.40 | 44.19 | 933.36 | 2.97 | 4774.20 | |
| Wusu City, Tacheng Prefecture | Corn | Denghai 550 | 12 | N_GY | 84.303 | 44.413 | 483 | 6.43 | 203.60 | 49.68 | 872.90 | 1.85 | 4828.40 | |
| Shanshan County, Turpan City | Corn | Sitai 159 | 12 | N_SY | 90.546 | 43.005 | 517 | 11.98 | 25.89 | 30.51 | 908.67 | 3.24 | 4805.60 | |
| Fukang City, Changji Hui Autonomous Prefecture | Corn | Sitai 112 | 7 | N_JY | 88.076 | 44.170 | 560 | 9.59 | 189.26 | 44.36 | 928.99 | 2.90 | 4782.40 | |
| Southern Xinjiang | Aksu City, Aksu Prefecture | Cotton | Xinluzhong 84 | 12 | S_AMr | 80.263 | 41.168 | 1162 | 13.58 | 57.37 | 31.85 | 894.88 | 2.69 | 4832.60 |
| Aksu City, Aksu Prefecture | Soybean | Fengchan 80 | 12 | S_LDr | 80.263 | 41.168 | 1162 | 13.58 | 57.37 | 31.85 | 894.88 | 2.69 | 4832.60 | |
| Xinhe County, Aksu Prefecture | Corn | Zhengdan 958 | 12 | S_XY | 82.577 | 41.546 | 1017 | 12.79 | 75.75 | 35.18 | 892.17 | 2.9 | 4821.20 | |
| Tumushuke City | Corn | Xianyu 335 | 12 | S_4414Y | 79.133 | 39.827 | 1164 | 13.64 | 88.23 | 33.53 | 892.17 | 2.91 | 4779.40 | |
| Tumushuke City | Soybean | Zhonghuang 35 | 12 | S_44D | 79.133 | 39.827 | 1164 | 13.64 | 88.23 | 33.53 | 892.17 | 2.91 | 4779.40 | |
| Tumushuke City | Soybean | Heihe 11 | 12 | S_53D | 79.089 | 39.868 | 1098 | 13.64 | 88.23 | 33.53 | 892.17 | 2.91 | 4779.40 | |
| Tumushuke City | Cotton | Tahe 2 | 12 | S_53M | 79.089 | 39.868 | 1098 | 13.64 | 88.23 | 33.53 | 892.17 | 2.91 | 4779.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, K.; Zhang, B.; Cai, Z.; Chen, J.; Zhang, J.; Su, J. Diversity and Influencing Factors of Endosymbiotic Bacteria in Tetranychus truncatus Sourced from Major Crops in Xinjiang. Insects 2025, 16, 1126. https://doi.org/10.3390/insects16111126
Mu K, Zhang B, Cai Z, Chen J, Zhang J, Su J. Diversity and Influencing Factors of Endosymbiotic Bacteria in Tetranychus truncatus Sourced from Major Crops in Xinjiang. Insects. 2025; 16(11):1126. https://doi.org/10.3390/insects16111126
Chicago/Turabian StyleMu, Kaiqin, Bing Zhang, Zhiping Cai, Jing Chen, Jianping Zhang, and Jie Su. 2025. "Diversity and Influencing Factors of Endosymbiotic Bacteria in Tetranychus truncatus Sourced from Major Crops in Xinjiang" Insects 16, no. 11: 1126. https://doi.org/10.3390/insects16111126
APA StyleMu, K., Zhang, B., Cai, Z., Chen, J., Zhang, J., & Su, J. (2025). Diversity and Influencing Factors of Endosymbiotic Bacteria in Tetranychus truncatus Sourced from Major Crops in Xinjiang. Insects, 16(11), 1126. https://doi.org/10.3390/insects16111126








