Putative Photosensitivity-Associated Sexual Dimorphism in Compound Eye Structure of Lymantria xylina (Lepidoptera: Erebidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Light Exposure and Dark Adaptation Protocols
2.3. Scanning Electron Microscopy Compound Eye Preparation
2.4. Transmission Electron Microscopy Compound Eye Preparation
2.5. Histological Preparation
2.6. Data Analysis
3. Results
3.1. External Morphology of Compound Eyes in L. xylina
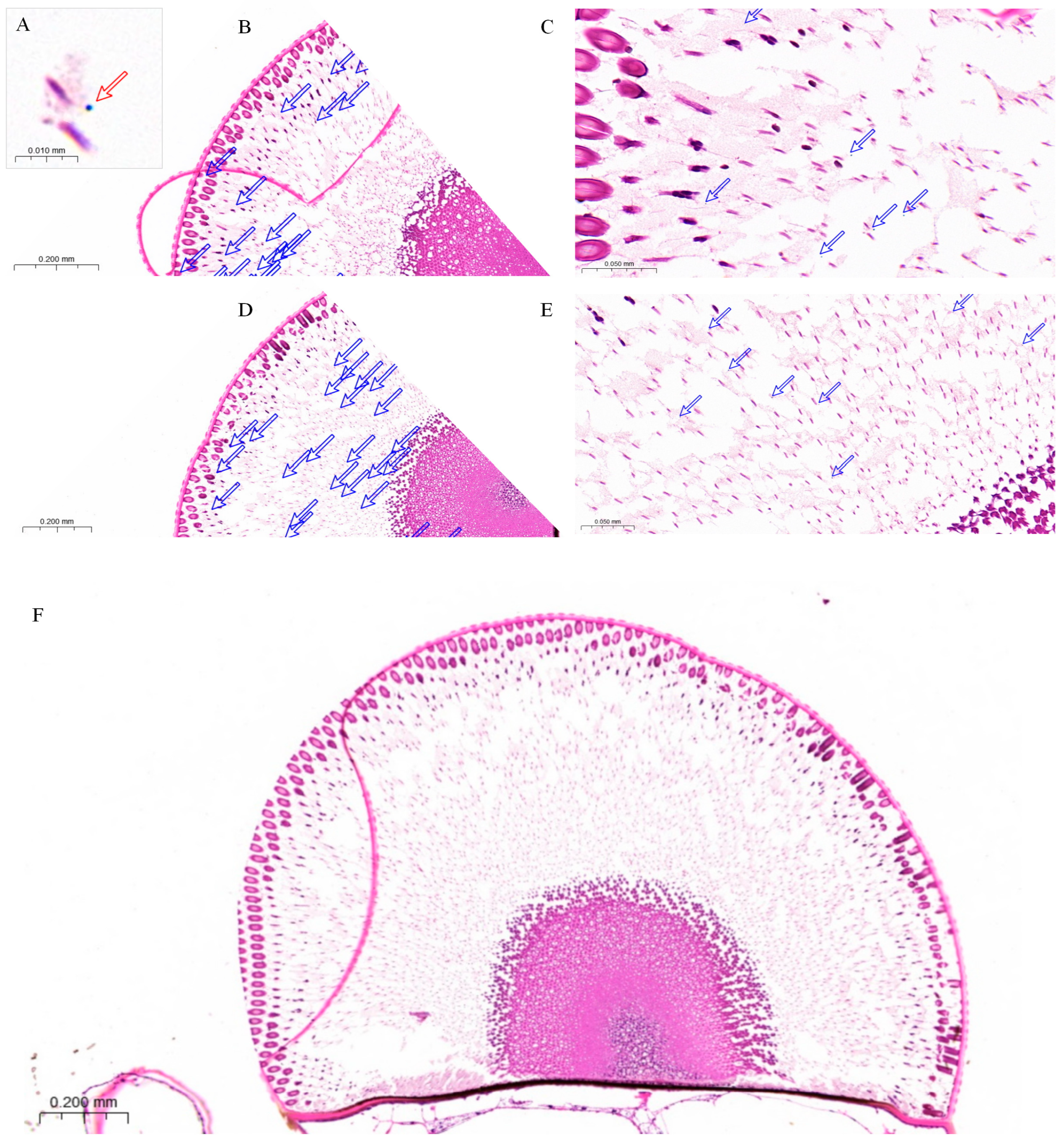
3.2. Internal Structure of the Compound Eyes in L. xylina
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Ye, G.; Gao, W.; You, L.; Nie, S.; Wang, Y.P. Ecological response of Casuarina equisetifolia to environmental stress in coastal dunes in China. J. For. Res. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, G.; Liu, J.; Chen, K.; Miao, X.; Hussain, J.; Liu, S.; Ren, H. Genome-wide identification of WRKY transcription factors in Casuarina equisetifolia and the function analysis of CeqWRKY11 in response to NaCl/NaHCO3 stresses. BMC Plant Biol. 2024, 24, 376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Wang, Y.; Peng, Y.; Wang, Y.; Guo, Y.; Hu, M.; Lin, W.; Wu, Z. Effect of continuous planting on archaeal community changes in rhizosphere of Casuarina equisetifolia plantations. For. Res. 2024, 37, 106–117. (In Chinese) [Google Scholar] [CrossRef]
- Chang, H.; Li, C.; Zhu, T.; Cai, S.; Chen, J.; Zhan, F.; Zeng, L.; Fang, Y.; Ye, G.; Li, J.; et al. GLR3.6T8071 mutation of Casuarina equisetifolia is associated with a decreased JA response to insect feeding by Lymantria xylina. Plant Cell Environ. 2025, 48, 3185–3198. [Google Scholar] [PubMed]
- Wang, R.; Zhang, Z.; Hu, X.; Wu, S.; Wang, J.; Zhang, F. Molecular detection and genetic diversity of casuarina moth, Lymantria xylina (Lepidoptera: Erebidae). J. Insect Sci. 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-López, M.A. Lymantria xylina. CABI Compend. 2022. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Sun, Y.; Ren, H.; Chen, J.; Zhang, F.; Wang, R. Rapid identification of the sex of Lymantria xylina pupae. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2023, 52, 628–631. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J. Distribution of Lymantria xylina Egg Masses and Effects of Extreme Environmental Factors. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. (In Chinese). [Google Scholar]
- Zhang, J.; Wang, B.; Ren, H.; Chen, J.; Li, J.; Sun, Y.; Cui, Y.; Wang, R.; Liu, M.; Zhang, F. Evaluation of the potential flight ability of the casuarina moth, Lymantria xylina (Lepidoptera: Erebidae). Insects 2024, 15, 506. [Google Scholar] [CrossRef] [PubMed]
- Pogue, M.; Schaefer, P.W. A Review of Selected Species of Lymantria Hübner (1819) (Lepidoptera: Noctuidae: Lymantriinae) from Subtropical and Temperate Regions of Asia, Including the Descriptions of Threenew Species, Some Potentially Invasive to North America; Forest Health Technology Enterprise Team: Washington, DC, USA, 2007. [Google Scholar]
- Zhang, J.; Wang, B.; Wang, L.; Zuo, C.; Li, J.; Cui, Y.; Wen, X.; Cowan, D.; Wu, S.; Liu, M.; et al. Reproductive and flight characteristics of Lymantria xylina (Lepidoptera: Erebidae) in Fuzhou, China. Insects 2024, 15, 894. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.T.; Liao, C.T.; Ko, W.F.; Pai, K.F. Light attractive evaluation and insecticide trials of casuarina moth (Lymantria xylina Swinhoe) in Bagua Mountain area. Field Taizhong Dist. 2010, 107, 47–59. [Google Scholar]
- Zhang, J.; Wang, B.; Wang, R.; Peng, X.; Li, J.; Xu, C.; Cui, Y.; Liu, M.; Zhang, F. Phototaxis characteristics of Lymantria xylina (Lepidoptera: Erebidae). Insects 2025, 16, 338. [Google Scholar] [CrossRef]
- Kim, K.; Huang, Q.; Lei, C. Advances in insect phototaxis and application to pest management: A review. Pest Manag. Sci. 2019, 75, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, Q.; Wen, C.; Wen, J. Comparison of fine structure of the compound eyes in Eucryptorrhynchus scrobiculatus and Eucryptorrhynchus brandti adults. Insects 2023, 14, 699. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Strickler, K.L. Finding and accepting host plants. In Chemical Ecology of Insects; Bell, W.J., Cardé, R.T., Eds.; Chapman & Hall: London, UK, 1984; pp. 127–157. [Google Scholar]
- Bell, W.J.; Cardé, R.T. Chemical Ecology of Insects; Springer: New York, NY, USA, 2013; 524p. [Google Scholar]
- Nilsson, D.E. Optics and evolution of the compound eye. In Facets of Vision; Stavenga, D.G., Hardie, R.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 30–73. [Google Scholar]
- Hou, W.W.; Ho, S.W. Studies on the phototactic behaviour of nocturnal moths: Change in behaviour during the transformation of compound eyes. Acta Entomol. Sin. 1979, 22, 34–40. [Google Scholar]
- Chapman, R.F. The Insects: Structure and Function; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Pichaud, F.; Casares, F. The size and shape of the insect compound eye. Semin. Cell Dev. Biol. 2022, 130, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, M.; Jiang, Y.; Meng, Z.; Tan, C.; Wang, G.; Bian, L. Ultrastructure of the compound eye of adult Ectropis grisescens (Lepidoptera: Geometridae). Acta Entomol. Sin. 2022, 65, 1277–1286. (In Chinese) [Google Scholar] [CrossRef]
- Lau, T.F.S.; Gross, E.M.; Meyer-Rochow, V.B. Sexual dimorphism and light/dark adaptation in the compound eyes of male and female Acentria ephemerella (Lepidoptera: Pyraloidea: Crambidae). Euroupe J. Entomol. 2007, 104, 459–470. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, R.; Kuang, R.P.; Sun, R. Phototactic behaviour of the parasitoid Encarsia formosa (Hymenoptera, Aphelinidae). Biocontrol Sci. Technol. 2016, 26, 250–262. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Xie, Q.; Cai, Q.; Wu, J.; Li, Y.; Zheng, X.; Zhu, Z.; Zhou, B.; Zheng, H. Studies on the lymantriid moth Lymantria xylina Swinhoe. Acta Entomol. Sin. 1981, 24, 174–183. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Tao, Y.; Ma, T. Observation on the external morphology and ultrastructure of compound eye of adult Diaphania glauculalis (Lepidoptera: Pyraustinae). Plant Prot. 2025, 51, 247–255+271. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, P.; Hu, K.; Qian, C.; Wang, S.; Wen, X.; Wang, S.; Ma, T. Observation on the external morphology and ultrastructure of compound eyes of adult Eutectona machaeralis (Lepidoptera: Crambidae). Acta Entomol. Sin. 2022, 65, 1056–1067. (In Chinese) [Google Scholar] [CrossRef]
- Lau, T.F.S.; Meyer-Rochow, V.B. The compound eye of Orgyia antiqua (Lepidoptera: Lymantriidae): Sexual dimorphism and light/dark adaptational changes. Euroupe J. Entomol. 2007, 104, 247–258. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Lau, T.F.S. Sexual dimorphism in the compound eye of the moth Operophtera brumata (Lepidoptera, Geometridae). Invertebr. Biol. 2008, 127, 201–216. [Google Scholar] [CrossRef]
- Yang, X. Study on Microstructure of the Compound Eye Andphototactic Behavior of Athetis lepigone. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2015. (In Chinese). [Google Scholar]
- Land, M.F. Visual acuity in insects. Annu. Rev. Entomol. 1997, 42, 147–177. [Google Scholar] [CrossRef]
- Land, M.F. Optics and vision in invertebrates. In Comparative Physiology and Evolution of Vision in Invertebrates; Autrum, H., Ed.; Handbook of Sensory Physiology; Springer: Berlin/Heidelberg, Germany, 1981; Volume 6, pp. 471–592. [Google Scholar]
- Warrant, E.; Dacke, M. Vision and visual navigation in nocturnal insects. Annu. Rev. Entomol. 2011, 56, 239–254. [Google Scholar] [CrossRef]
- Yang, X.; Ran, H.; Jiang, Y.; Lu, Z.; Wei, G.; Li, J. Fine structure of the compound eyes of the crepuscular moth Grapholita molesta (Busck 1916) (Lepidoptera: Tortricidae). Front. Physiol. 2024, 15, 1343702. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Chen, Y.W.; Li, W.L. Ultrastructural comparison of the compound eyes of the asian corn borer Ostrinia furnacalis (Lepidoptera: Crambidae) under light/dark adaptation. Arthropod Struct. Dev. 2019, 53, 100901. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Wood, E.J.; Wu, M. Developmental evolution of the insect retina: Insights from standardized numbering of homologous photoreceptors. J. Exp. Zool. Part B Mol. Dev. Evol. 2011, 316, 484–499. [Google Scholar] [CrossRef]
- Zhang, L. Study on External Morphology and Microstructure of the Compound Eye of the Moth. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2010. (In Chinese). [Google Scholar]
- Chen, Q.X.; Lyu, Q.H.; Chen, Y.W.; Song, Y.Q. Phylogenetic implications based on an ultrastructural study with emphasis on the tracheal system of the compound eyes of three species of nymphalid butterflies. Arthropod Struct. Dev. 2023, 72, 101230. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, E. Retinular fine structure in compound eyes of diurnal and nocturnal sphingid moths. Cell Tissue Res. 1982, 223, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Ning, S.; Feng, J. Ultrastructure and morphology of the compound eyes of the predatory bug Montandoniola moraguesi (Insecta: Hemiptera: Anthocoridae). Arthropod Struct. Dev. 2021, 61, 101030. [Google Scholar] [CrossRef]
- Giglio, A.; Vommaro, M.L.; Agostino, R.G. Exploring compound eyes in adults of four coleopteran species using synchrotron X-ray phase-contrast microtomography (SR-PhC Micro-CT). Life 2022, 12, 741. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.P.; Liang, A.P. An apposition-like compound eye with a layered rhabdom in the small diving beetle Agabus japonicus (Coleoptera, Dytiscidae). J. Morphol. 2014, 275, 1273–1283. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Compound eyes of insects and crustaceans: Some examples that show there is still a lot of work left to be done. Insect Sci. 2015, 22, 461–481. [Google Scholar] [CrossRef]
- Takemura, S.Y.; Arikawa, K. Ommatidial type-specific interphotoreceptor connections in the lamina of the swallowtail butterfly, Papilio xuthus. J. Comp. Neurol. 2006, 494, 663–672. [Google Scholar] [CrossRef]
- Sison-Mangus, M.P.; Briscoe, A.D.; Zaccardi, G. The lycaenid butterfly Polyommatus icarus uses a duplicated blue opsin to see green. J. Exp. Biol. 2008, 211, 361–369. [Google Scholar] [CrossRef]
- Zaccardi, G.; Kelber, A.; Sison-Mangus, M.P. Color discrimination in the red range with only one long-wavelength sensitive opsin. J. Exp. Biol. 2006, 209, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Chen, Q.X.; Yi, Q.; Hua, B.Z. Ultrastructure of the larval eyes of the hangingfly Terrobittacus implicatus (Mecoptera: Bittacidae). Micron 2022, 152, 103176. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wei, C. Morphology, histology and ultrastructure of the compound eyes of the last instar nymphs and adults of Meimuna mongolica (Hemiptera: Cicadidae). Acta Entomol. Sin. 2021, 63, 1441–1451. [Google Scholar]
- Land, M.F.; Nilsson, D.E. Animal Eyes, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Lo, M.V.; Pak, W.L. Light-induced pigment granule migration in the retinular cells of Drosophila melanogaster. Comparison of wild type with ERG-defective mutants. J. Gen. Physiol. 1981, 77, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Stewart, F.J.; Koshitaka, H.; Akashi, H.D.; Pirih, P.; Sato, Y.; Arikawa, K. Red-shift of spectral sensitivity due to screening pigment migration in the eyes of a moth, Adoxophyes orana. Zool. Lett. 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, Q.X. Ultrastructural and light/dark adaptational characteristics of the compound eyes in the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Arthropod Struct. Dev. 2025, 86, 101449. [Google Scholar] [CrossRef] [PubMed]
- Alba-Alejandre, I.; Alba-Tercedor, J.; Vega, F.E. Anatomical study of the coffee berry borer (Hypothenemus hampei) using micro-computed tomography. Sci. Rep. 2019, 9, 17150. [Google Scholar]
- Smith, D.B.; Bernhardt, G.; Raine, N.E. Exploring miniature insect brains using micro-CT scanning techniques. Sci. Rep. 2016, 6, 21768. [Google Scholar] [CrossRef]
- Wipfler, B.; Pohl, H.; Yavorskaya, M.I. A review of methods for analysing insect structures—The role of morphology in the age of phylogenomics. Curr. Opin. Insect Sci. 2016, 18, 60–68. [Google Scholar] [CrossRef]
- Betz, O.; Wegst, U.; Weide, D. Imaging applications of synchrotron X-ray phase-contrast microtomography in biological morphology and biomaterials science. I. General aspects of the technique and its advantages in the analysis of millimetre-sized arthropod structure. J. Microsc. 2007, 227, 51–71. [Google Scholar] [CrossRef] [PubMed]

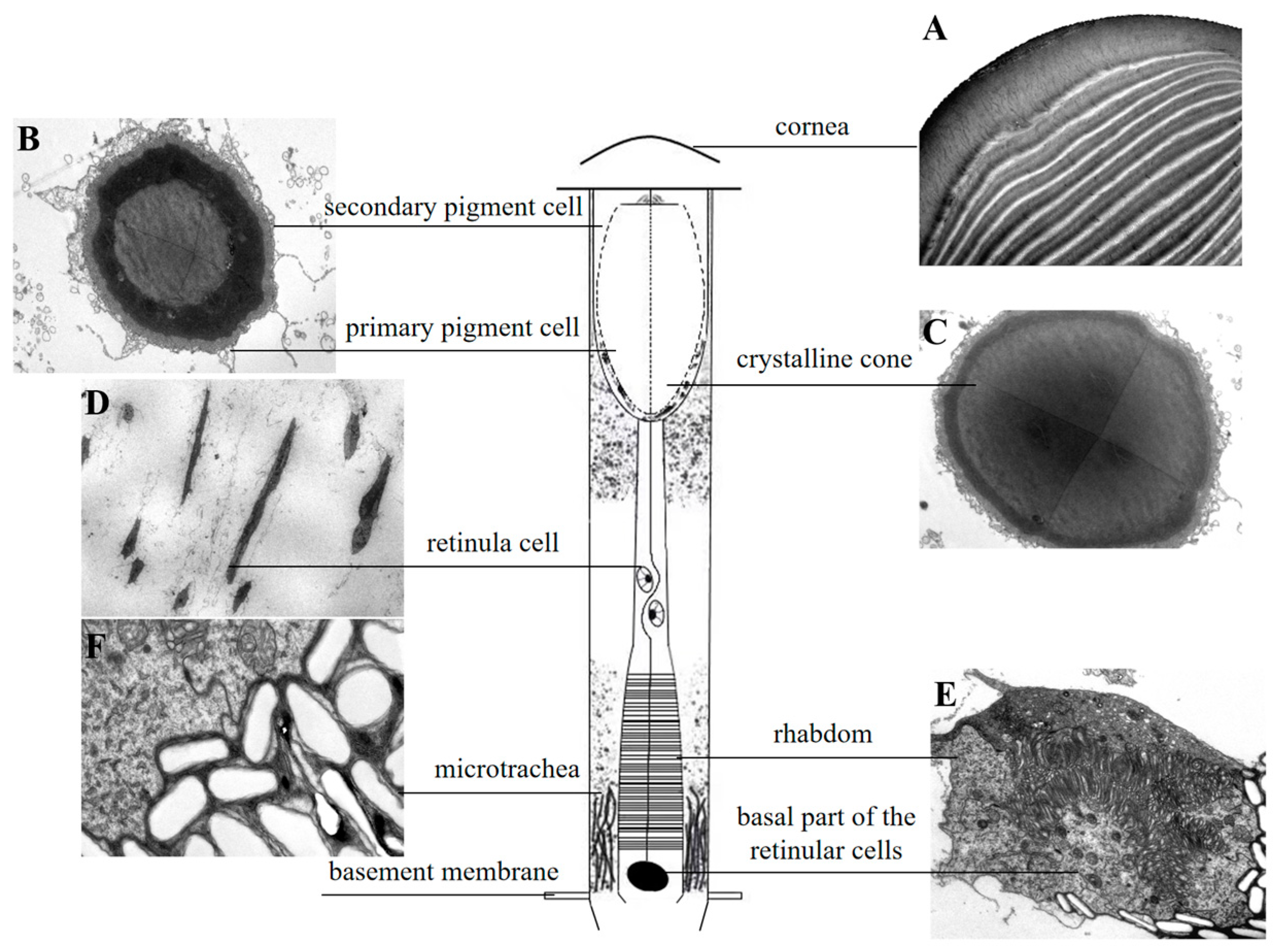

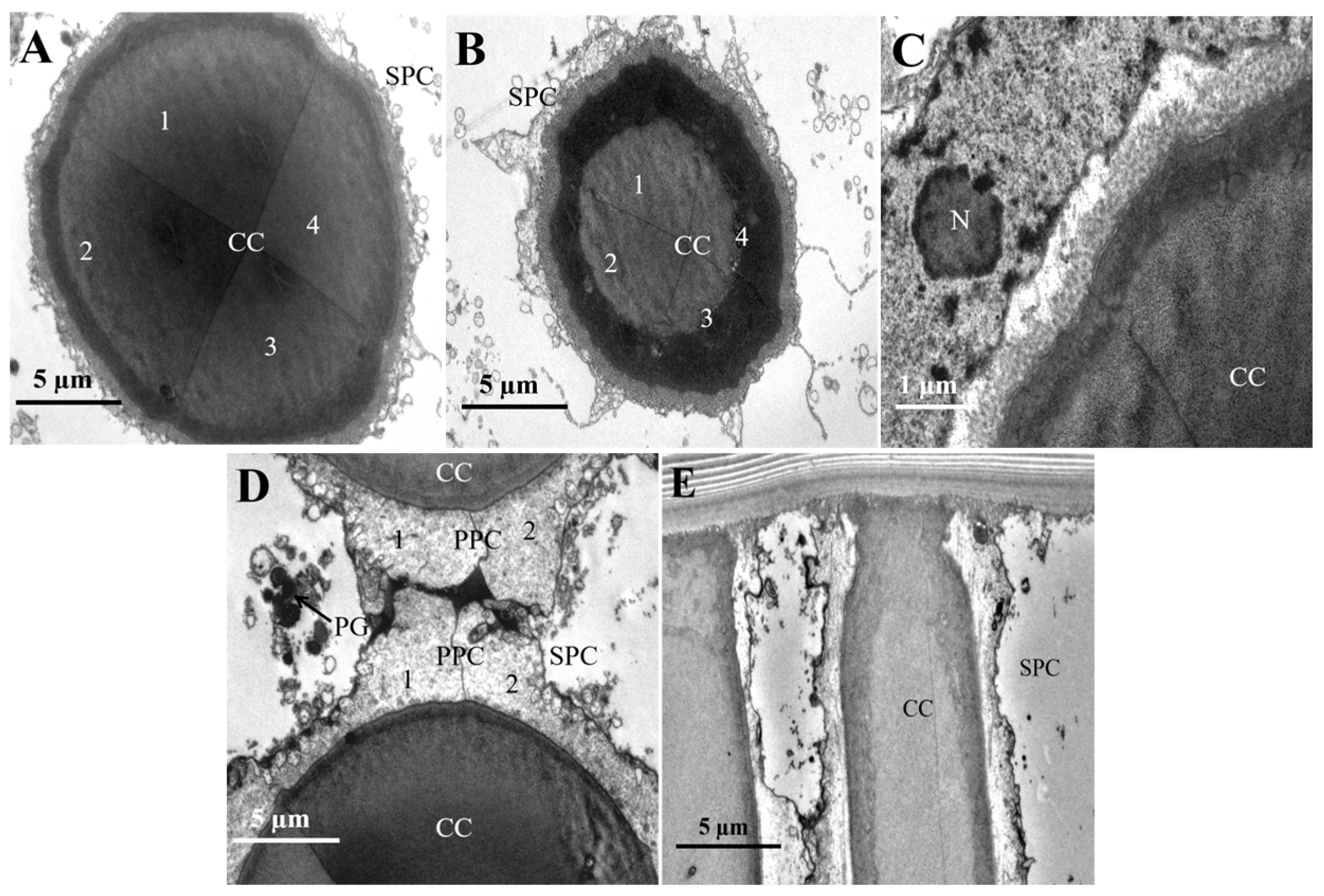
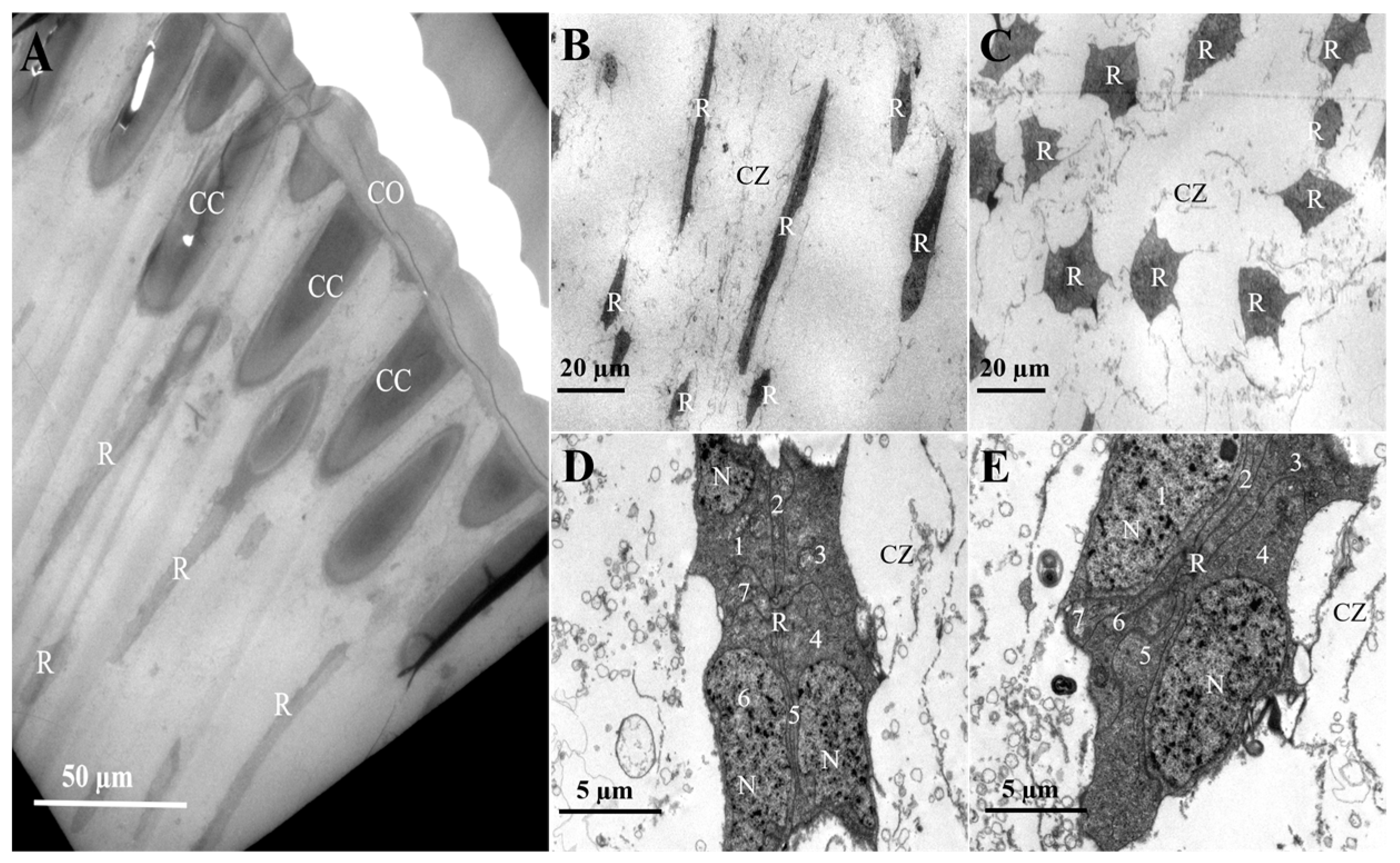

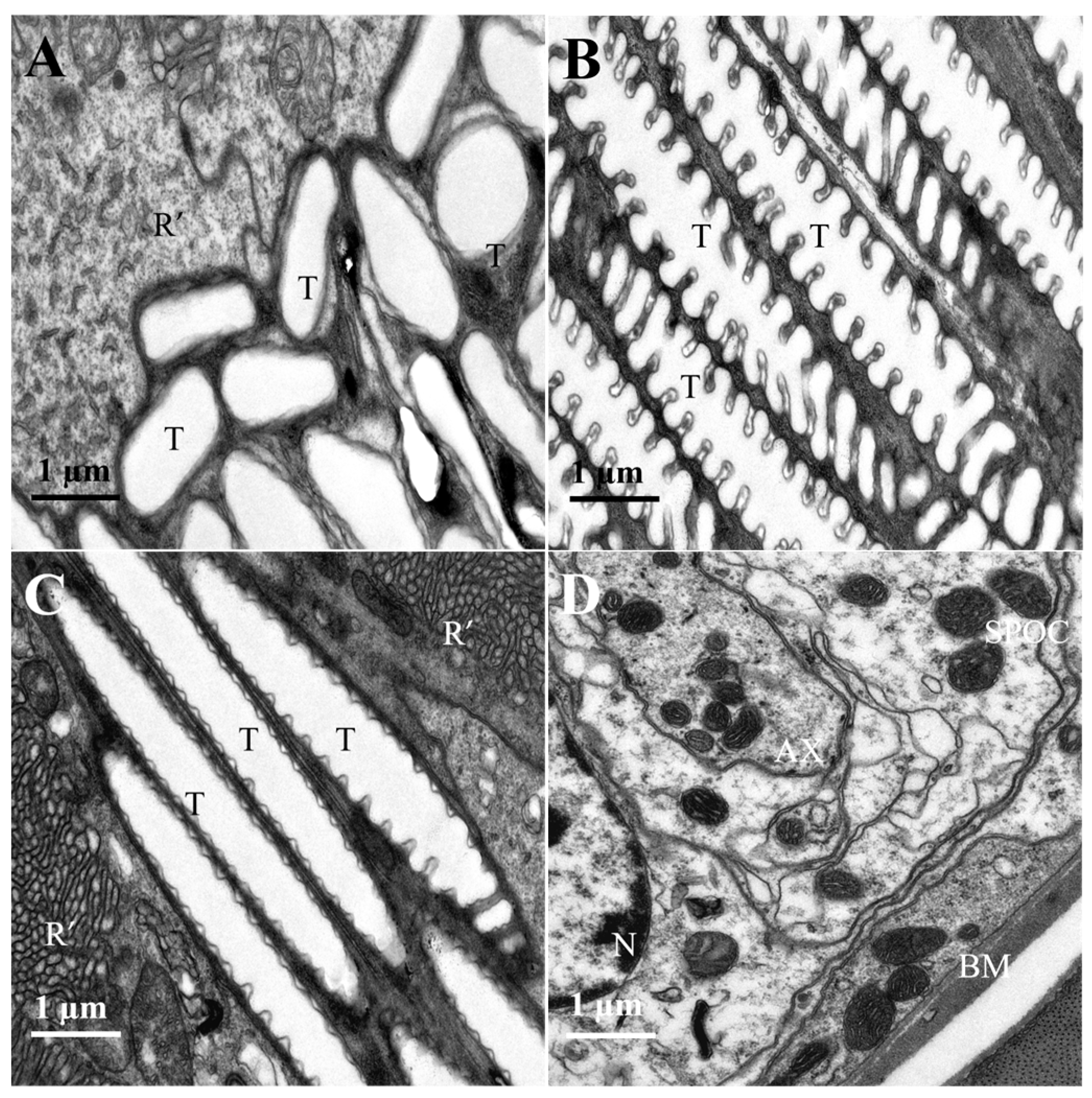
| Parameter | Gender | p-Value | |
|---|---|---|---|
| Female | Male | ||
| Compound eye area (mm2) | 1.85 ± 0.02 | 2.67 ± 0.09 | <0.001 |
| Compound eye perimeter (mm) | 4.84 ± 0.05 | 6.54 ± 0.12 | <0.001 |
| Facet number per eye | 7062.17 ± 42.33 | 7752.83 ± 119.87 | 0.014 |
| Facet area (μm2) | 356.4 ± 64.63 | 562.79 ± 9.66 | 0.002 |
| Facet perimeter (μm) | 74.62 ± 0.94 | 91.83 ± 1.29 | <0.001 |
| Facet spacing (μm) | 0.45 ± 0.06 | 0.57 ± 0.03 | 0.124 |
| Parameter | Gender | p-Value | |
|---|---|---|---|
| Female | Male | ||
| Crystalline cone width (μm) | 13.66 ± 0.70 | 12.33 ± 1.44 | 0.610 |
| Crystalline cone length (μm) | 57.99 ± 1.29 | 61.48 ± 1.19 | 0.026 |
| Corneal thickness (μm) | 16.3 ± 0.41 | 13.43 ± 0.53 | 0.002 |
| Corneal width (μm) | 19.92 ± 1.77 | 19.86 ± 1.63 | 0.981 |
| Interommatidial angle (°) | 3.62 ± 0.25 | 3.49 ± 0.27 | 0.725 |
| Number of Corneal Layers | 28.00 ± 0.58 | 25.00 ± 1.15 | 0.079 |
| Family | Insect | Distal Retinula Cells | Basal Retinula Cells | References |
|---|---|---|---|---|
| Geometridae | Ectropis grisescens | 14 | 1 | [22] |
| Operophtera brumata | 14 | 1 | [29] | |
| Pyralidae | Eutectona machaeralis | 11 | 1 | [27] |
| Ostrinia furnacalis | 11 | 1 | [35] | |
| Nymphalidae | Childrena zenobia | 8 | 1 | [38] |
| Neptis beroe | 8 | 1 | [38] | |
| Sphingidae | Cephonodes hylas | 8 | 1 | [39] |
| Cechenena lineosa | 8 | 1 | [39] | |
| Tortricidae | Grapholita molesta | 7 | 1 | [34] |
| Acleris fimbriana | 7 | 1 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Ni, T.; Liu, S.; Wang, M.; Zheng, J.; Wang, B.; Wu, S.; Zhang, F.; Wang, R. Putative Photosensitivity-Associated Sexual Dimorphism in Compound Eye Structure of Lymantria xylina (Lepidoptera: Erebidae). Insects 2025, 16, 1122. https://doi.org/10.3390/insects16111122
Jiang H, Ni T, Liu S, Wang M, Zheng J, Wang B, Wu S, Zhang F, Wang R. Putative Photosensitivity-Associated Sexual Dimorphism in Compound Eye Structure of Lymantria xylina (Lepidoptera: Erebidae). Insects. 2025; 16(11):1122. https://doi.org/10.3390/insects16111122
Chicago/Turabian StyleJiang, Hui, Tao Ni, Siyi Liu, Meng Wang, Jialing Zheng, Baode Wang, Songqing Wu, Feiping Zhang, and Rong Wang. 2025. "Putative Photosensitivity-Associated Sexual Dimorphism in Compound Eye Structure of Lymantria xylina (Lepidoptera: Erebidae)" Insects 16, no. 11: 1122. https://doi.org/10.3390/insects16111122
APA StyleJiang, H., Ni, T., Liu, S., Wang, M., Zheng, J., Wang, B., Wu, S., Zhang, F., & Wang, R. (2025). Putative Photosensitivity-Associated Sexual Dimorphism in Compound Eye Structure of Lymantria xylina (Lepidoptera: Erebidae). Insects, 16(11), 1122. https://doi.org/10.3390/insects16111122






