CHS-2 Is Involved in the Response of Aedes albopictus (Diptera: Culicidae) Larvae to Cadmium Stress by Mediating the Formation of the Peritrophic Membrane
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Cd Stress on Ae. albopictus
2.3. Microinjection
2.4. Gene Expression Analysis
2.5. Determination of Chitin Content in the Midgut
2.6. Antioxidant Enzyme Activity Assay
2.7. Observation of the PM of the Midgut
2.8. Data Analysis
3. Results
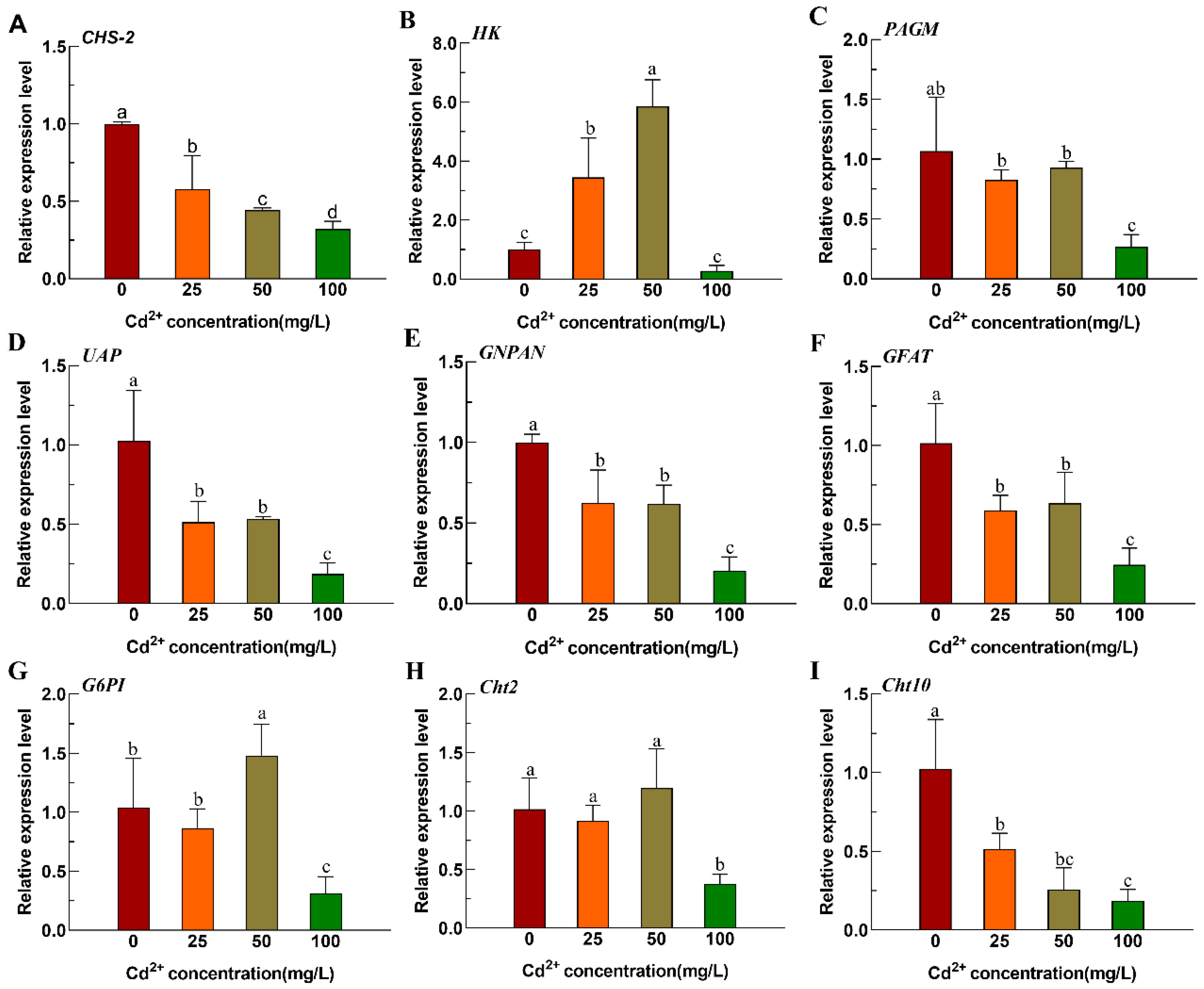
3.1. Expression Changes in Genes Related to Chitin Metabolism Pathway in Ae. albopictus After Cd Stress
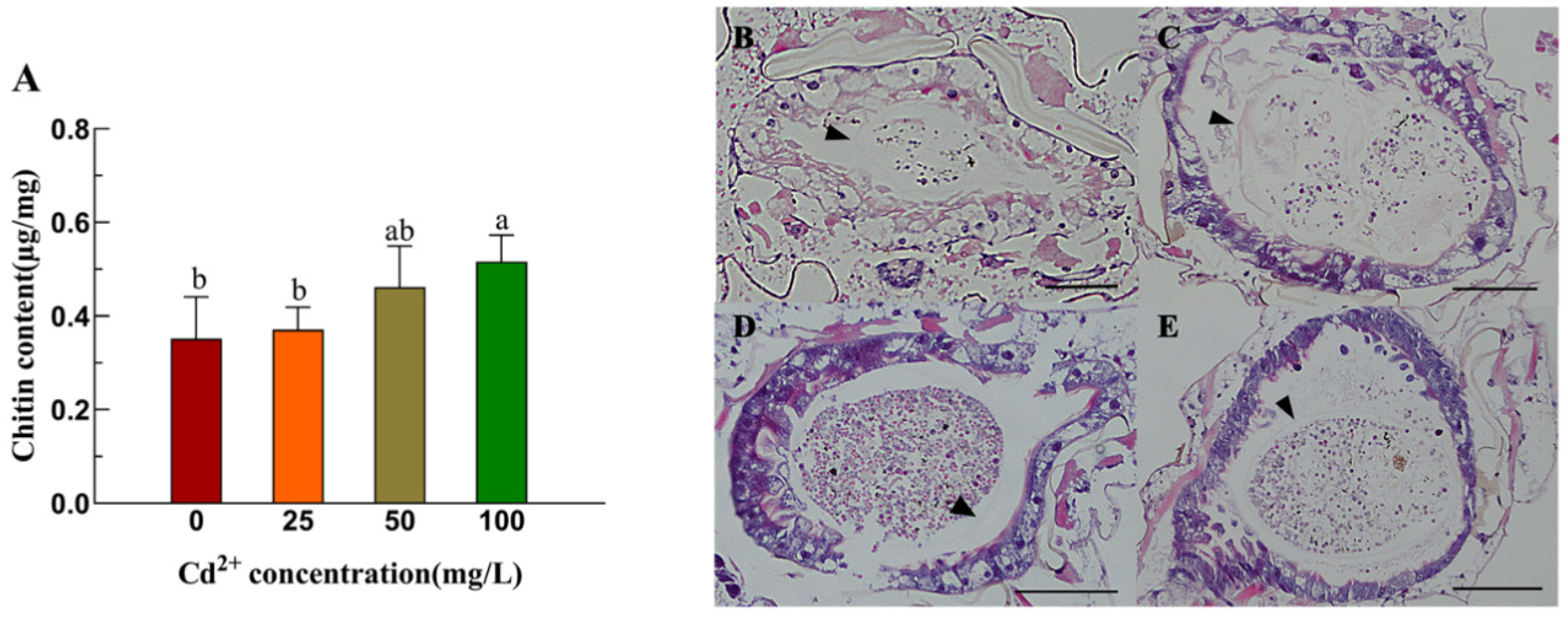
3.2. Determination of Chitin Content and Observation of Midgut Structure of Aedes albopictus Larvae Under Cadmium Stress
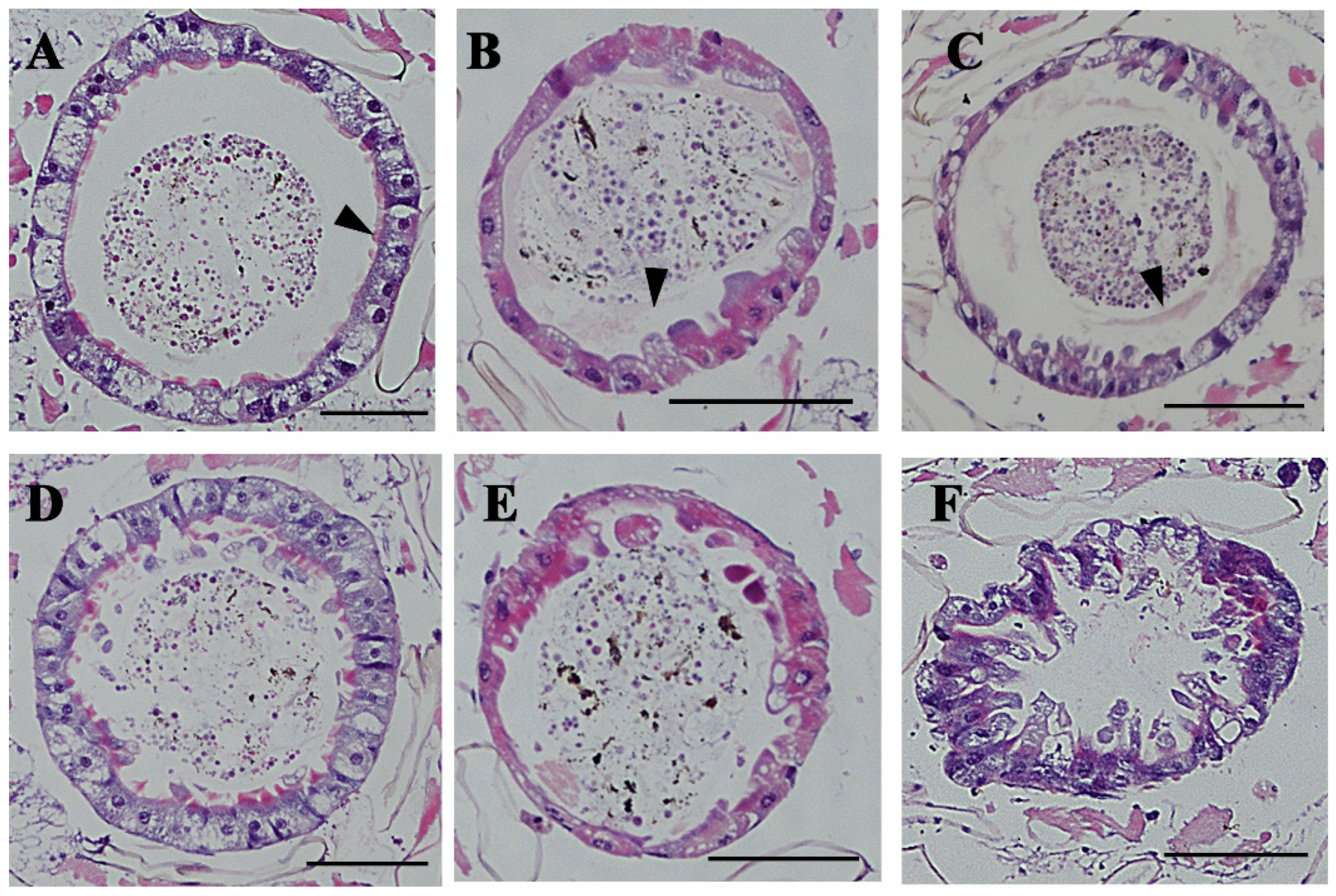
3.3. Morphological Changes in the Midgut of Ae. albopictus Under Cd Stress After CHS-2 Silencing
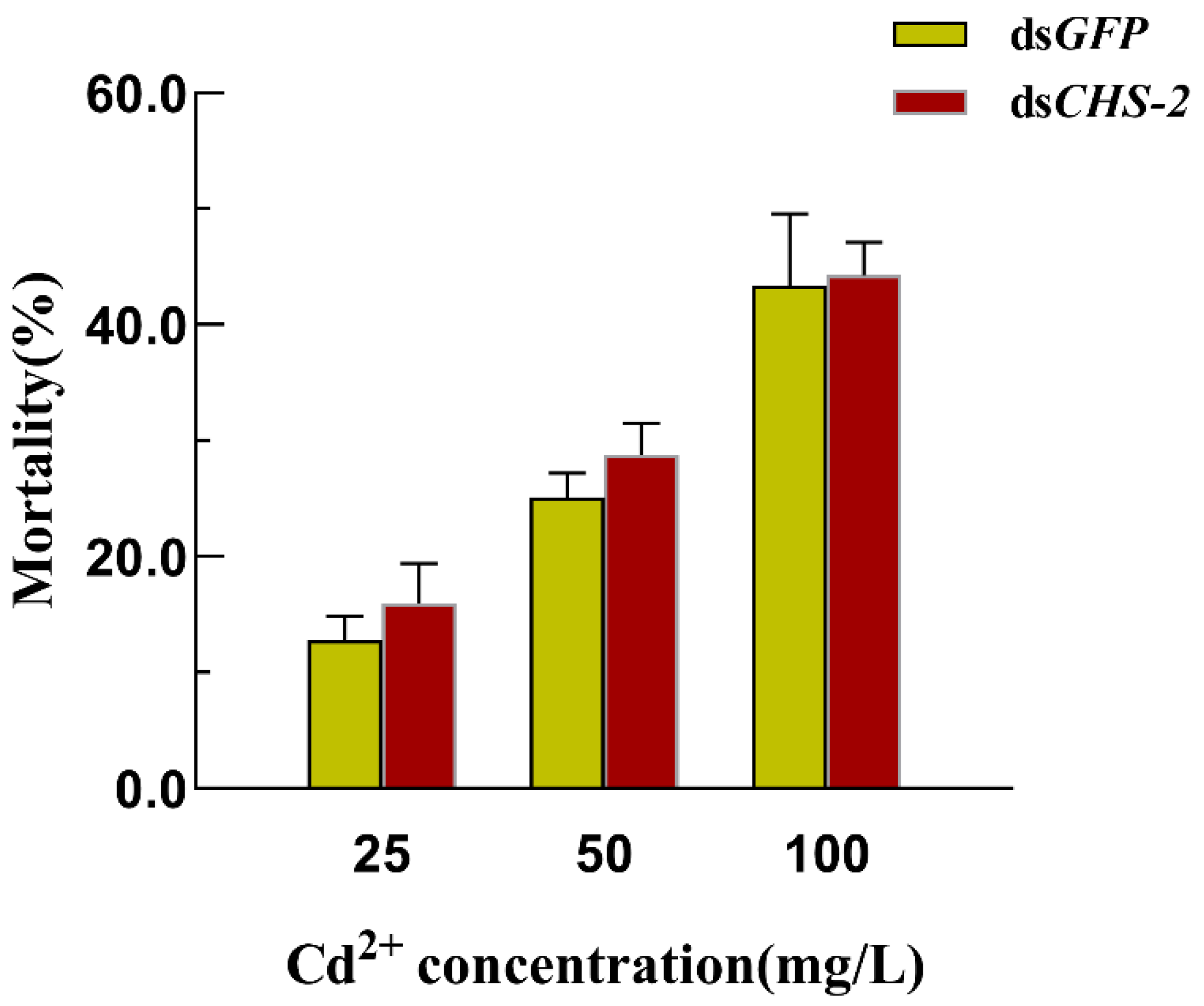
3.4. Mortality of Ae. albopictus After CHS-2 Silencing Combined with Cd Stress
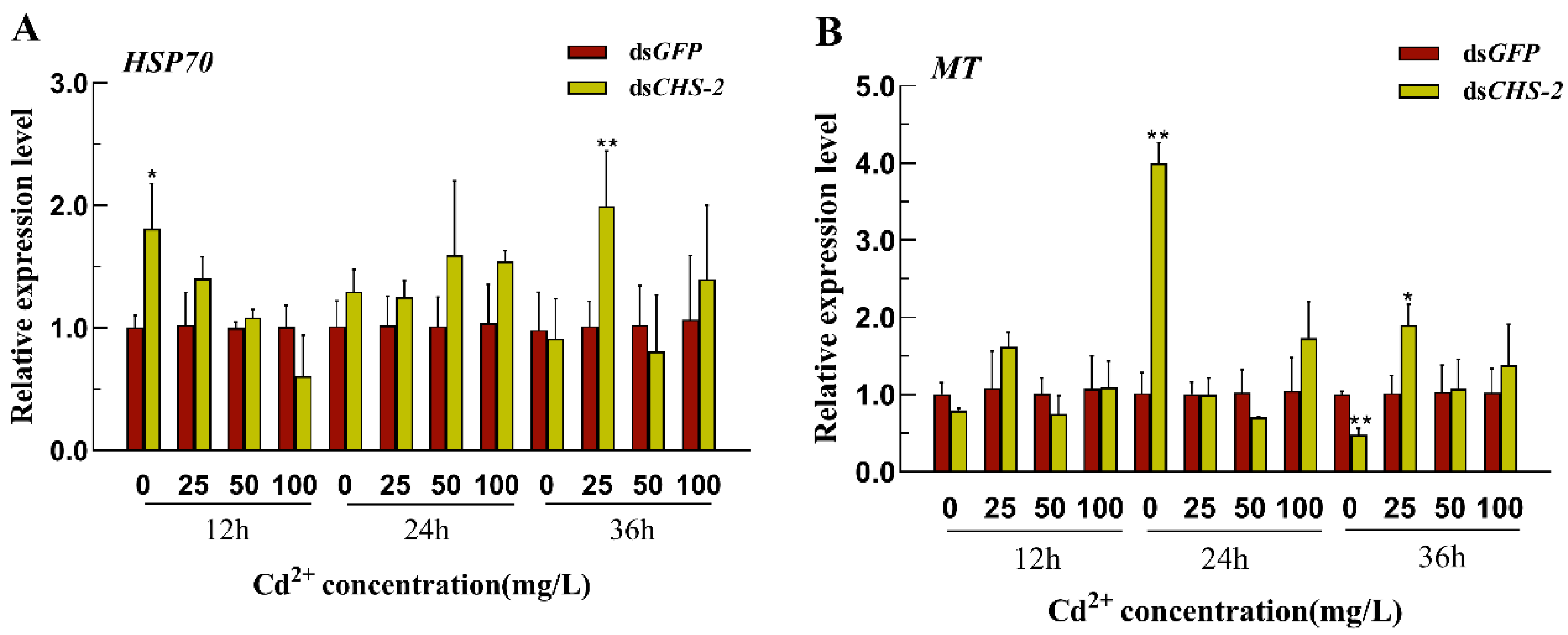
3.5. Changes in HSP70 and MT Expression in Ae. albopictus After CHS-2 Silencing Combined with Acute Cd Stress
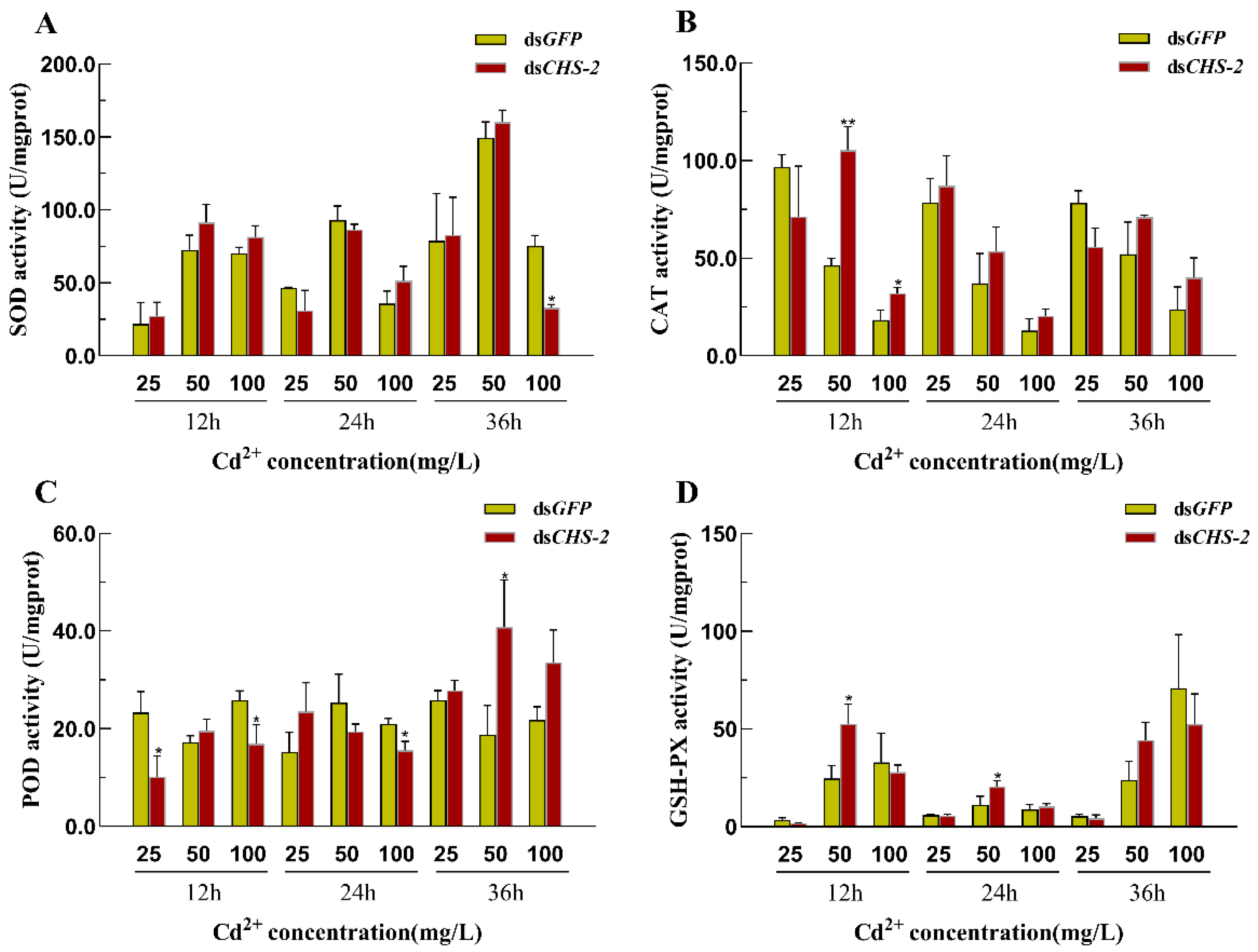
3.6. Changes in the Activities of Antioxidant Enzymes in Ae. albopictus After CHS-2 Silencing Combined with Cd Stress
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, Y.H.; Tao, Y.Q. Pollution status of heavy metals and metalloids in Chinese lakes: Distribution, bioaccumulation and risk assessment. Ecotoxicol. Environ. Saf. 2022, 248, 114293. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Costa, M. Metals and molecular carcinogenesis. Carcinogenesis 2020, 41, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Farías, P.; Hernández-Bonilla, D.; Moreno-Macías, H.; Montes-López, S.; Schnaas, L.; Texcalac-Sangrador, J.L.; Ríos, C.; Riojas-Rodríguez, H. Prenatal Co-Exposure to Manganese, Mercury, and Lead, and Neurodevelopment in Children during the First Year of Life. Int. J. Environ. Res. Public Health 2022, 19, 13020. [Google Scholar] [CrossRef]
- Ma, C.C.; Iwai-Shimada, M.; Nakayama, S.F.; Isobe, T.; Kobayashi, Y.; Tatsuta, N.; Taniguchi, Y.; Sekiyama, M.; Michikawa, T.; Yamazaki, S.; et al. Association of prenatal exposure to cadmium with neurodevelopment in children at 2 years of age: The Japan Environment and Children’s Study. Environ. Int. 2021, 156, 106762. [Google Scholar] [CrossRef]
- Fu, Z.S.; Xi, S.H. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Lian, G.Q.; Lee, X.Q. Concentrations, Distribution, and Pollution Assessment of Metals in River Sediments in China. Int. J. Environ. Res. Public Health 2021, 18, 6908. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.W.; Shu, Y.H. Review on the effects of heavy metal pollution on herbivorous insects. Ying Yong Sheng Tai Xue Bao 2020, 31, 1773–1782. [Google Scholar] [CrossRef]
- Jiang, D.; Tan, M.T.; Guo, Q.X.; Yan, S.C. Transfer of heavy metal along food chain: A mini-review on insect susceptibility to entomopathogenic microorganisms under heavy metal stress. Pest Manag. Sci. 2021, 77, 1115–1120. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, M.I.; Saeed, M.F.; Khalid, S.; Saqib, M.; Arshad, M.; Afzal, M.; Damalas, C.A. Heavy metal exposure through artificial diet reduces growth and survival of Spodoptera litura (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2019, 26, 14426–14434. [Google Scholar] [CrossRef]
- Arambourou, H.; Llorente, L.; Moreno-Ocio, I.; Herrero, O.; Barata, C.; Fuertes, I.; Delorme, N.; Méndez-Fernández, L.; Planelló, R. Exposure to heavy metal-contaminated sediments disrupts gene expression, lipid profile, and life history traits in the midge. Water Res. 2020, 168, 115165. [Google Scholar] [CrossRef]
- Gao, H.H.; Zhao, H.Y.; Du, C.; Deng, M.M.; Du, E.X.; Hu, Z.Q.; Hu, X.S. Life table evaluation of survival and reproduction of the aphid, Sitobion avenae, exposed to cadmium. J. Insect Sci. 2012, 12, 44. [Google Scholar] [CrossRef]
- Lefcort, H.; Vancura, J.; Lider, E.L. 75 years after mining ends stream insect diversity is still affected by heavy metals. Ecotoxicology 2010, 19, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Hensbergen, P.J.; van Velzen, M.J.M.; Nugroho, R.A.; Donker, M.H.; van Straalen, N.M. Metallothionein-bound cadmium in the gut of the insect Orchesella cincta (Collembola) in relation to dietary cadmium exposure. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2000, 125, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.A.; Kristiansen, E.; Andersen, R.A.; Zachariassen, K.E. Cadmium is deposited in the gut content of larvae of the beetle Tenebrio molitor and involves a Cd-binding protein of the low cysteine type. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2008, 148, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Su, H.H.; Hu, M.M.; Harvey-Samuel, T.; Yang, Y.Z. Accumulation and Excretion of Cadmium in Three Successive Generations of Spodoptera exigua (Lepidoptera: Noctuidae) and Impact on the Population Increase. J. Econ. Entomol. 2014, 107, 223–229. [Google Scholar] [CrossRef]
- Hegedus, D.; Erlandson, M.; Gillott, C.; Toprak, U. New Insights into Peritrophic Matrix Synthesis, Architecture, and Function. Annu. Rev. Entomol. 2009, 54, 285–302. [Google Scholar] [CrossRef]
- Liu, X.J.; Cooper, A.M.W.; Zhang, J.Z.; Zhu, K.Y. Biosynthesis, modifications and degradation of chitin in the formation and turnover of peritrophic matrix in insects. J. Insect Physiol. 2019, 114, 109–115. [Google Scholar] [CrossRef]
- Xu, C.D.; Liu, Y.K.; Qiu, L.Y.; Wang, S.S.; Pan, B.Y.; Li, Y.; Wang, S.G.; Tang, B. GFAT and PFK genes show contrasting regulation of chitin metabolism in Nilaparvata lugens. Sci. Rep. 2021, 11, 5246. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.Q.; Li, G.Y.; Song, Q.S.; Stanley, D.; Wei, S.J.; Zhu, J.Y. Genomic and transcriptomic analyses of chitin metabolism enzymes in Tenebrio molitor. Arch. Insect Biochem. Physiol. 2022, 111, e21950. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Y.J.; Zhou, M.; Tang, Y.; Chen, R.F.; Chen, Y.R.; Wen, Y.T.; Wang, S.G. RNAi-mediated CHS-2 silencing affects the synthesis of chitin and the formation of the peritrophic membrane in the midgut of Aedes albopictus larvae. Parasites Vectors 2023, 16, 259. [Google Scholar] [CrossRef]
- Joly, A.L.; Wettstein, G.; Mignot, G.; Ghiringhelli, F.; Garrido, C. Dual Role of Heat Shock Proteins as Regulators of Apoptosis and Innate Immunity. J. Innate Immun. 2010, 2, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Planelló, R.; Martínez-Paz, P.; Herrero, O.; Cortés, E.; Martínez-Guitarte, J.L.; Morcillo, G. Characterization of Hsp70 gene in Chironomus riparius: Expression in response to endocrine disrupting pollutants as a marker of ecotoxicological stress. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2011, 153, 150–158. [Google Scholar] [CrossRef]
- Gu, B.N.; Liang, W.; Yang, T.Z.; Hu, Z.J.; Shen, H.D. Metallothionein, hemocyte status and superoxide dismutase/aspartate aminotransferase activity are sensitive biomarkers of cadmium stress in. Aquat. Toxicol. 2019, 215, 105284. [Google Scholar] [CrossRef]
- El-Gendy, A.H.; Augustyniak, M.; Toto, N.A.; Al Farraj, S.; El-Samad, L.M. Oxidative stress parameters, DNA damage and expression of HSP70 and MT in midgut of Trachyderma hispida(Forskal, 1775) (Coleoptera: Tenebrionidae) from a textile industry area. Environ. Pollut. 2020, 267, 115661. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Y.; Chen, X.M.; Wei, Y.; Ding, Y.J.; Wang, Q.W.; Wang, S.H.; Tang, B.; Wang, S.G. Effects of long-term cadmium exposure on trehalose metabolism, growth, and development of Aedes albopictus (Diptera: Culicidae). Ecotoxicol. Environ. Saf. 2020, 204, 111034. [Google Scholar] [CrossRef]
- Puggioli, A.; Balestrino, F.; Damiens, D.; Lees, R.S.; Soliban, S.M.; Madakacherry, O.; Dindo, M.L.; Bellini, R.; Gilles, J.R.L. Efficiency of Three Diets for Larval Development in Mass Rearing Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 819–825. [Google Scholar] [CrossRef]
- GB/T 602-2002; Chemical Reagent—Preparations of Standard Solutions for Impurities. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2002.
- Liu, X.Y.; Wang, S.S.; Yu, Y.Y.; Cheng, Y.S.; Hu, C.X.; Zhou, M.; Li, C.; Tang, B.; Wu, Y. Effects of Inhibiting the Expression of Chitin Synthase Gene sfCHSB on the Metabolism of Trehalose and Chitin in Spodoptera frugiperda Larvae. Agriculture 2022, 12, 2019. [Google Scholar] [CrossRef]
- Gregor, K.M.; Becker, S.C.; Hellhammer, F.; Schön, K.; Baumgärtner, W.; Puff, C. Histochemical staining techniques in Culex pipiens and Drosophila melanogaster (Diptera) with a comparison to mammals. Vet. Pathol. 2022, 59, 836–849. [Google Scholar] [CrossRef]
- Braeckman, B.; Raes, H.; VanHoye, D. Heavy-metal toxicity in an insect cell line. Effects of cadmium chloride, mercuric chloride and methylmercuric chloride on cell viability and proliferation in Aedes albopictus cells. Cell Biol. Toxicol. 1997, 13, 389–397. [Google Scholar] [CrossRef]
- Mireji, P.O.; Keating, J.; Hassanali, A.; Mbogo, C.M.; Muturi, M.N.; Githure, J.I.; Beier, J.C. Biological cost of tolerance to heavy metals in the mosquito Anopheles gambiae. Med. Vet. Entomol. 2010, 24, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Abedi, Z.H.; Brown, A.W.A. Peritrophic Membrane as Vehicle for DDT and DDE Excretion in Aedes aegypti Larvae. Ann. Entomol. Soc. Am. 1961, 54, 539–542. [Google Scholar] [CrossRef]
- Lin, X.W.; Xu, W.H. Hexokinase is a key regulator of energy metabolism and ROS activity in insect lifespan extension. Aging 2016, 8, 245–259. [Google Scholar] [CrossRef]
- Zimoch, L.; Hogenkamp, D.G.; Kramer, K.J.; Muthukrishnan, S.; Merzendorfer, H. Regulation of chitin synthesis in the larval midgut of Manduca sexta. Insect Biochem. Mol. Biol. 2005, 35, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.X.; Gao, X.; Ye, G.Y.; Li, K.; Hu, C.; Cheng, J.A. Ultrastructural alterations in midgut and Malpighian tubules of Boettcherisca peregrina exposure to cadmium and copper. Ecotoxicol. Environ. Saf. 2009, 72, 1137–1147. [Google Scholar] [CrossRef]
- Dabour, K.; Al Naggar, Y.; Masry, S.; Naiem, E.; Giesy, J.P. Cellular alterations in midgut cells of honey bee workers (Apis millefera L.) exposed to sublethal concentrations of CdO or PbO nanoparticles or their binary mixture. Sci. Total Environ. 2019, 651, 1356–1367. [Google Scholar] [CrossRef]
- Luo, M.; Finet, C.; Cong, H.S.; Wei, H.Y.; Chung, H. The evolution of insect metallothioneins. Proc. R. Soc. B-Biol. Sci. 2020, 287, 20202189. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.H.; Gao, Z.H.; Wen, Y.T.; Wang, W.Q.; Liu, W.; Wang, X.P.; Zhu, F. Identification of three metallothioneins in the black soldier fly and their functions in Cd accumulation and detoxification. Environ. Pollut. 2021, 286, 117146. [Google Scholar] [CrossRef]
- Aoki, Y.; Suzuki, K.T.; Kubota, K. Accumulation of Cadmium and Induction of Its Binding-Protein in the Digestive-Tract of Fleshfly (Sarcophaga-Peregrina) Larvae. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 1984, 77, 279–282. [Google Scholar] [CrossRef]
- Kafel, A.; Nowak, A.; Bembenek, J.; Szczygiel, J.; Nakonieczny, M.; Swiergosz-Kowalewska, R. The localisation of HSP70 and oxidative stress indices in heads of Spodoptera exigua larvae in a cadmium-exposed population. Ecotoxicol. Environ. Saf. 2012, 78, 22–27. [Google Scholar] [CrossRef]
- Somasundaram, S.; Abraham, J.S.; Maurya, S.; Toteja, R.; Gupta, R.; Makhija, S. Expression and molecular characterization of stress-responsive genes (hsp70 and Mn-sod) and evaluation of antioxidant enzymes (CAT and GPx) in heavy metal exposed freshwater ciliate, Tetmemena sp. Mol. Biol. Rep. 2019, 46, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Wu, W.J.; Guo, J.X.; Xiao, R.; Jiang, F.Z.; Zheng, L.Y.; Zhang, G.R. Effects of nickel exposure on testicular function, oxidative stress, and male reproductive dysfunction in Spodoptera litura Fabricius. Chemosphere 2016, 148, 178–187. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Liang, H.W.; Zou, L.H.; Sun, J.J.; Zhang, Y.Y.; Qin, F.; Liu, S.Z.; Wang, Z.Z. Molecular cloning and characterization of cat, gpx1 and Cu/Zn-sod genes in pengze crucian carp (Carassius auratus var. Pengze) and antioxidant enzyme modulation induced by hexavalent chromium in juveniles. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2013, 157, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, A.; Mrdakovic, M.; Ilijin, L.; Vlahovic, M.; Todorovic, D.; Grcic, A.; Peric-Mataruga, V. Effect of fluoranthene on antioxidative defense in different tissues of Lymantria dispar and Euproctis chrysorrhoea larvae. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2019, 224, 108565. [Google Scholar] [CrossRef]
- Peric-Mataruga, V.; Ilijin, L.; Mrdakovic, M.; Todorovic, D.; Prokic, M.; Matic, D.; Vlahovic, M. Parameters of oxidative stress, cholinesterase activity, Cd bioaccumulation in the brain and midgut of Lymantria dispar (Lepidoptera: Lymantriidae) caterpillars from unpolluted and polluted forests. Chemosphere 2019, 218, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Song, D.N.; Wu, H.H.; Yang, H.M.; Zhang, J.Z.; Li, L.J.; Ma, E.B.; Guo, Y.P. Effect of Dietary Cadmium on the Activity of Glutathione S-Transferase and Carboxylesterase in Different Developmental Stages of the Oxya chinensis (Orthoptera: Acridoidea). Environ. Entomol. 2014, 43, 171–177. [Google Scholar] [CrossRef]
- Stdimitrova, M.; Tishinova, V.; Velcheva, V. Combined Effect of Zinc and Lead on the Hepatic Superoxide Dismutase-Catalase System in Carp (Cyprinus-Carpio). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 1994, 108, 43–46. [Google Scholar] [CrossRef]
- Ma, C.P.; Guo, Z.M.; Zhang, F.L.; Su, J.Y. Molecular identification, expression and function analysis of peroxidasin in. Insect Sci. 2020, 27, 1173–1185. [Google Scholar] [CrossRef]
- Terra, W.R. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch. Insect Biochem. Physiol. 2001, 47, 47–61. [Google Scholar] [CrossRef]
| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | NCBI |
|---|---|---|---|
| dsCHS-2 | GGATCCTAATACGACTCACTATAGGAGTCAACGCACCGTACAGGA | GGATCCTAATACGACTCACTATAGGGAAGAGCAACACCAAACCGA | LOC109405983 |
| dsGFP | GGATCCTAATACGACTCACTATAGGAAGGGCGAGGAGCTGTTCACCG | GGATCCTAATACGACTCACTATAGGCAGCAGGACCATGTGATCGCGC | XM_013480425.1 |
| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | NCBI |
|---|---|---|---|
| actin | GCTACGTCGCCCTGCACTT | AGGAACGACGGCTGGAAGA | DQ657949.1 |
| CHS-2 | GGAGACCAAAGGATGGGACG | CCTGTAAGGACGATGACGAATGT | XM_019679038.3 |
| Cht2 | GACTCGGATGACAAGGGTTT | CAGGGCTTCACATACTTCGTT | XM_029879282.1 |
| Cht10 | GCCACGGATACTTAGAATAGCG | CTGTTTGACGGTCGTTGATTT | XM_029869372.1 |
| HK | GGGAGAAGCCAGCGAAGATA | GATGTCTGCTTCGGGATGTG | AY705876.1 |
| PAGM | GCCATTTCTGACATGCTACT | CGTTTCGGTCTTCTACTTTGA | XM_019671844.2 |
| UAP | AGTGCTCTATTTACACGCTCAT | ACTCCGACCGCTTCATTT | GAPW01001510.1 |
| GNPNA | CAGTCCTCCCATTTCAGCC | AACGAAACATCGCCCACC | XM_019707567.2 |
| GFAT | TCAACGGGCAACATCCAG | AAGCGTCCGATGCAAAGA | XM_019671518.2 |
| G6PI | GGAGGATGACATTCGCTTCG | CGGTGATACGGTTCTTGGAG | XM_019673634.3 |
| HSP70 | GATGTGTCCCTTTTGACCATT | CGACTTCACGACGCAGTTTCT | JN132154.1 |
| MT | ATGCCTTGCCCATGCGA | CAGTCAGATCCGCAGTTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Ding, Y.; Lan, R.; Zhou, M.; Chen, Y.; Tang, B.; Qiao, G.; Wang, S. CHS-2 Is Involved in the Response of Aedes albopictus (Diptera: Culicidae) Larvae to Cadmium Stress by Mediating the Formation of the Peritrophic Membrane. Insects 2025, 16, 1102. https://doi.org/10.3390/insects16111102
Zhang C, Ding Y, Lan R, Zhou M, Chen Y, Tang B, Qiao G, Wang S. CHS-2 Is Involved in the Response of Aedes albopictus (Diptera: Culicidae) Larvae to Cadmium Stress by Mediating the Formation of the Peritrophic Membrane. Insects. 2025; 16(11):1102. https://doi.org/10.3390/insects16111102
Chicago/Turabian StyleZhang, Chen, Yanjuan Ding, Ruoyun Lan, Min Zhou, Yanrong Chen, Bin Tang, Gexia Qiao, and Shigui Wang. 2025. "CHS-2 Is Involved in the Response of Aedes albopictus (Diptera: Culicidae) Larvae to Cadmium Stress by Mediating the Formation of the Peritrophic Membrane" Insects 16, no. 11: 1102. https://doi.org/10.3390/insects16111102
APA StyleZhang, C., Ding, Y., Lan, R., Zhou, M., Chen, Y., Tang, B., Qiao, G., & Wang, S. (2025). CHS-2 Is Involved in the Response of Aedes albopictus (Diptera: Culicidae) Larvae to Cadmium Stress by Mediating the Formation of the Peritrophic Membrane. Insects, 16(11), 1102. https://doi.org/10.3390/insects16111102








