Distinct Modulation of Feeding Behavior in the Whitefly Vector Bemisia tabaci MED by ToCV Single-Infection Versus Synergistic Co-Infection with TYLCV
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants, Whitefly Population, and Viruses
2.2. Establishment of ToCV-Infected and ToCV&TYLCV Co-Infected Tomato Plants
2.3. Establishment of ToCV-Viruliferous and ToCV&TYLCV-Viruliferous Whitefly Colonies
2.4. Electrical Penetration Graph (EPG) Recording
2.5. Data Analysis
3. Results
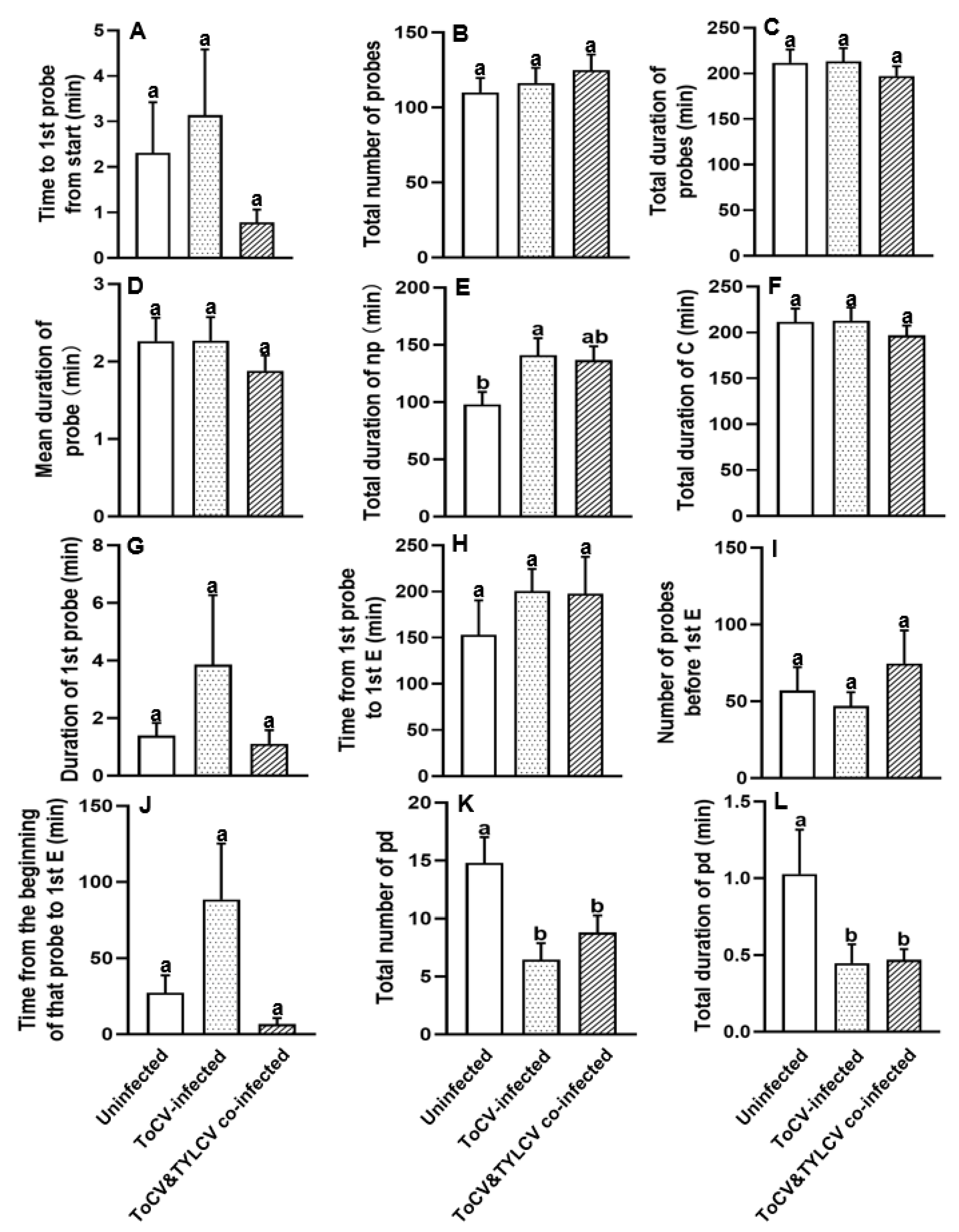
3.1. Feeding Behavior at Non-Phloem Phase of Bemisia tabaci on ToCV-Infected, ToCV&TYLCV Co-Infected, and Un-Infected Tomato Plants
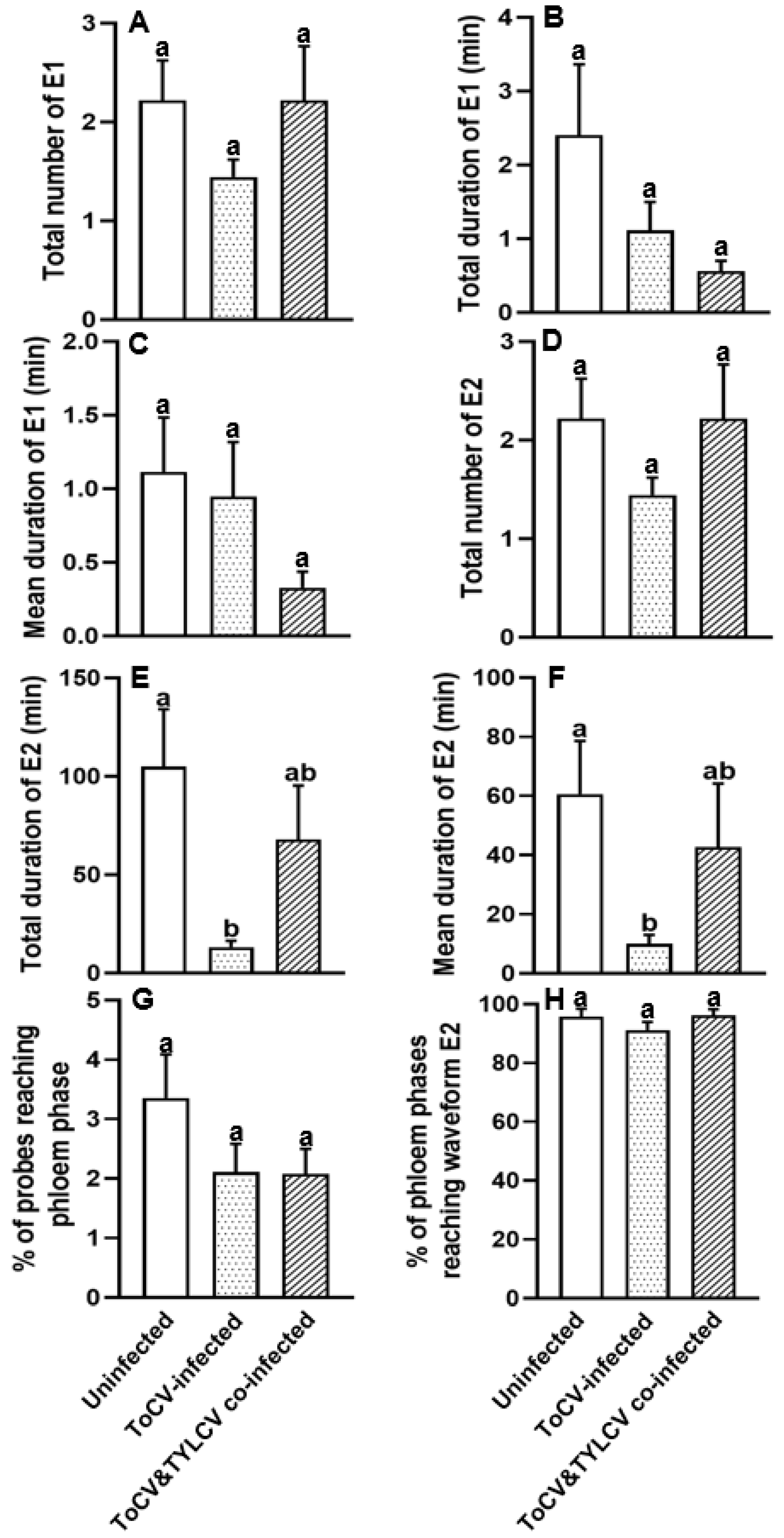
3.2. Feeding Behavior at Phloem Phase of Bemisia tabaci on ToCV-Infected, ToCV&TYLCV Co-Infected, and Un-Infected Tomato Plants
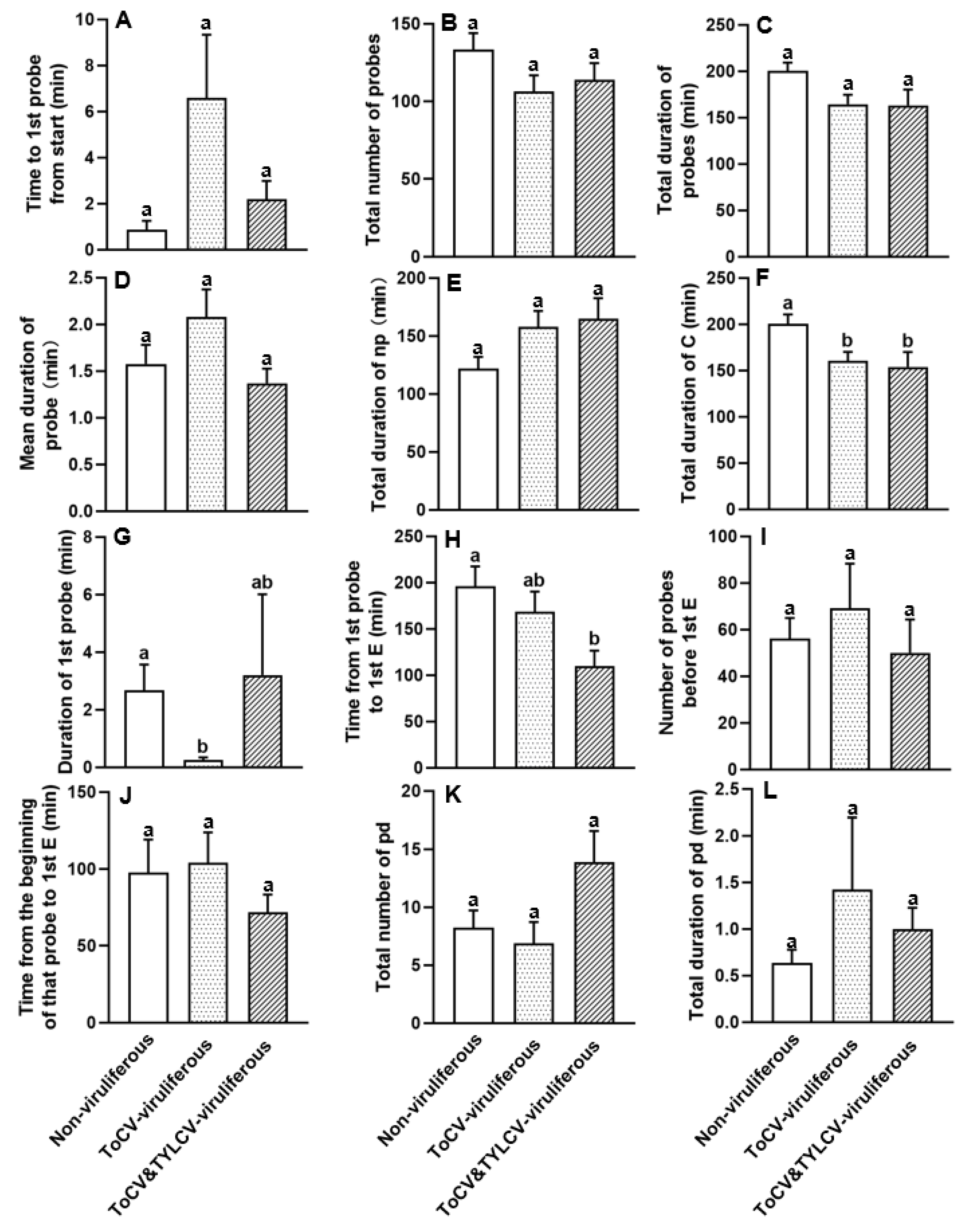
3.3. Feeding Behavior at Non-Phloem Phase of ToCV-Viruliferous, ToCV&TYLCV-Viruliferous, and Non-Viruliferous Bemisia tabaci on Cotton Plants
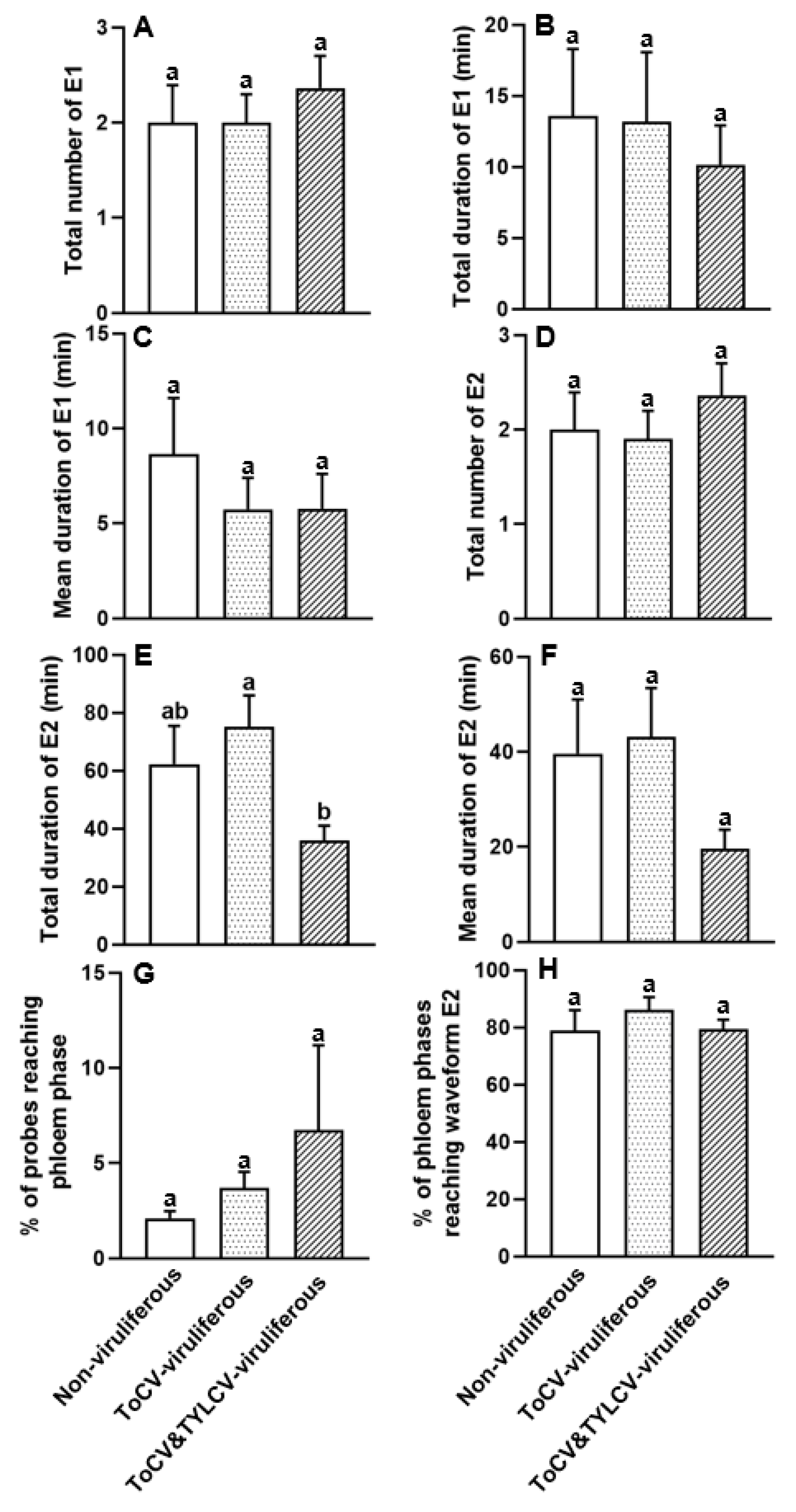
3.4. Feeding Behavior at Phloem Phase of ToCV-Viruliferous, ToCV&TYLCV-Viruliferous, and Non-Viruliferous Bemisia tabaci on Cotton Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanakala, S.; Ghanim, M. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS ONE 2019, 14, e0213946. [Google Scholar] [CrossRef] [PubMed]
- Oliveria, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status and collaborative research projects for Bemisia tabaci. Crop. Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef]
- Dalton, R. Whitefly infestations: The Christmas invasion. Nature 2006, 443, 898–900. [Google Scholar] [CrossRef]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olive, E.; Sanchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Polston, J.E.; De-Barro, P.J.; Boykin, L.M. Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Manag. Sci. 2014, 70, 1547–1552. [Google Scholar] [CrossRef]
- Guo, C.L.; Zhu, Y.Z.; Zhang, Y.J.; Keller, M.A.; Liu, T.X.; Chu, D. Invasion biology and management of sweetpotato whitefly (Hemiptera: Aleyrodidae) in China. J. Integr. Pest Manag. 2021, 12, 2. [Google Scholar] [CrossRef]
- Pan, H.P.; Chu, D.; Yan, W.Q.; Su, Q.; Liu, B.M.; Wang, S.L.; Wu, Q.J.; Xie, W.; Jiao, X.G.; Li, R.M.; et al. Rapid spread of Tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS ONE 2012, 7, e34817. [Google Scholar] [CrossRef]
- Ning, W.X.; Shi, X.B.; Liu, B.M.; Pan, H.P.; Wei, W.T.; Zeng, Y.; Sun, X.P.; Xie, W.; Wang, S.L.; Wu, Q.J.; et al. Transmission of Tomato yellow leaf curl virus by Bemisia tabaci as affected by whitefly sex and biotype. Sci. Rep. 2015, 5, 10744. [Google Scholar] [CrossRef]
- Dai, H.J.; Liu, Y.G.; Zhu, X.P.; Liu, Y.J.; Zhao, J. Tomato chlorosis virus (ToCV) transmitted by Bemisia tabaci biotype Q of Shouguang in Shandong Province. J. Plant Prot. 2016, 43, 162–167. [Google Scholar] [CrossRef]
- Wisler, G.C.; Li, R.H.; Liu, H.Y.; Lowry, D.; Duffus, J.E. Tomato chlorosis virus: A new whitefly-transmitted, phloem-limited, bipartite closterovirus of tomato. Phytopathology 1998, 88, 402–409. [Google Scholar] [CrossRef]
- Accotto, G.; Vaira, A.; Vecchiati, M.; Finetti-Sialer, M.; Gallitelli, D.; Davino, M. First report of Tomato chlorosis virus in Italy. Plant Dis. 2001, 85, 1208. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.S.; Shih, S.L.; Green, S.K.; Hanson, P.; Liu, H.Y. First report of the occurrence of Tomato chlorosis virus and Tomato infectious chlorosis virus in Taiwan. Plant Dis. 2004, 88, 311. [Google Scholar] [CrossRef] [PubMed]
- Lett, J.M.; Hoareau, M.; Reynaud, B.; Saison, A.; Hostachy, B.; Lobin, K.; Benimadhu, S.P. First report of Tomato chlorosis virus in tomato on Mauritius Island. Plant Dis. 2009, 93, 111. [Google Scholar] [CrossRef] [PubMed]
- Arruabarrena, A.; Rubio, L.; Gonzálezarcos, M.; Maeso, D.; Fonseca, M.E.N.; Boiteux, L.S. First report of tomato chlorosis virus infecting tomato crops in Uruguay. Plant Dis. 2014, 98, 1445. [Google Scholar] [CrossRef]
- Zhao, L.M.; Li, G.; Liu, Y.G.; Guo, J.J.; Wei, J.P.; Zhu, X.P. Molecular identification on mixed infections of Tomato chlorosis virus and Tomato yellow leaf curl virus. China Veg. 2014, 12, 15–20. [Google Scholar] [CrossRef]
- Wu, S.H.; Li, T.F.; Zhao, W.H.; Cheng, Z.B.; Guo, Q.Y.; Zhao, T.M.; Yu, W.G.; Zhu, Y.Q.; Ji, Y.H. Molecular identification on mixed infection of Tomato yellow leaf curl virus and Tomato chlorosis virus on tomato in Jiangsu Province. Acta Hortic. Sin. 2016, 43, 89–99. [Google Scholar]
- Liu, W.; Shi, X.B.; Tang, X.; Zhang, Y.; Zhang, D.Y.; Zhou, X.G.; Liu, Y. Molecular identification of Tomato chlorosis virus and Tomato yellow leaf curl virus in Yunnan Province. Acta Hortic. Sin. 2018, 45, 552–560. [Google Scholar] [CrossRef]
- Chen, G.; Pan, H.P.; Xie, W.; Wang, S.L.; Wu, Q.J.; Fang, Y.; Shi, X.B.; Zhang, Y.J. Virus infection of a weed increases vector attraction to and vector fitness on the weed. Sci. Rep. 2013, 3, 2253. [Google Scholar] [CrossRef]
- Su, Q.; Preisser, E.L.; Zhou, X.M.; Xie, W.; Liu, B.M.; Wang, S.L.; Wu, Q.J.; Zhang, Y.J. Manipulation of host quality and defense by a plant virus improves performance of whitefly vectors. J. Econ. Entomol. 2015, 108, 11–19. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.X.; Liu, X.M.; Michaud, J.P.; Zhi, H.J.; Li, K.; Li, X.R.; Li, Z. Host plant infection by soybean mosaic virus reduces the fitness of its vector, Aphis glycines (Hemiptera: Aphididae). J. Econ. Entomol. 2018, 111, 2017–2023. [Google Scholar] [CrossRef]
- Ding, T.B.; Zhou, X.; Yang, N.; Yang, Y.; Tang, Y.; Chu, D. Host adaptability of Bemisia tabaci on tomato plants with ToCV single infection and TYLCV&ToCV co-infection and the changes in the nutrient contents and defense responses of host plants. Acta Entomol. Sin. 2021, 64, 384–391. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Ammar, E.D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 9350–9355. [Google Scholar] [CrossRef]
- Moreno-Delafuente, A.; Garzo, E.; Moreno, A.; Fereres, A. A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. PLoS ONE 2013, 8, e61543. [Google Scholar] [CrossRef] [PubMed]
- Wamonje, F.O.; Donnelly, R.; Tungadi, T.D.; Murphy, A.M.; Pate, A.E.; Woodcock, C.; Caulfield, J.; Musembi-Mutuku, J.; Bruce, T.J.A.; Gilligan, C.A.; et al. Different plant viruses induce changes in feeding behavior of specialist and generalist aphids on common bean that are likely to enhance virus transmission. Front. Plant Sci. 2020, 10, 1811. [Google Scholar] [CrossRef]
- Fan, X.F.; Liu, Y.; Tan, X.Q.; Zhang, Z.H.; Zhang, Z.; Shi, X.B. Effects of Tomato chlorosis virus on the host preference and feeding behaviors of Bemisia tabaci MED (Hemiptera: Aleyrodidae) Adults on different host plants. Acta Entomol. Sin. 2022, 65, 1018–1025. [Google Scholar] [CrossRef]
- Mauck, K.; Nilsa, A.; Bosque-Pérez Eigenbrode, S.D.; Consuelo, M.; Moraes, D.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host-vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Liu, B.M.; Preisser, E.L.; Chu, D.; Pan, H.P.; Xie, W.; Wang, S.L.; Wu, Q.J.; Zhou, X.G.; Zhang, Y.J. Multiple forms of vector manipulation by a plant-infecting virus: Bemisia tabaci and Tomato yellow leaf curl virus. J. Virol. 2013, 87, 4929–4937. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, S.S.; Wang, X.W.; Yang, J.G.; Pan, L.L. Manipulation of whitefly behavior by plant viruses. Microorganisms 2022, 10, 2410. [Google Scholar] [CrossRef]
- Maluta, N.; Fereres, A.; Lopes, J.R.S. Plant-mediated indirect effects of two viruses with different transmission modes on Bemisia tabaci feeding behavior and fitness. J. Pest Sci. 2019, 92, 405–416. [Google Scholar] [CrossRef]
- Ontiveros, I.; López-Moya, J.J.; Díaz-Pendón, J.A. Coinfection of tomato plants with Tomato yellow leaf curl virus and Tomato chlorosis virus affects the interaction with host and whiteflies. Phytopathology 2022, 112, 944–952. [Google Scholar] [CrossRef]
- Chu, D.; Hu, X.; Gao, X.; Zhao, H.; Nichols, R.L. LX Use of of mitochondrial cytochrome oxidase I polymerase chain reaction-restriction fragment length polymorphism for identifying subclades of Bemisia tabaci Mediterranean group. J. Econ. Entomol. 2012, 105, 242–251. [Google Scholar] [CrossRef]
- Chu, D.; Zhang, Y.J.; Wan, F.H. Cryptic invasion of the exotic Bemisia tabaci biotype Q occurred widespread in Shandong Province of China. Fla. Entomol. 2010, 93, 203–207. [Google Scholar] [CrossRef]
- Ma, W.H.; Li, X.C.; Dennehy, T.J.; Lei, C.L.; Wang, M.; Degain, B.A.; Nichols, R.L. Utility of MtCOI polymerase chain reaction-restriction fragment length polymorphism in differentiating between Q and B whitefly Bemisia tabaci biotypes. Insect Sci. 2009, 16, 107–114. [Google Scholar] [CrossRef]
- Dovas, C.I.; Katis, N.I.; Avgelis, A.D. Multiplex detection of criniviruses associated with epidemics of a yellowing disease of tomato in Greece. Plant Dis. 2002, 86, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Li, C.B.; Cui, Y.L.; Zhang, L.Y.; Li, C.Y. Molecular detection of tomato yellow leaf curl virus (TYLCV). Hereditas 2012, 34, 366–370. [Google Scholar] [CrossRef]
- Ding, T.B.; Li, J.; Chen, E.H.; Niu, J.Z.; Chu, D. Transcriptome profiling of the whitefly Bemisia tabaci MED in response to single infection of Tomato yellow leaf curl virus, Tomato chlorosis virus, and their co-infection. Front. Physiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Maluta, N.K.P.; Garzo, E.; Moreno, A.; Navascastillo, J.; Fialloolive, E.; Lopes, J.R.; Fereres, A. Stylet penetration activities of the whitefly Bemisia tabaci associated with inoculation of the crinivirus Tomato chlorosis virus. J. Gen. Virol. 2017, 98, 1515–1520. [Google Scholar] [CrossRef]
- Jiang, Y.; Lei, H.; Collar, J.; Martin, B.; Muniz, M.; Fereres, A. Probing and feeding behavior of two distinct biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on tomato plants. J. Econ. Entomol. 1999, 92, 357–366. [Google Scholar] [CrossRef]
- Jiang, Y.; de Blas, C.; Barrios, L.; Fereres, A. Correlation between whitefly (Homoptera: Aleyrodidae) feeding behavior and transmission of Tomato yellow leaf curl virus. Ann. Entomol. Soc. Am. 2000, 93, 573–579. [Google Scholar] [CrossRef]
- Lu, S.H.; Chen, M.S.; Li, J.J.; Shi, Y.; Gu, Q.S.; Yan, F.M. Changes in Bemisia tabaci feeding behaviors caused directly and indirectly by Cucurbit chlorotic yellows virus. Virol. J. 2019, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.; Cunniffe, N.J.; Carr, J.P.; Gilligan, C.A. Pathogenic modification of plants enhances long-distance dispersal of nonpersistently transmitted viruses to new hosts. Ecology 2019, 100, e02725. [Google Scholar] [CrossRef]
- Zhang, T.; Luan, J.B.; Qi, J.F.; Huang, C.J.; Li, M.; Zhou, X.P.; Liu, S.S. Begomovirus-whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol. Ecol. 2012, 21, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Weldegergis, R.T.; Li, J.; Jung, C.; Jing, Q.; Sun, Y.; Qian, H.; Tee, C.; van Loon, J.J.A.; Dicke, M.; et al. Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell 2014, 26, 4991–5008. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Jiao, X.G.; Xie, W.; Wang, S.L.; Wu, Q.J.; Shi, X.B.; Chen, G.; Su, Q.; Yang, X.; Pan, H.P.; et al. Tomato yellow leaf curl virus alters the host preferences of its vector Bemisia tabaci. Sci. Rep. 2013, 3, 2876. [Google Scholar] [CrossRef]
- Fereres, A.; Penaflor, M.F.G.; Favaro, C.F.; Azevedo, K.E.X.; Landi, C.H.; Maluta, N.K.P.; Bento, J.M.S.; Lopes, J.R. Tomato infection by whitefly-transmitted circulative and non-circulative viruses induce contrasting changes in plant volatiles and vector behaviour. Viruses 2016, 8, 225. [Google Scholar] [CrossRef]
- Luan, J.B.; Yao, D.M.; Zhang, T.; Walling, L.L.; Yang, M.; Wang, Y.J.; Liu, S.S. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef]
- Seo, J.K.; Kim, M.K.; Kwak, H.R.; Choi, H.S.; Nam, M.; Choe, J.; Choi, B.; Han, S.J.; Kang, J.H.; Jung, C. Molecular dissection of distinct symptoms induced by tomato chlorosis virus and tomato yellow leaf curl virus based on comparative transcriptome analysis. Virology 2018, 516, 1–20. [Google Scholar] [CrossRef]
- Gabrys, B.; Tjallingii, W.F.; Van Beek, T.A. Analysis of EPG recorded probing by cabbage aphid on host plant parts with different glucosinolate contents. J. Chem. Ecol. 1997, 23, 1661–1673. [Google Scholar] [CrossRef]
- Li, J.; Ding, T.B.; Chi, H.; Chu, D. Effects of Tomao chlorosis virus on the performance of its key vector, Bemisia tabaci, in China. J. Appl. Entomol. 2018, 142, 296–304. [Google Scholar] [CrossRef]
- Lu, S.H.; Li, J.J.; Wang, X.L.; Song, D.Y.; Bai, R.E.; Shi, Y.; Gu, Q.S.; Kuo, Y.W.; Falk, B.W.; Yan, F.M. A semipersistent plant virus differentially manipulates feeding behaviors of different sexes and biotypes of its whitefly vector. Viruses 2017, 9, 4. [Google Scholar] [CrossRef]
- Jacob-Pitt, W.; Kairy, L.; Villa, E.; Nalam, V.J.; Nachappa, P. Virus infection and host plant suitability affect feeding behaviors of cannabis aphid (Hemiptera: Aphididae), a newly described vector of potato virus Y. Environ. Entomol. 2022, 51, 322–331. [Google Scholar] [CrossRef]
- Gao, D.M.; Qiao, J.H.; Gao, Q.; Zhang, J.W.; Zang, Y.; Xie, L.; Zhang, Y.; Wang, Y.; Fu, J.Y.; Zhang, H.; et al. A plant cytorhabdovirus modulates locomotor activity of insect vectors to enhance virus transmission. Nat. Commun. 2023, 14, 5754. [Google Scholar] [CrossRef]
| Parameters | p Value a | |
|---|---|---|
| Tomato Plants b | Bemisia tabaci MED c | |
| Non-phloem parameters | ||
| 1. Time to 1st probe from start | 0.686 | 0.152 |
| 2. Total number of probes | 0.592 | 0.183 |
| 3. Total duration of probes | 0.646 | 0.064 |
| 4. Mean duration of probe | 0.467 | 0.201 |
| 5. Total duration of np | 0.035 | 0.086 |
| 6. Total duration of C | 0.644 | 0.016 |
| 7. Duration of 1st probe | 0.071 | 0.003 |
| 8. Time from 1st probe to 1st E | 0.574 | 0.014 |
| 9. Number of probes before 1st E | 0.477 | 0.594 |
| 10. Time from the beginning of that probe to 1st E | 0.192 | 0.396 |
| 11. Total number of pd | 0.022 | 0.093 |
| 12. Total duration of pd | 0.023 | 0.302 |
| Phloem parameters | ||
| 13. Total number of E1 | 0.350 | 0.657 |
| 14. Total duration of E1 | 0.200 | 0.979 |
| 15. Mean duration of E1 | 0.242 | 0.827 |
| 16. Total number of E2 | 0.350 | 0.582 |
| 17. Total duration of E2 | 0.003 | 0.028 |
| 18. Mean duration of E2 | 0.016 | 0.142 |
| 19. Percentage of probes reaching phloem phase | 0.203 | 0.452 |
| 20. Percentage of phloem phases reaching waveform E2 | 0.117 | 0.307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, T.; Huang, H.; Liu, X.; Zhang, M.; Yu, J.; Xia, G.; Chu, D. Distinct Modulation of Feeding Behavior in the Whitefly Vector Bemisia tabaci MED by ToCV Single-Infection Versus Synergistic Co-Infection with TYLCV. Insects 2025, 16, 1091. https://doi.org/10.3390/insects16111091
Ding T, Huang H, Liu X, Zhang M, Yu J, Xia G, Chu D. Distinct Modulation of Feeding Behavior in the Whitefly Vector Bemisia tabaci MED by ToCV Single-Infection Versus Synergistic Co-Infection with TYLCV. Insects. 2025; 16(11):1091. https://doi.org/10.3390/insects16111091
Chicago/Turabian StyleDing, Tianbo, Hong Huang, Xiaobei Liu, Min Zhang, Jianmei Yu, Guoxu Xia, and Dong Chu. 2025. "Distinct Modulation of Feeding Behavior in the Whitefly Vector Bemisia tabaci MED by ToCV Single-Infection Versus Synergistic Co-Infection with TYLCV" Insects 16, no. 11: 1091. https://doi.org/10.3390/insects16111091
APA StyleDing, T., Huang, H., Liu, X., Zhang, M., Yu, J., Xia, G., & Chu, D. (2025). Distinct Modulation of Feeding Behavior in the Whitefly Vector Bemisia tabaci MED by ToCV Single-Infection Versus Synergistic Co-Infection with TYLCV. Insects, 16(11), 1091. https://doi.org/10.3390/insects16111091





