1. Introduction
Agricultural straw, a massive lignocellulosic by-product with annual output exceeding 700 million tons in China, faces critical challenges in high-value utilization. Traditional disposal (open burning, low-efficiency composting) wastes resources and causes environmental issues, making straw conversion into high value products a priority for circular agriculture and carbon neutrality [
1].
Protaetia brevitarsis (PB) larvae, a saprophagous insect, offer a promising solution by bio-converting straw into dual high value products: insect protein [
2,
3] and frass rich in humic acid (HA) [
4]. Unlike energy intensive chemical methods, larvae decompose straw lignocellulose via gut microbiota endogenous enzyme synergy [
5]. The straw derived frass has HA contents far exceeding traditional compost [
6], and the larval frass HA was considered as a candidate plant biostimulants. Gao et al. (2023) [
7] identified six HA related aromatic compounds from the frass that significantly stimulate plant growth, and all six compounds demonstrated the ability to enhance seed germination, and the piperic acid which was first reported exhibits root growth promotion in plants. Furthermore, the frass extract can be used to develop Eco-friendly pH-responsive hexaconazole nano delivery system for controlling fungal disease and promoting crop growth [
8]. These data suggested that further extraction and processing of frass can further enhance its value and promote the high value utilization of straw.
However, the application of frass-derived HA is limited by inefficient extraction. HA is a mixture of macromolecular organic compounds that possess numerous reactive functional groups, such as carboxyl and phenolic hydroxyl groups, which make it readily soluble in NaOH or KOH solutions. Therefore, alkali dissolution methods are commonly used to extract HA from organic materials such as coal [
9]. During the extraction process, parameters such as dissolution temperature, dissolution time, alkali concentration, and solid-to-liquid ratio are key factors influencing extraction efficiency. Response Surface Methodology (RSM) has been widely applied to optimize production processes. In this study, RSM was employed to investigate the interactions among multiple parameters and to optimize the HA extraction process.
This study was conducted in response to the growing need for high-value utilization of P. brevitarsis larvae frass. It primarily focuses on three aspects: (1) optimization of the extraction process via RSM, (2) physicochemical characterization of the extracted products, and (3) evaluation of the effects of the extracted products on plant growth. The findings of this work are expected to provide technical support for the further development of high-value frass-derived products.
2. Materials and Methods
2.1. Collection and Processing of Insect Frass Samples
The insect frass used in this experiment was obtained from the population of P. brevitarsis reared at the Langfang Pilot Experimental Base of the Institute of Plant Protection, Chinese Academy of Agricultural Sciences. The insects were raised in open plastic boxes with dimensions of 65 cm × 41 cm × 16 cm (length × width × height), fed with fermented Flammulina velutipes culture substrate residue, and maintained under conditions of 25 °C, 70% relative humidity, and total darkness. When most of the feed was consumed, the insect frass and larvae were separated by sieving, and the remaining feed was removed. The sieved insect frass was oven-dried at 75 °C to a constant weight (24–48 h) for subsequent use.
2.2. Experimental Design for Response Surface Methodology (RSM) Optimization
2.2.1. RSM Optimization of Factors Affecting Dissolution Rate
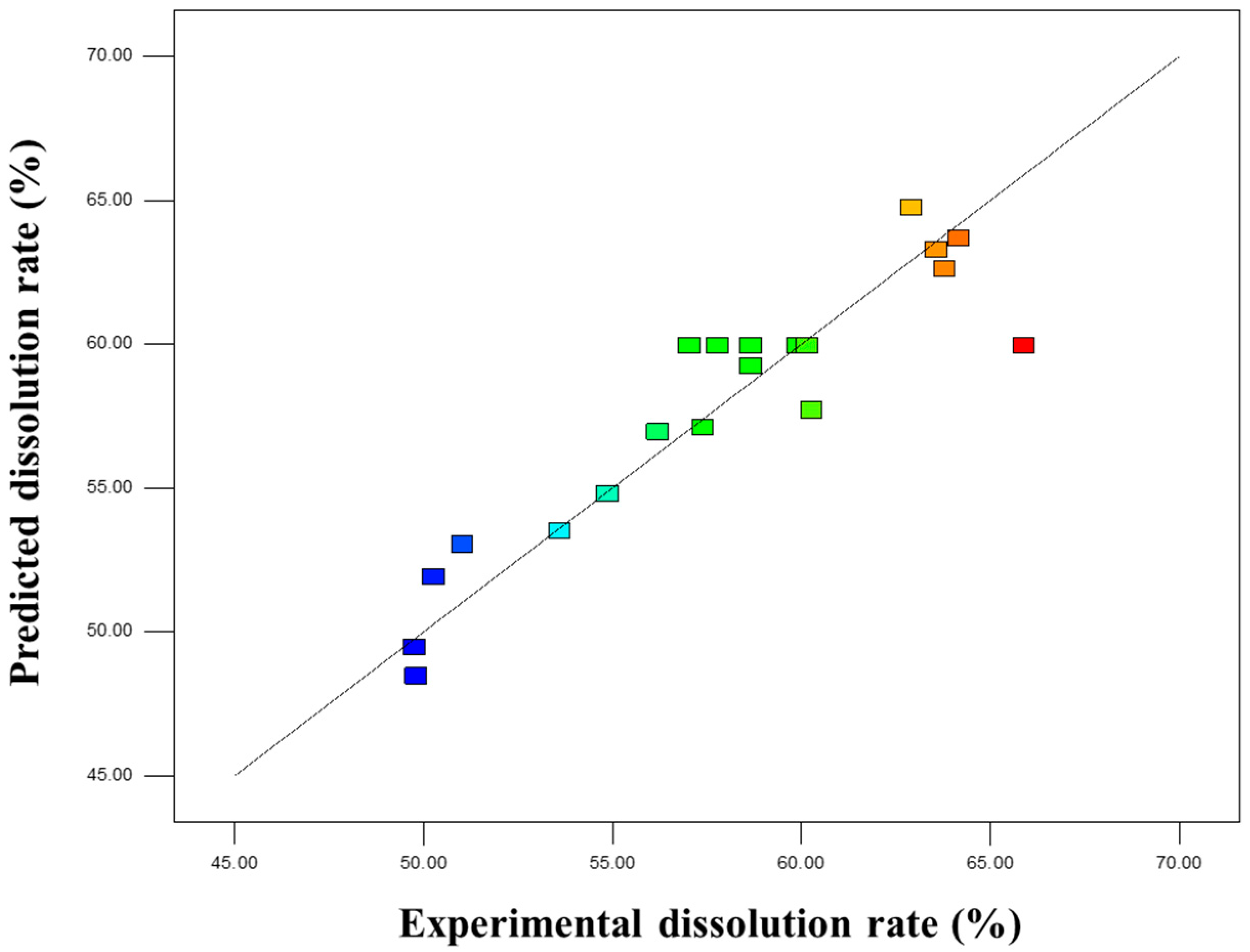
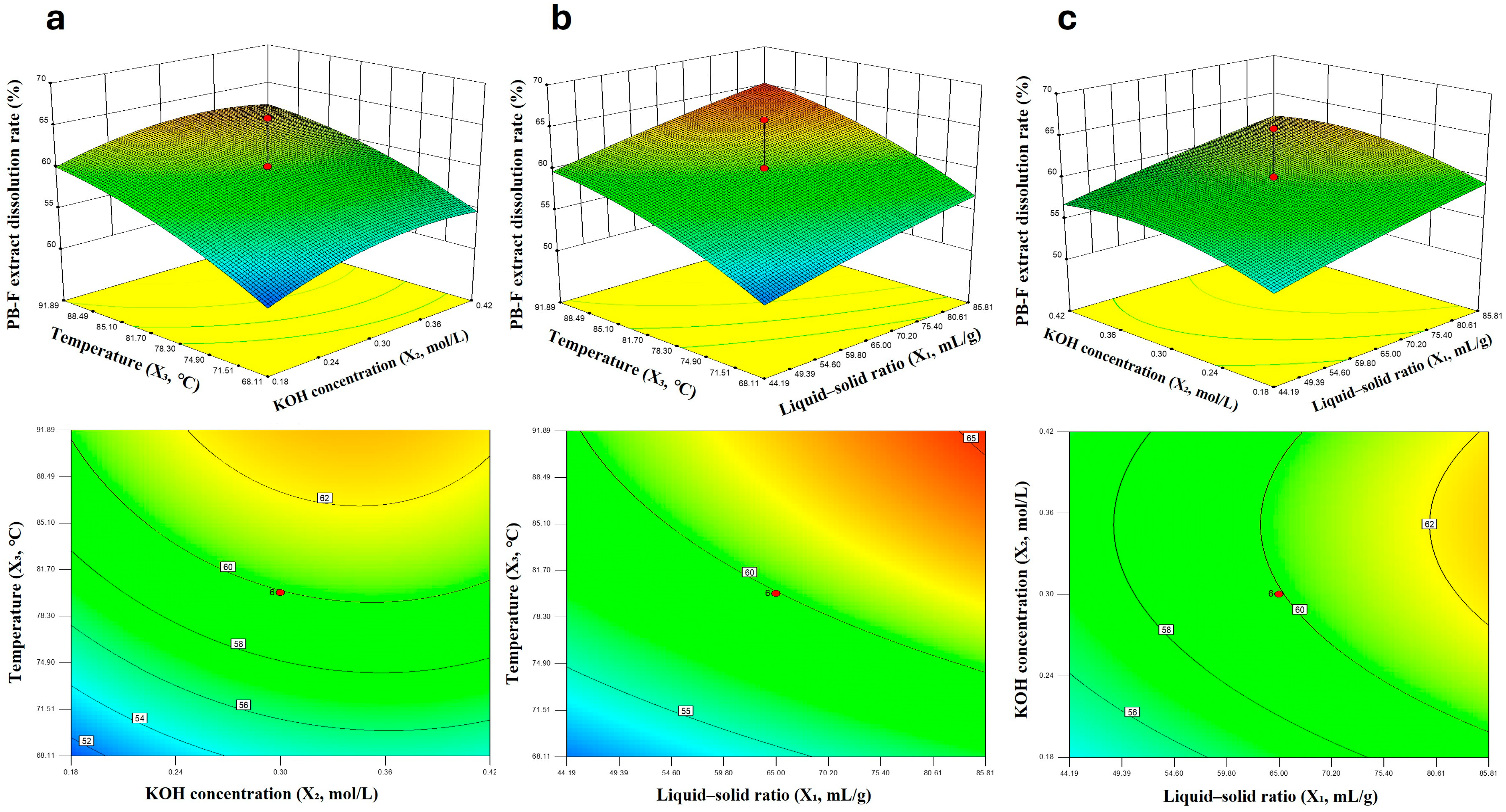
To investigate the dissolution properties of insect frass, a three-factor, five-level rotatable central composite design (CCD) was employed in this study, and a quadratic regression model was established. Based on the results of single-factor experiments, liquid-to-solid ratio, KOH concentration, temperature, and time all affected the dissolution rate of insect frass. Three variables, namely X
1 (liquid-to-solid ratio, g·mL
−1), X
2 (extraction temperature, °C), and X
3 (extraction time, h), were selected as independent variables, with the dissolution rate (%) as the response value. The actual and coded levels of each factor are listed in
Table 1, and the experimental design scheme is presented in
Table 2.
The experiment consisted of 20 treatments, including 8 full-factorial points, 6 axial points, and 6 center points. The experimental sequence was randomized, and each treatment was repeated three times to minimize systematic errors. The obtained data were used to fit a quadratic regression equation, and analysis of variance (ANOVA) was performed to evaluate the significance and goodness-of-fit of the model. Finally, response surface plots and contour plots were generated to analyze the effects of individual factors and their interactions on the dissolution rate.
The experimental procedure was as follows: According to the liquid-to-solid ratios specified in the table (with a fixed addition of 40 mL KOH solution), insect frass and KOH solution were added to a 150 mL Erlenmeyer flask, and heated with stirring at a constant and moderate speed on a magnetic stirrer for 1 h. Each treatment was repeated three times. After stirring, the dissolved solution was transferred to a 50 mL centrifuge tube, and the residues in the Erlenmeyer flask were rinsed with a small amount of water, which was then added to the same centrifuge tube. Centrifugation was performed at 5000 r/min for 15 min, and the supernatant was discarded; this washing process was repeated five times with ultrapure water. Finally, the obtained precipitate was dried in a constant-temperature oven at 80 °C until its weight remained nearly unchanged, and then weighed to calculate the dissolution rate of insect frass by comparing its weight with the initial weight of the insect frass.
2.2.2. RSM Optimization of Factors Affecting Organic Carbon Content
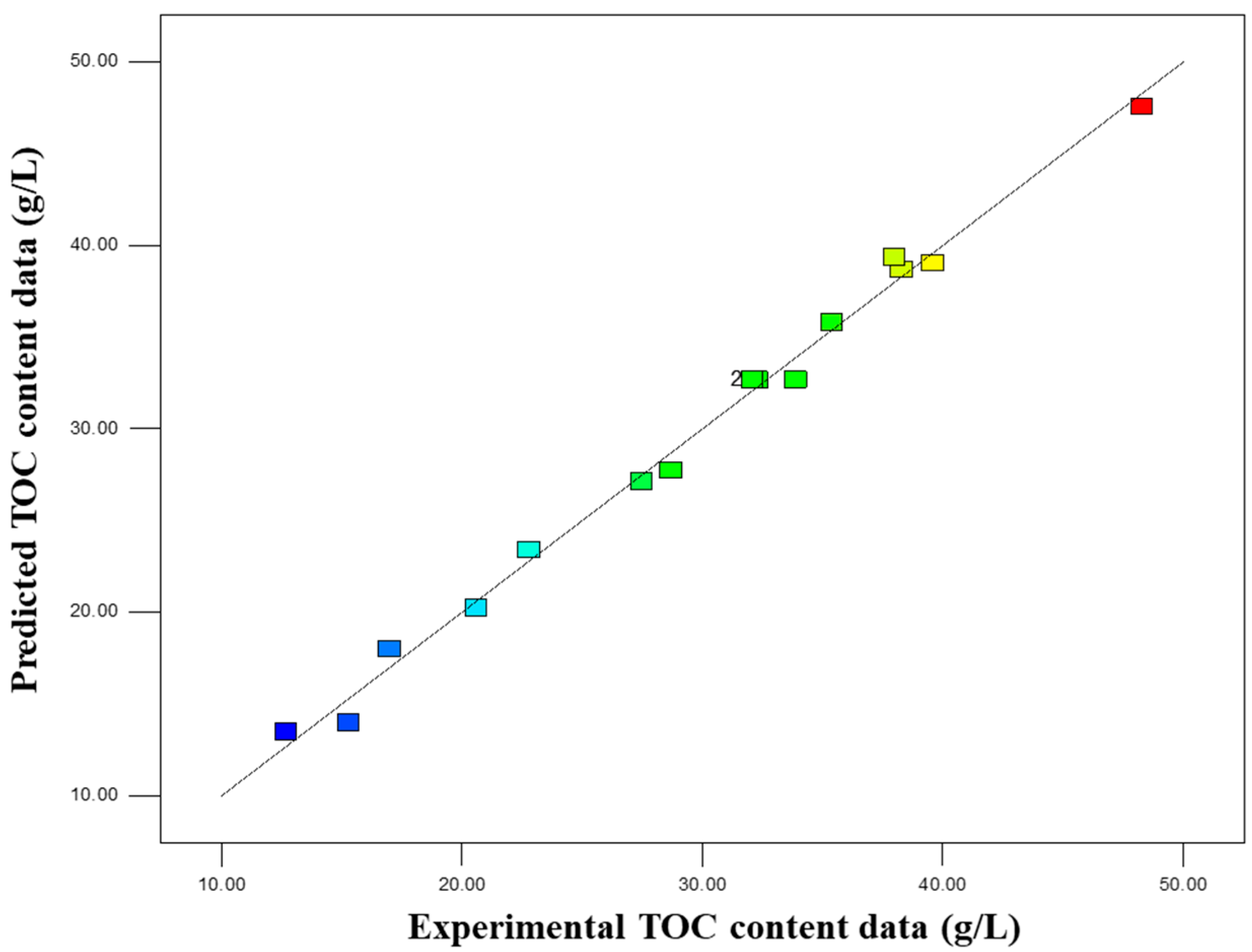
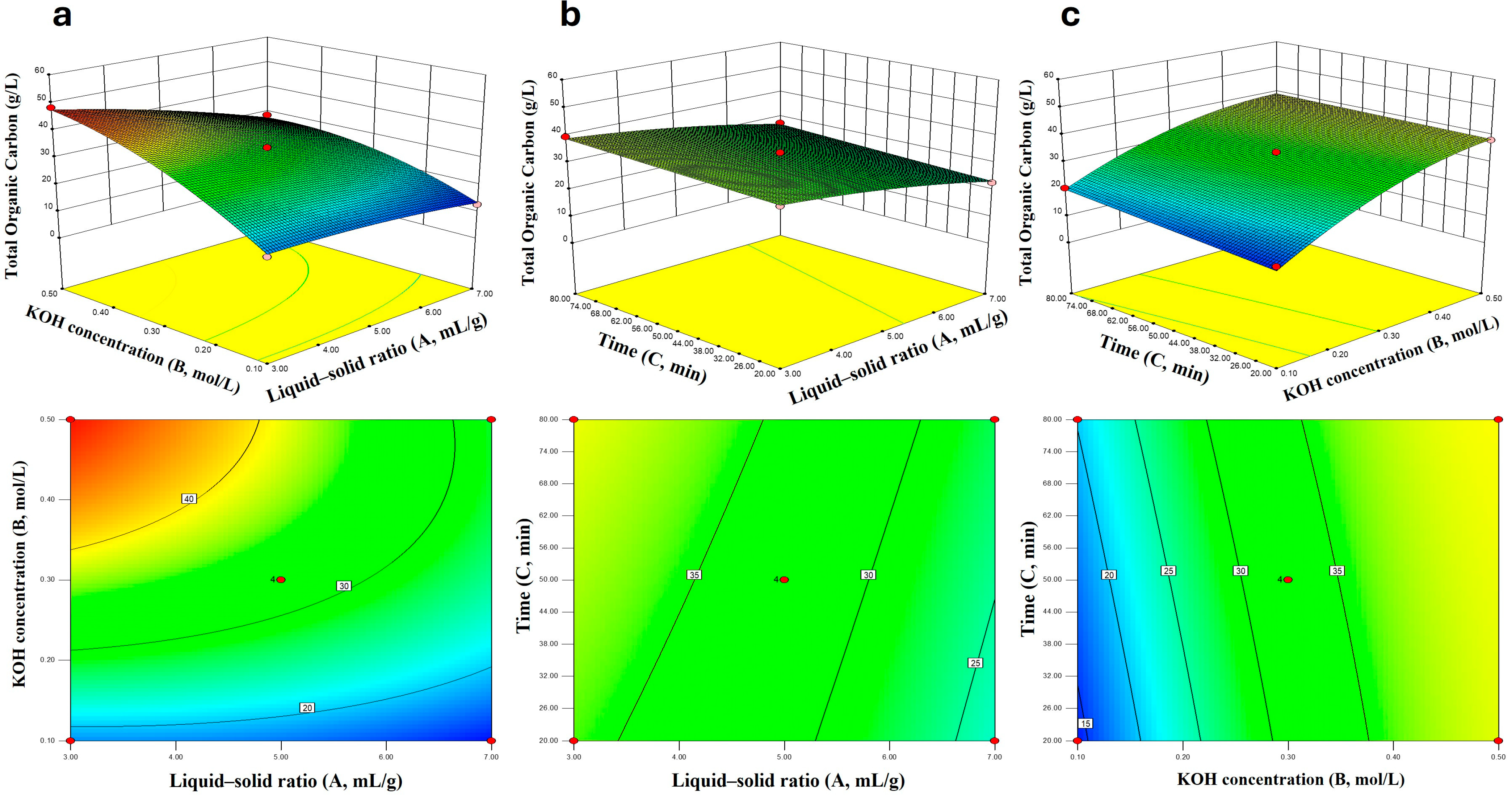
To optimization of factors to produce a high content extract, a three-factor, three-level Box–Behnken design (BBD) was used to investigate the effects of liquid-to-solid ratio (A, mL/g), KOH concentration (B, mol/L), and extraction time (C, min) on the mass fraction of organic carbon (%) in the extract. The coded levels of factors and the experimental design scheme are shown in
Table 3 and
Table 4, respectively.
The experimental procedure was as follows: 4 g of insect frass was placed in a 150 mL Erlenmeyer flask, and KOH solution with the corresponding concentration and volume was added according to the liquid-to-solid ratio specified in the table. The mixture was heated and stirred at a constant temperature of 80 °C on a magnetic stirrer at a moderate speed, with the stirring time set according to the experimental design. After the reaction, the solution was transferred to a 50 mL centrifuge tube, and the residues in the Erlenmeyer flask were rinsed with a small amount of ultrapure water, which was then combined with the solution in the centrifuge tube. Centrifugation was conducted at 5000 r/min for 15 min, and the supernatant was collected for the determination of organic carbon content.
2.3. Component Analysis (Organic Carbon and Metal Elements)
The organic carbon content was determined according to the standard method described in NY/T 4606-2025 [
10].
For the determination of elements including phosphorus (P), potassium (K), copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), boron (B), calcium (Ca), magnesium (Mg), sulfur (S), sodium (Na), as well as heavy metals such as arsenic (As), mercury (Hg), lead (Pb), cadmium (Cd), and chromium (Cr), the procedure followed the Water Industry Standard of the People’s Republic of China SL 394.1-2007 [
11], employing inductively coupled plasma atomic emission spectrometry (ICP-AES).
Approximately 0.1–0.5 g of each sample (passed through a 100-mesh sieve) was accurately weighed into polytetrafluoroethylene (PTFE) crucibles. A few drops of ultrapure water were added to moisten the samples, followed by 5 mL of hydrochloric acid (HCl). The mixture was gently heated on an electric hot plate at a low temperature until the volume was reduced to about 2 mL. Then, 10 mL of nitric acid (HNO3) was added and heating continued until the solution became nearly viscous. Subsequently, 5 mL of hydrofluoric acid (HF) was added, and the mixture was heated further with frequent shaking to promote silicon removal. Finally, 2 mL of perchloric acid (HClO4) was added, and heating was continued until dense white fumes were almost completely expelled.
The inner wall and lid of the crucible were rinsed with 2% nitric acid, and the residue was dissolved while warm. After cooling, the solution was transferred into a 50 mL volumetric flask and diluted to volume with 2% nitric acid. Three reagent blanks were prepared in parallel. The resulting solutions were filtered through 0.22 μm PTFE membranes and analyzed using an inductively coupled plasma atomic emission spectrometer (ICP-AES, Thermo Fisher iCAP 7400 Dual Channel, Thermo Fisher Scientific, Waltham, MA, USA) and an inductively coupled plasma mass spectrometer (ICP-MS, Thermo Fisher iCAP RQ).
The chloride ion content was determined by the silver nitrate titration method, referring to the Agricultural Industry Standard of the People’s Republic of China NY/T 1121.17-2006 [
12]. The total nitrogen content was measured using the Kjeldahl method, in compliance with the Agricultural Industry Standard of the People’s Republic of China NY/T 1121.24-2012 [
13].
2.4. Solid-State 13C Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
The 13C cross-polarization magic-angle spinning nuclear magnetic resonance (13C CP-MAS NMR) experiments were performed using a JNM-ECZ600R spectrometer (JEOL Ltd., Tokyo, Japan), with the 13C resonance frequency set at 150 MHz. Samples were loaded into a 3.2 mm diameter MAS rotor, and data were acquired under magic-angle spinning at 12 kHz. The experimental parameters were as follows: relaxation delay time of 3 s, contact time of 2 ms, and a total of 1200 cumulative scans, resulting in a total acquisition time of approximately 1 h. The line broadening factor was set to 80 Hz.
2.5. Preparation of Insect Frass Extract for Plant Experiments
The insect frass, after the aforementioned treatment, was extracted with 0.33 mol·L−1 KOH at a concentration of 135 g·L−1 and heated at 80 °C for 1 h. After cooling, the mixture was aliquoted and centrifuged at 5000 r·min−1 for 15 min. The supernatant was collected, adjusted to neutral pH by dropwise addition of 85% phosphoric acid, and stored at 4 °C. The stock frass extract thus prepared was used for plant experiments in this study. Relevant components of the stock extract were determined, with the total organic carbon content being 25.5 g/L.
2.6. Plant Toxicity Assay
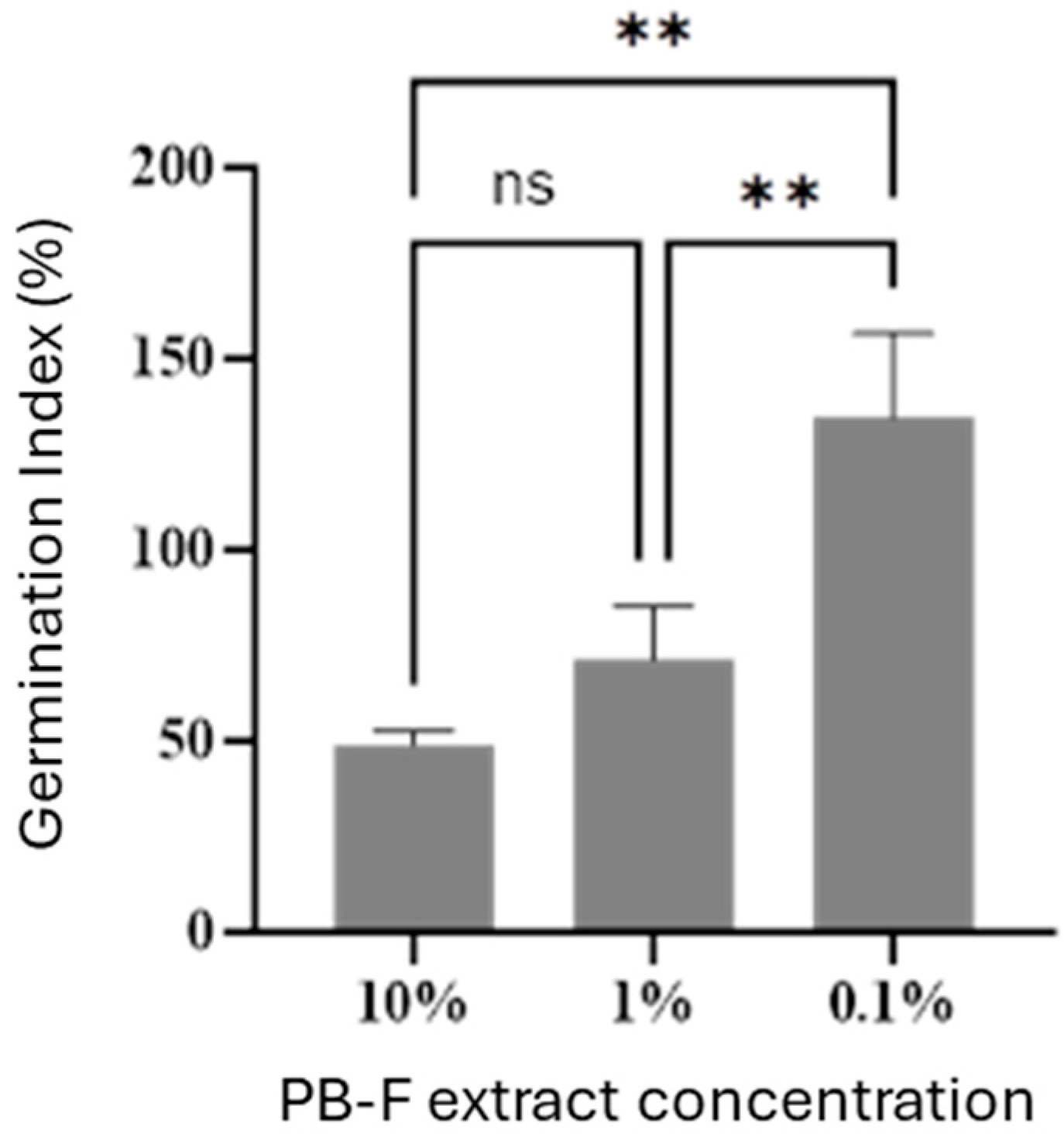
The determination of the germination index (GI) was performed following the Agricultural Industry Standard NY 525-2021 [
14]. A 10 mL aliquot of the frass extract at the tested concentration was added to 9 mm Petri dishes lined with two layers of qualitative filter paper, and 10 plump, uniformly sized non-coated radish seeds were placed in each dish. Petri dishes containing pure water served as the control. The dishes were covered and incubated in the dark at 27 °C and 60% relative humidity for 48 h. After incubation, the number of germinated seeds was counted, and the primary root length of each seed was measured using a vernier caliper.
The seed germination index (GI) was calculated using the following formula:
where
A1 is the percentage of germinated seeds in the frass extract treatment relative to the total number of seeds (%);
A2 is the average root length of all germinated seeds in the frass extract treatment (mm);
B1 is the percentage of germinated seeds in the pure water control relative to the total number of seeds (%);
B2 is the average root length of all germinated seeds in the pure water control (mm).
The arithmetic mean of parallel determinations was taken as the final result, which was retained to one decimal place. The absolute difference between parallel analysis results did not exceed 5.0%.
2.7. Plant Cultivation and Treatment Conditions
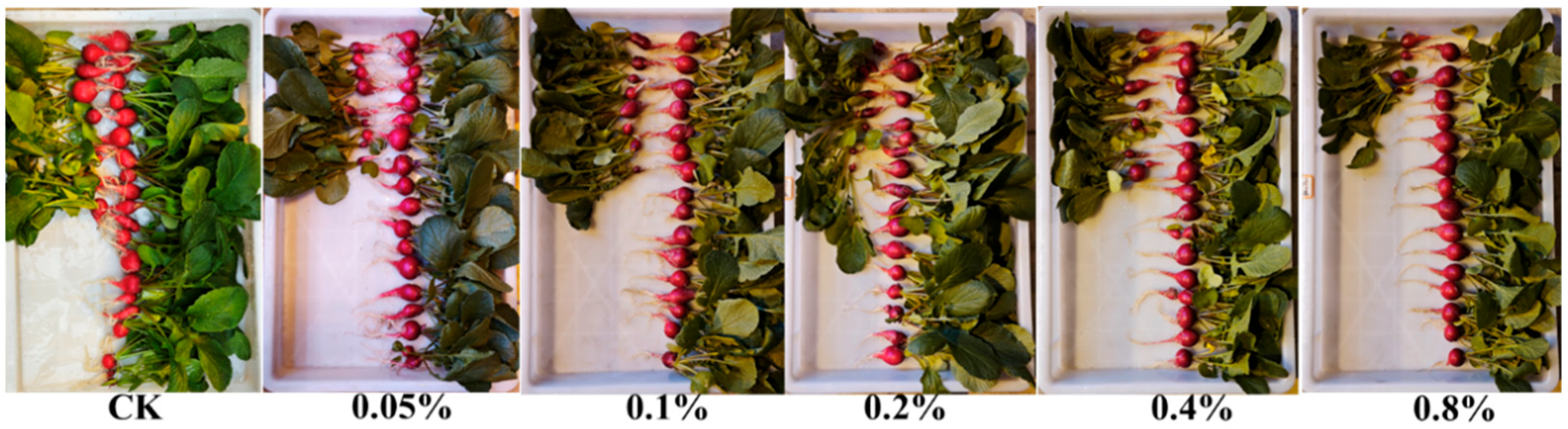
Cherry radish (Raphanus sativus L.) was used as the material for the cultivation experiment. Uniformly sized seeds were first sterilized with 75% ethanol for 1 min, then soaked in 1% sodium hypochlorite for 5 min, and finally rinsed 3–4 times with sterile distilled water. The treated seeds were soaked in pure water for 6 h, then sown in 11 cm diameter pots containing vermiculite, with 2 seeds evenly sown per pot and 15 pots per treatment group.
On the sowing day, each treatment group was supplied with 2 L of Hoagland nutrient solution with standard formulation. Thereafter, 1 L of 1/2 strength Hoagland nutrient solution was applied every 3 days. PB-F extract, with an original organic carbon content of 25.5 g L
−1 (prepared as described in
Section 2.5), was applied at concentrations of 0.05%, 0.1%, 0.2%, 0.4%, and 0.8% for each treatment group every 6 days together with the nutrient solution, while no PB-F extract was applied to the control group.
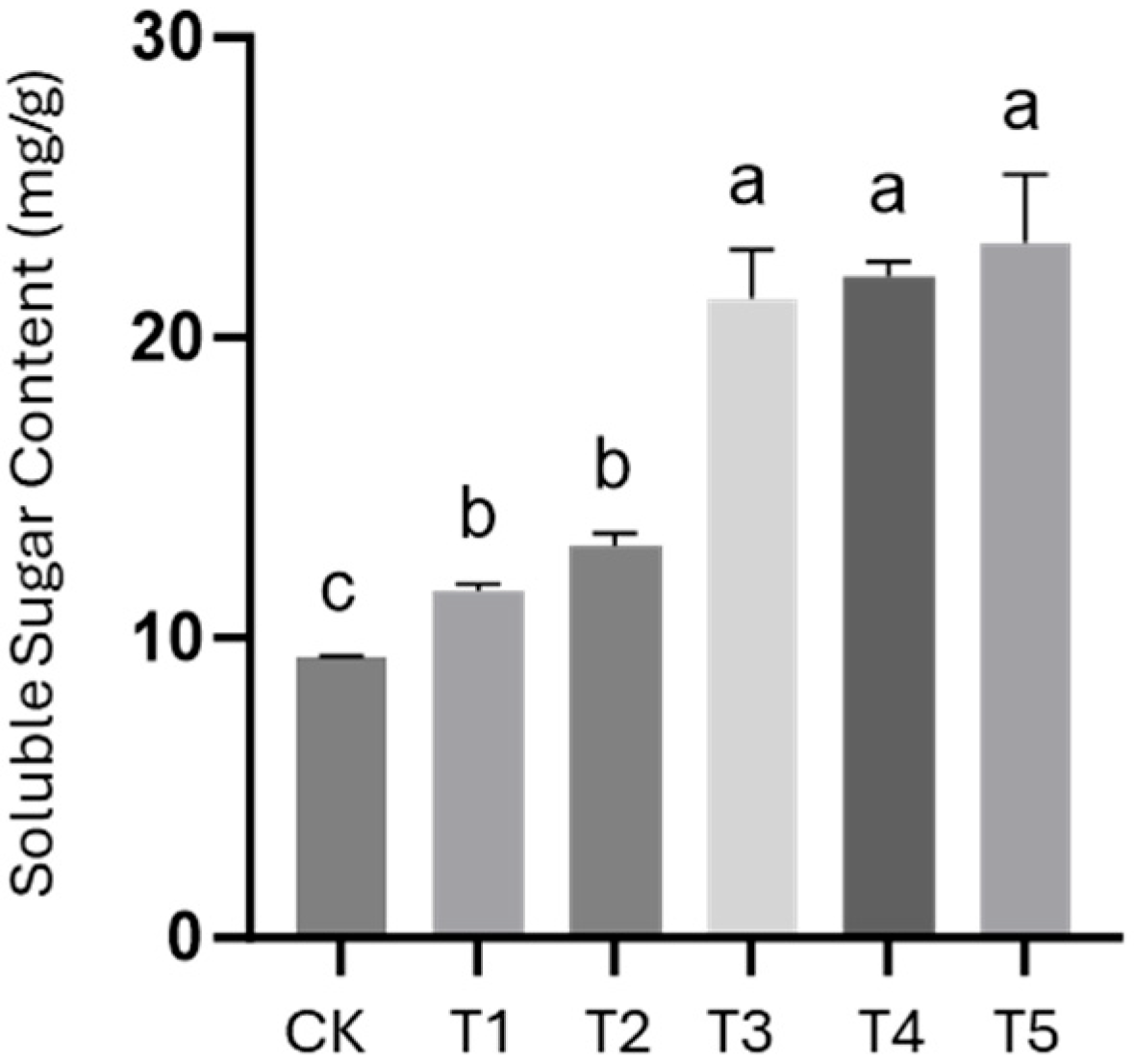
Plants were grown in a greenhouse with temperatures maintained at 10–15 °C and a photoperiod of 16 h light/8 h dark. Harvesting and sampling measurements were conducted on day 30. Soluble sugar content was determined using the anthrone colorimetric method; 3 samples were selected from each treatment and assayed using the BC0030 Plant Soluble Sugar Content Assay kit (Solarbio, Beijing, China).
4. Discussion
The global population growth has driven up food demand, intensifying the overexploitation of arable land [
19]. This overexploitation leads to land degradation issues such as salinization and organic matter loss, posing a threat to food security [
20]. HA can improve soil structure, enhance soil fertility, and boost crop stress resistance, making it a core material for remediating degraded arable land. As an agricultural waste, crop straw can be used as a raw material for artificial HA production [
21]. This approach not only addresses straw pollution but also replaces fossil-derived HA, reducing costs and being environmentally friendly. This study was conducted to meet the demands in this field, holding significant implications for environmental sustainability.
Artificial HA is currently a sustainable source of HA, and the mainstream preparation method is microbial transformation, which simulates the natural humification process. However, conventional artificial HA production methods have limitations: biological fermentation (e.g., straw composting) is time-consuming and yields low HA content. In contrast,
P. brevitarsis plays a crucial role in the ecosystem by transforming plant litter into soil humus [
5,
22]. When used as a bioreactor, its gut microbiota synergizes with its own metabolism to complete the transformation within a few hours [
5]. Moreover, the HA content in the product is higher than that in compost [
6]. This approach not only simulates natural humification but also overcomes efficiency bottlenecks and enables straw resource utilization, thereby providing a new pathway for the efficient and environmentally friendly production of biological HA.
In this study, response surface methodology (RSM) was employed to optimize the extraction process. RSM has been widely applied in similar studies (e.g., coal HA extraction) and achieved favorable results [
9]. Here, the predicted values of the polynomial model for insect frass dissolution rate showed good consistency with the actual test results. The HA content prepared via the RSM-optimized extraction process reached 47.8 g/L, meeting the industrial standards for soluble organic fertilizers. Further elemental analysis of the spray-dried HA revealed a total NPK content of 163.4 g/kg, and the contents of heavy metals (e.g., Pb, Cd, Cr) were below the limit requirements specified in national standards. Additionally, phytotoxicity tests at the application dose (0.1% concentration of PB-F extract stock solution) showed that the extract exhibited a promotive effect on seed germination, with a germination index (GI) exceeding 100%. Collectively, these data demonstrate that this process is feasible for artificial HA production.
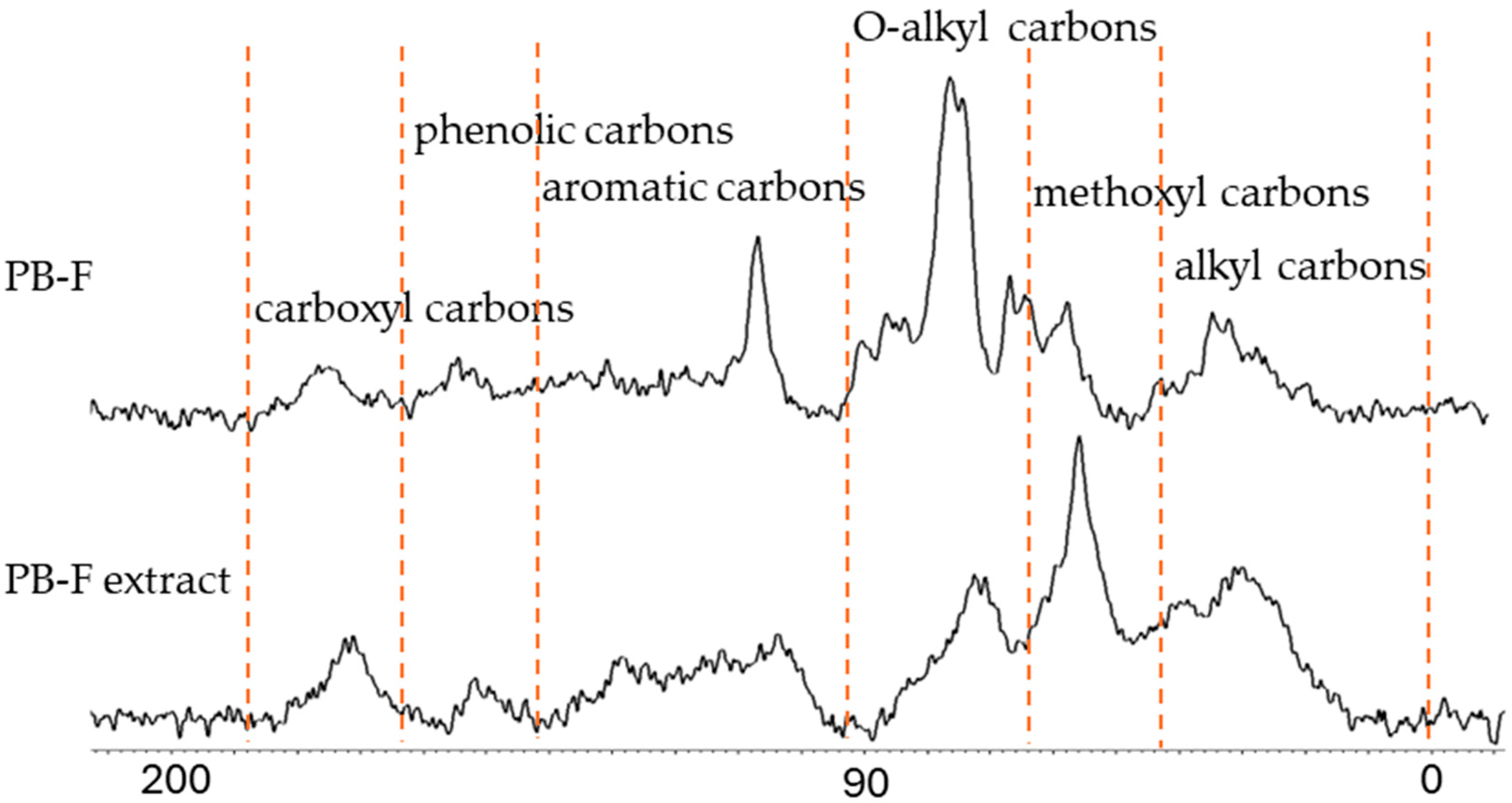
13C NMR analysis of the spray-dried extract showed that aliphatic and methoxyl carbons were significantly enriched in the sample after extraction. Compared with industrial natural HA, the artificial HA prepared in this study contains more bioactive molecules. In the pot experiment with cherry tomatoes, frass-derived HA significantly promoted belowground biomass accumulation at an application rate of 0.4–0.8%, which effectively increased crop yield. Furthermore, soluble sugar content analysis indicated that all concentrations of frass-derived HA increased soluble sugar levels; specifically, at 0.8% concentration, the soluble sugar content was 147.3% of that in the control group. This confirms that frass-derived HA can effectively improve crop quality.
In conclusion, this study demonstrates the production of artificial HA via straw transformation by the resource insect P. brevitarsis, which provides scientific support for circular agriculture, arable land protection, and the “dual carbon” goals, and thus possesses both academic value and broad application prospects.