Optimizing Foliar Spray Intervals and Rates of Isocycloseram and Cyantraniliprole Plus Thiamethoxam Application on Hydrangea paniculata to Combat Adult Systena frontalis (F) (Coleoptera: Chrysomelidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Assay, Procedure, and Evaluation
2.2. Field Site, Plant, and Insect
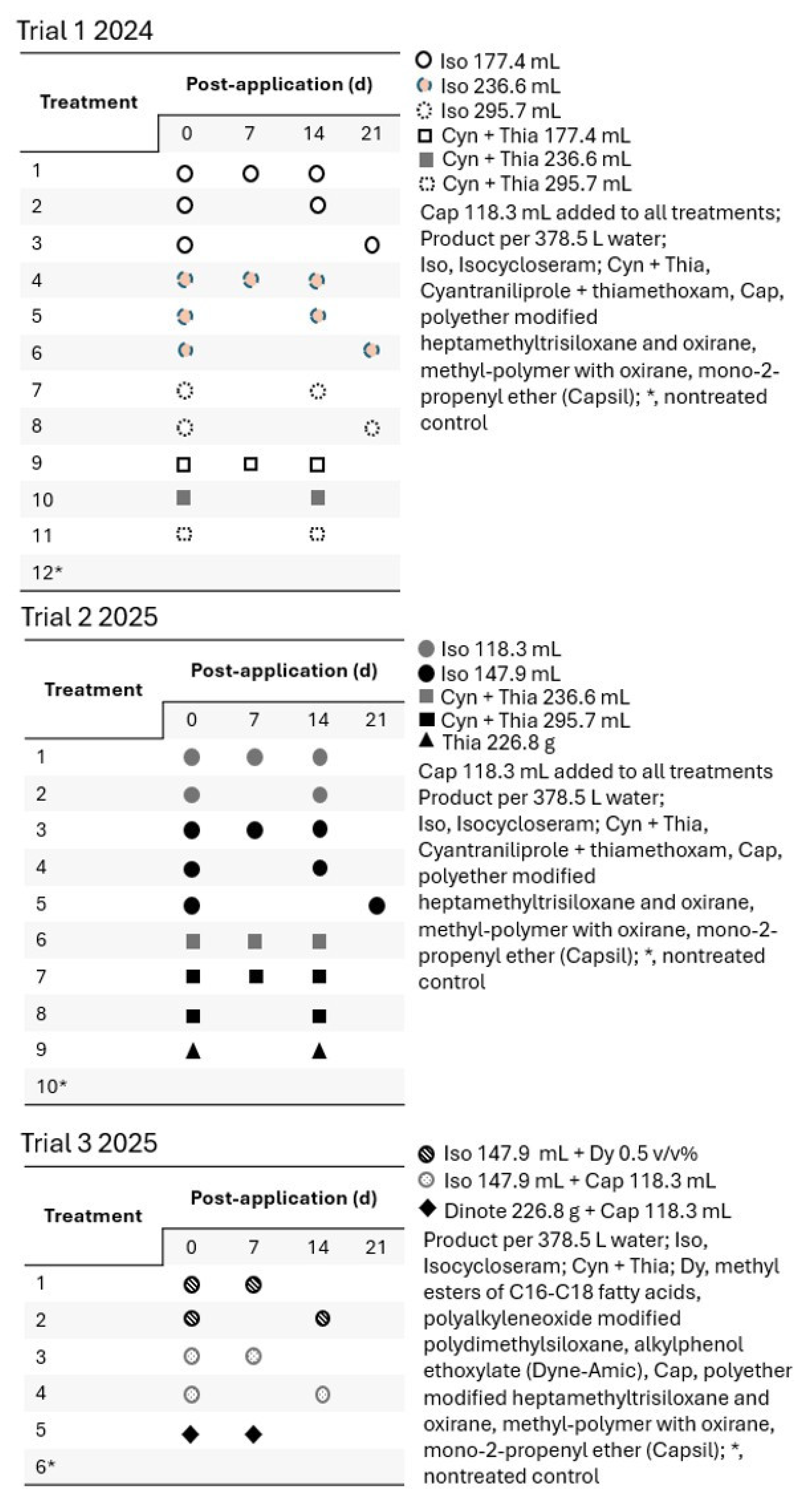
2.3. Field Trial Design, Procedure, and Evaluation
2.4. Statistical Analyses
3. Results
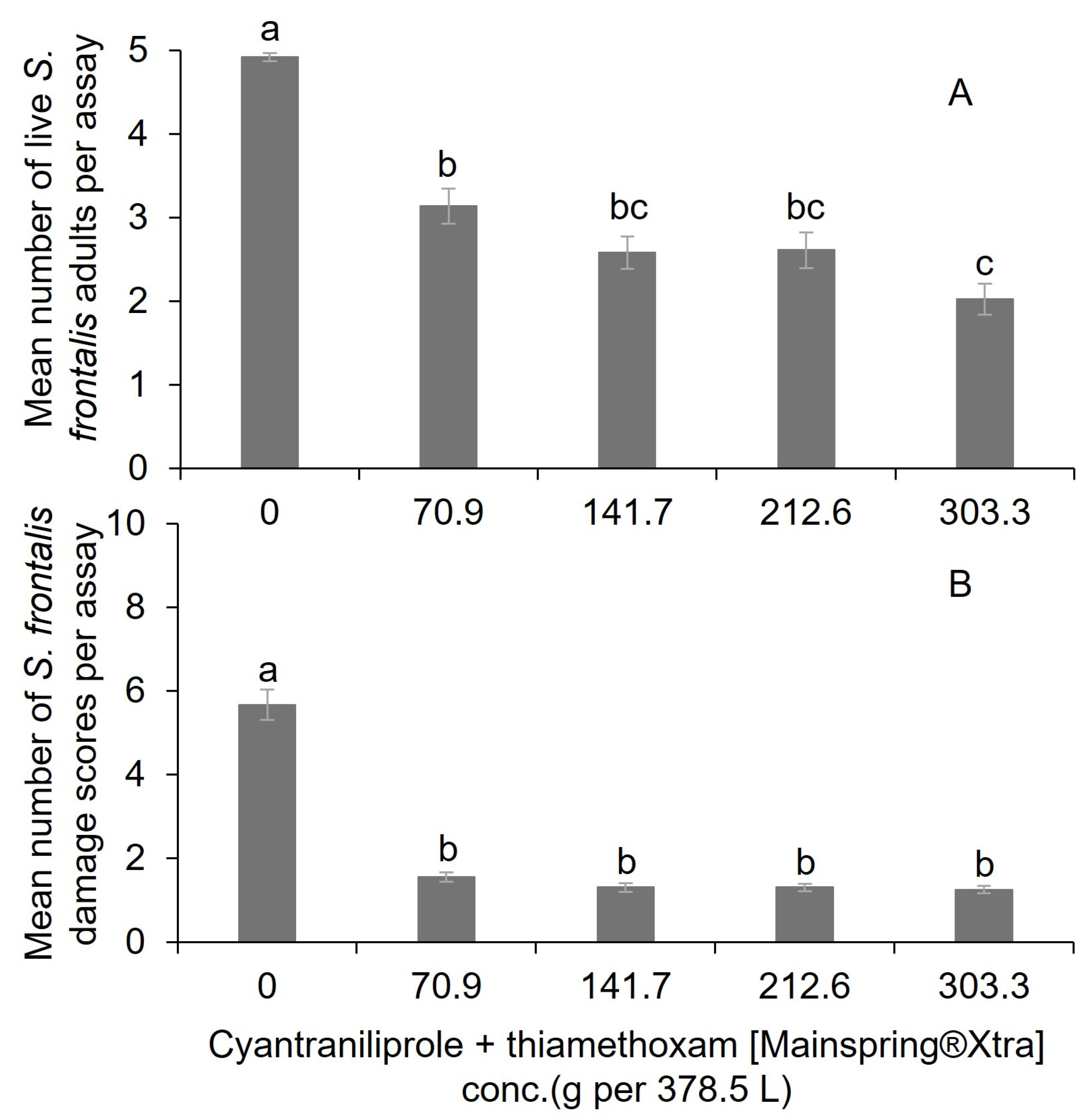
3.1. Laboratory Assay
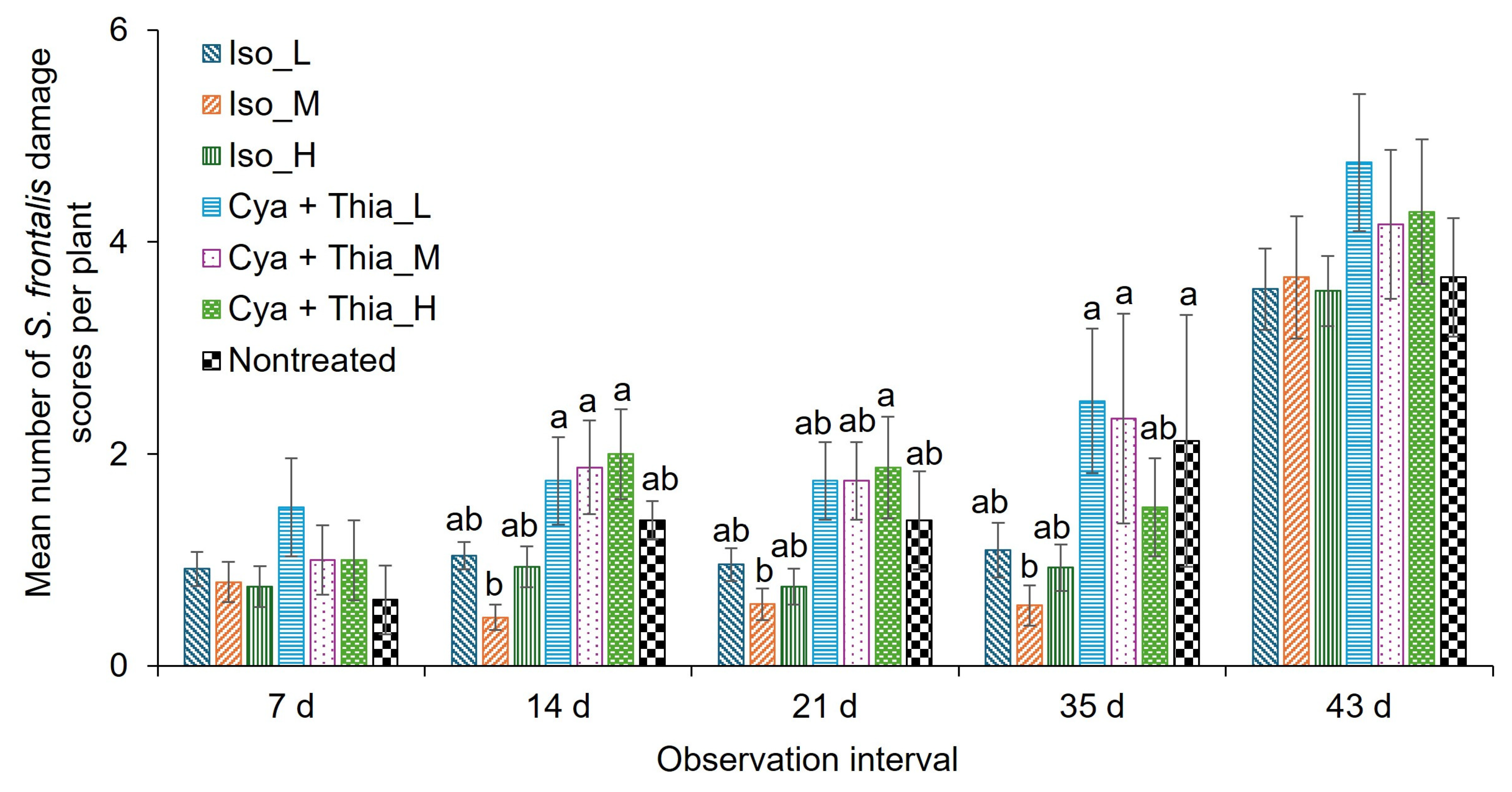
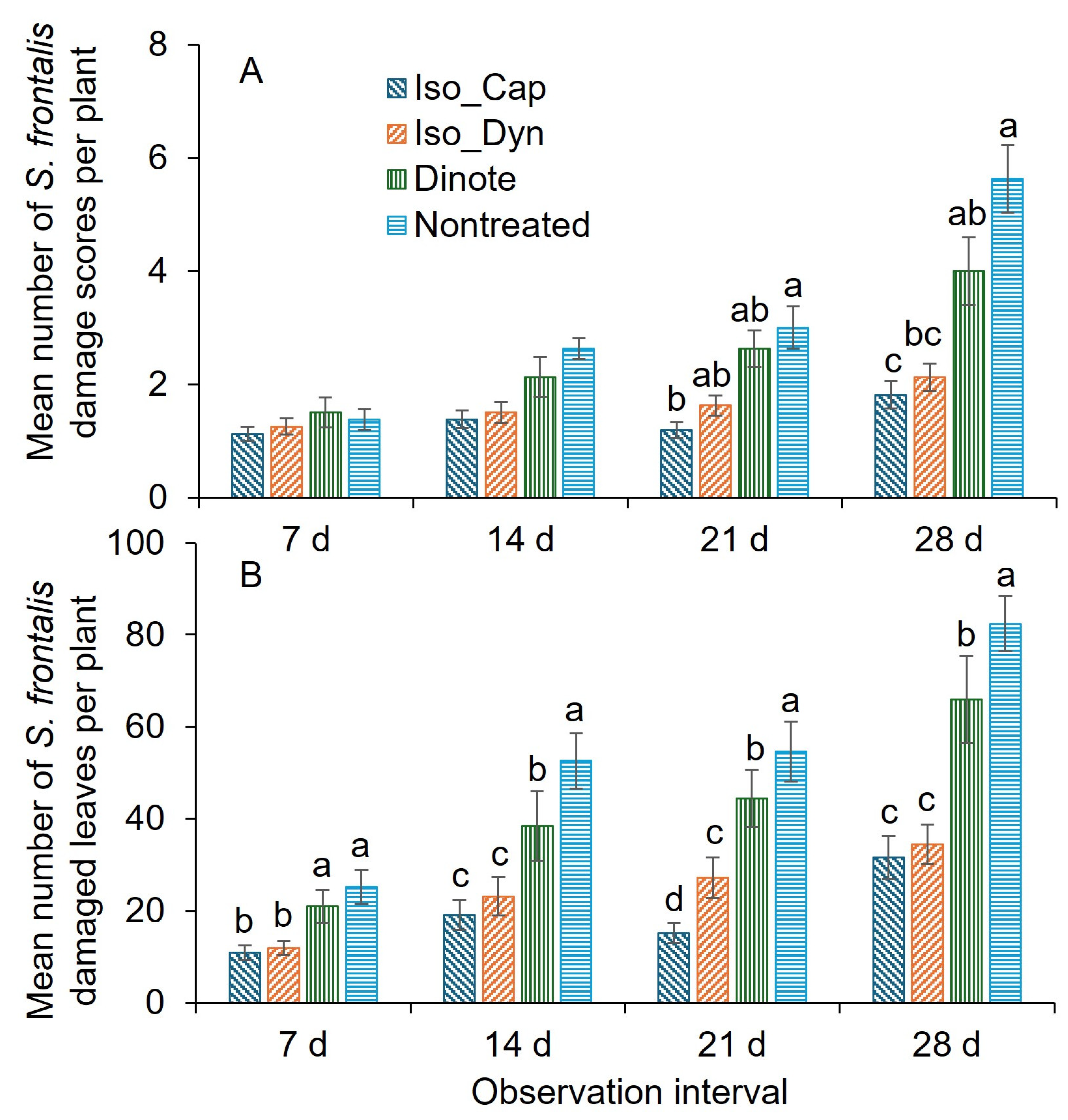
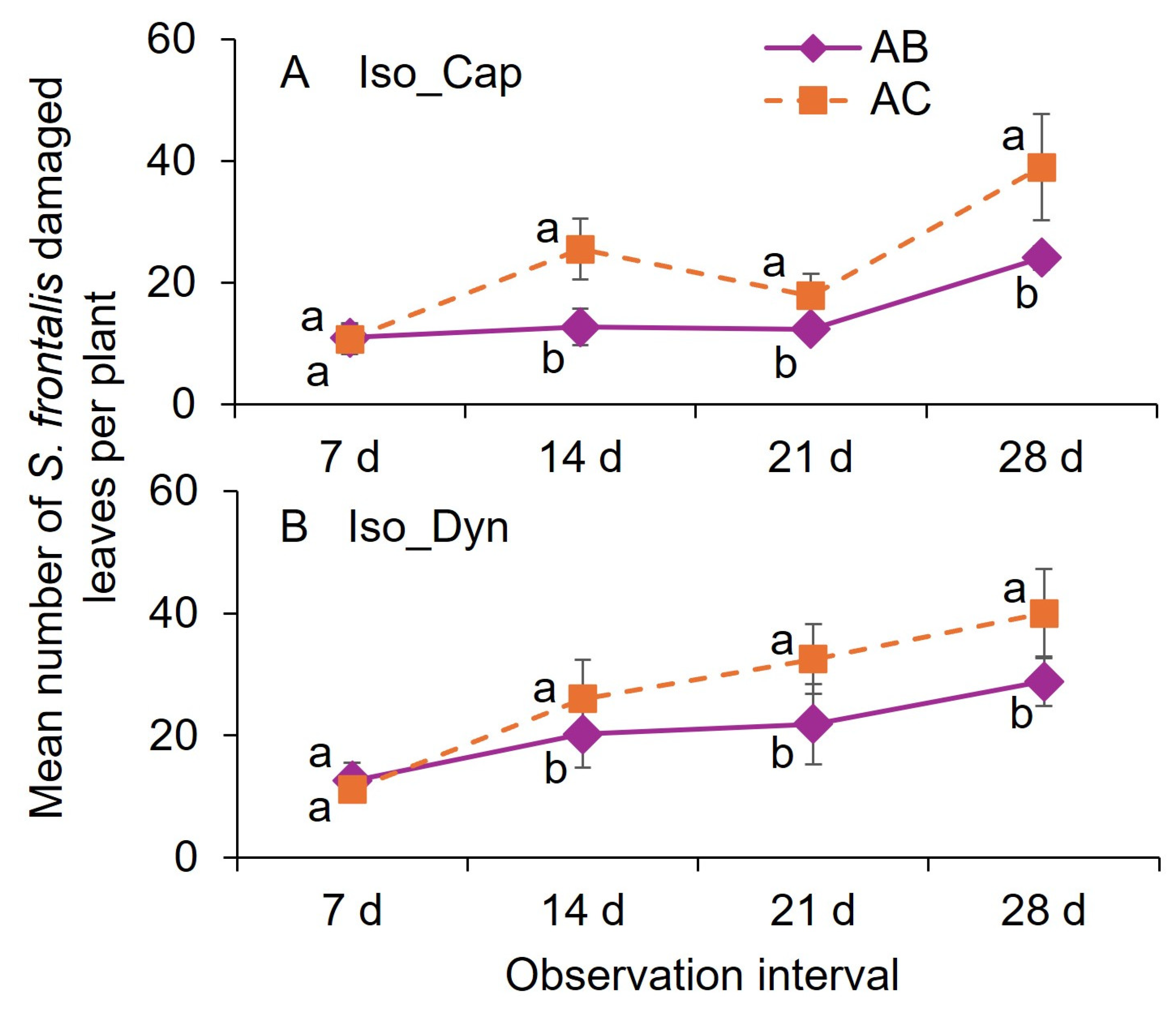
3.2. Field Trial 1 (2024)
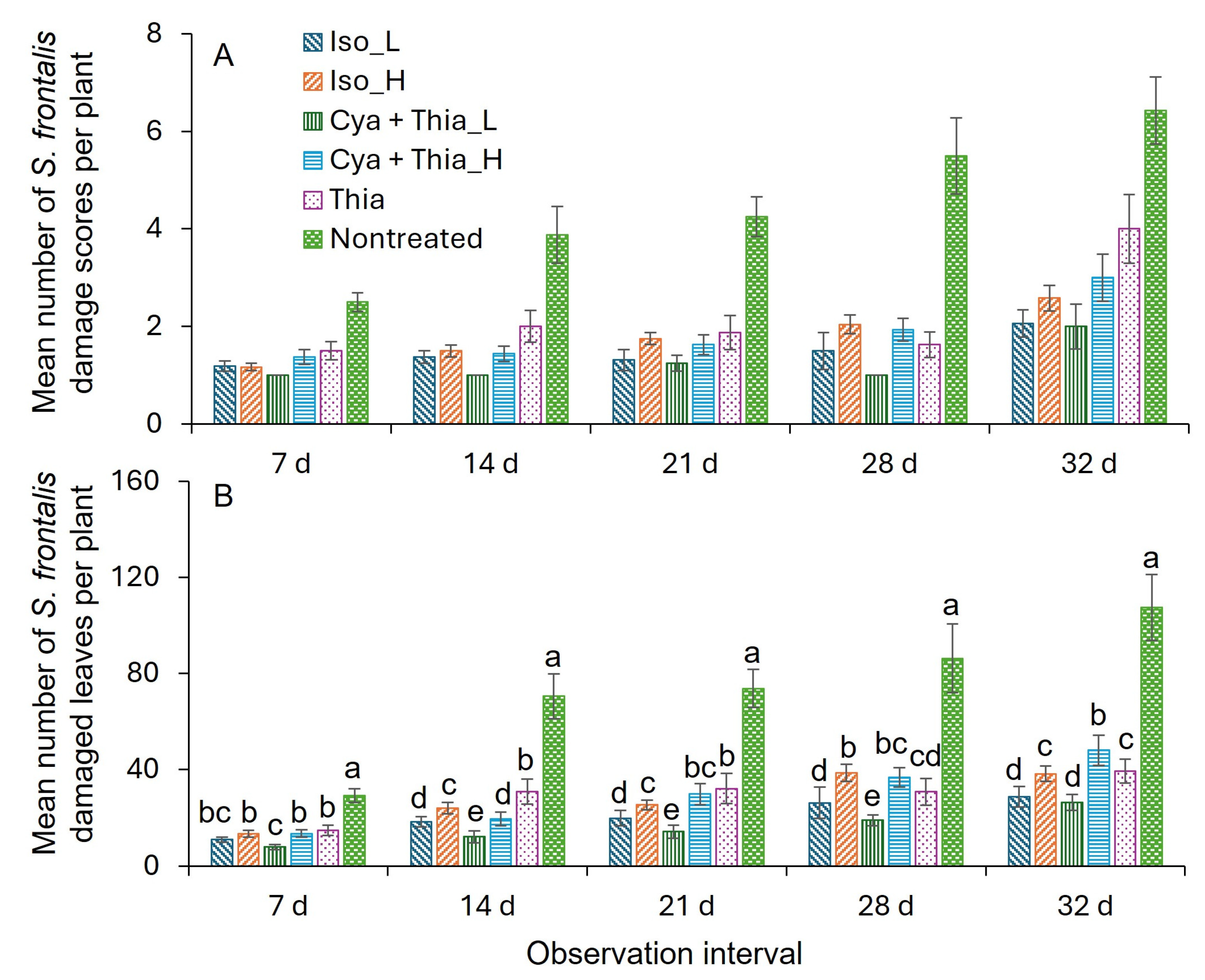
3.3. Field Trial 2 (2025)
3.4. Field Trial 3 (2025)
4. Discussion
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauderdale, D. Red-Headed Flea Beetle Biology and Management. Nursery & Landscape Notes, 2017, pp. 33–35. Available online: https://wilson.ces.ncsu.edu/wp-content/uploads/2017/02/2017-Nursery-Landscape-Notes-RHFB-Article.pdf?fwd=no (accessed on 26 August 2025).
- Joseph, S.V.; Chong, J.H.; Campbell, B.; Kunkel, B.; Lauderdale, D.; Jones, S.; Gill, S.; Chen, Y.; Schultz, P.; Held, D.; et al. Current pest status and management practices for Systena frontalis (Coleoptera: Chrysomelidae) in ornamental plants in the eastern United States: An online survey. J. Integr. Pest Manag. 2021, 12, 17. [Google Scholar] [CrossRef]
- Kunkel, B. Redheaded Flea Beetles: An Invasive Native. Growers Talk. 2021. Available online: https://www.growertalks.com/Article/?articleid=25339 (accessed on 10 February 2025).
- Arshad, R.; Chong, J.-H.; Lauderdale, D.; Kunkel, B.; Joseph, S.V. Biology and management of Systena frontalis (Coleoptera: Chrysomelidae) in ornamental plant nurseries. J. Integr. Pest Manag. 2023, 14, 7. [Google Scholar] [CrossRef]
- Vavilapalli, R.; Joseph, S.V. Emerging threat: Lysathia ludoviciana (Coleoptera: Chrysomelidae) as a pest of container-grown roses in ornamental nurseries. J. Econ. Entomol. 2025, 118, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Chong, J.-H.; Pozo-Valdivia, A.D.; Joseph, S.V. Growing media is the major source of damaging population of Systena frontalis (Coleoptera: Chrysomelidae) in ornamental plant nurseries. J. Econ. Entomol. 2023, 116, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Hudson, W. Redheaded Flea Beetle: An Ornamental Nursery Pest; University of Georgia Extension: Athens, GA, USA, 2020; p. C1187. Available online: https://fieldreport.caes.uga.edu/publications/C1187/red-headed-flea-beetle-an-ornamental-nursery-pest/ (accessed on 20 October 2025).
- Lane, E.L.; Del Pozo-Valdivia, A.I. Bioassays comparing different insecticides against Systena frontalis adults on Hydrangea paniculata, 2022. Arthropod Manag. Tests 2022, 47, tasc129. [Google Scholar] [CrossRef]
- Joseph, S.V. Efficacy of insecticides against Systena frontalis in containerized panicled hydrangea in nursery, 2023. Arthropod Manag. Tests 2023, 48, tsad092. [Google Scholar] [CrossRef]
- Arshad, R.; Joseph, S.V. Residual activity of insecticides against adult Systena frontalis (Coleoptera: Chrysomelidae) under semi-field conditions. J. Econ. Entomol. 2024, 117, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Pozo-Valdivia, A.D. Effects of insecticide drench application against immatures of Systena frontalis in containerized Hydrangea paniculata. J. Environ. Hortic. 2023, 41, 161–169. [Google Scholar] [CrossRef]
- Joseph, S.V. Response to the winter drench application and dosage of cyclaniliprole on Systena frontalis (Coleoptera: Chrysomelidae) in containerized panicled hydrangea. Crop Prot. 2025, 197, 107345. [Google Scholar] [CrossRef]
- IRAC, Insecticide Resistance Action Committee. Mode of Action Classification Scheme. 2025. Available online: https://irac-online.org/mode-of-action/classification-online/ (accessed on 24 June 2025).
- SAS Institute. Statistical Analysis Software (SAS) User’s Guide Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2024. [Google Scholar]
- Cloyd, R.A.; Herrick, N.J. Evaluation of systemic insecticides in protecting container-grown nursery plants from damage caused by field-collected populations of redheaded flea beetle, Systena frontalis (Coleoptera: Chrysomelidae), adults. J. Entomol. Sci. 2023, 58, 294–306. [Google Scholar] [CrossRef]
- Lauderdale, D. Incorporating Granular Imidacloprid to Reduce Red-Headed Flea Beetle Injury; North Carolina Cooperative Extension: Wilson County, NC, USA, 2022; Available online: https://go.ncsu.edu/readext?827451 (accessed on 20 October 2025).


| Insecticide Product | Active Ingredient (%) | IRAC Group | Rate (Product Per 378.5 L Water) | Bioassay | Field Trial | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 (2024) c | 2 (2025) c | 3 (2025) d | ||||
| Plinazolin® Technology | Isocycloseram 1.67 SC | 30 | 59.1 mL | * | ||||
| 118.3 mL | * | * | ||||||
| 147.9 mL | * | * | ||||||
| 177.4 mL | * | * | ||||||
| 236.6 mL | * | * | ||||||
| 295.7 mL | * | * | ||||||
| Mainspring® Xtra a | Cyantraniliprole (20%) + thiamethoxam (20%) | 28 + 4A | 70.9 g | * | ||||
| 141.7 g | * | |||||||
| 170.1 g | * | |||||||
| 212.6 g | * | |||||||
| 226.8 g | * | * | ||||||
| 283.5 g | * | * | ||||||
| 303.3 g | * | |||||||
| Flagship® 25WG a | Thiamethoxam (25%) | 4A | 226.8 g | * | ||||
| Safari® 20 SG b | Dinotefuran (20%) | 4A | 226.8 g | * | ||||
| Insecticide | Variable | Live Beetle | Damage Score | ||||
|---|---|---|---|---|---|---|---|
| F | df | p | F | df | p | ||
| Isocycloseram | |||||||
| Rate | 8.9 | 5,231 | <0.001 | 31.3 | 5,232 | <0.001 | |
| Time | 16.6 | 1,231 | <0.001 | 0.7 | 1,232 | 0.421 | |
| Rate × time | 1.3 | 5,231 | 0.268 | 1.7 | 5,232 | 0.127 | |
| Cyantraniliprole + thiamethoxam | |||||||
| Rate | 14.5 | 4,153 | <0.001 | 47.2 | 4,153 | <0.001 | |
| Time | 11.7 | 1,153 | <0.001 | 1.5 | 1,153 | 0.217 | |
| Rate × time | 1.1 | 4,153 | 0.353 | 1.4 | 4,153 | 0.251 | |
| Trial | Variable | Damage Score | Damaged Leaves | ||||
|---|---|---|---|---|---|---|---|
| F | df | p | F | df | p | ||
| 2024 trial 1 | |||||||
| Insecticide | 7.4 | 5,424 | <0.001 | - | - | - | |
| Application interval | 1.1 | 2,424 | 0.339 | - | - | - | |
| Insecticide × application interval | 2.9 | 3,424 | 0.031 | - | - | - | |
| Observation time | 63.3 | 4,424 | <0.001 | - | - | - | |
| 2025 trial 2 | |||||||
| Insecticide | 1.7 | 4,378 | 0.143 | 44.8 | 4,378 | <0.001 | |
| Application interval | 2.2 | 2,378 | 0.112 | 53.6 | 2,378 | <0.001 | |
| Insecticide × application interval | 1.5 | 2,378 | 0.217 | 15.6 | 2,378 | <0.001 | |
| Observation time | 15.2 | 4,378 | <0.001 | 322.1 | 4,378 | <0.001 | |
| 2025 trial 3 | |||||||
| Insecticide | 8.8 | 2,176 | <0.001 | 241.9 | 2,176 | <0.001 | |
| Application interval | 1.5 | 1,176 | 0.229 | 82.4 | 1,176 | <0.001 | |
| Insecticide × application interval | 0.5 | 1,176 | 0.499 | 4.5 | 1,176 | 0.036 | |
| Observation time | 11.4 | 3,176 | <0.001 | 244.7 | 3,176 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, S.V. Optimizing Foliar Spray Intervals and Rates of Isocycloseram and Cyantraniliprole Plus Thiamethoxam Application on Hydrangea paniculata to Combat Adult Systena frontalis (F) (Coleoptera: Chrysomelidae). Insects 2025, 16, 1082. https://doi.org/10.3390/insects16111082
Joseph SV. Optimizing Foliar Spray Intervals and Rates of Isocycloseram and Cyantraniliprole Plus Thiamethoxam Application on Hydrangea paniculata to Combat Adult Systena frontalis (F) (Coleoptera: Chrysomelidae). Insects. 2025; 16(11):1082. https://doi.org/10.3390/insects16111082
Chicago/Turabian StyleJoseph, Shimat V. 2025. "Optimizing Foliar Spray Intervals and Rates of Isocycloseram and Cyantraniliprole Plus Thiamethoxam Application on Hydrangea paniculata to Combat Adult Systena frontalis (F) (Coleoptera: Chrysomelidae)" Insects 16, no. 11: 1082. https://doi.org/10.3390/insects16111082
APA StyleJoseph, S. V. (2025). Optimizing Foliar Spray Intervals and Rates of Isocycloseram and Cyantraniliprole Plus Thiamethoxam Application on Hydrangea paniculata to Combat Adult Systena frontalis (F) (Coleoptera: Chrysomelidae). Insects, 16(11), 1082. https://doi.org/10.3390/insects16111082






