Toxicity of Hypaconitine from Aconitum coreanum (H. Lév.) Rapaics Against the Oriental Armyworm, Mythimna separata (Walker)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Sources
2.2. Chemicals
2.3. Larvicidal Bioassay
2.4. Determination of the Antifeedant Activity of Hypaconitine on M. separata Larvae
2.5. Analysis of the Growth Inhibitory Activity of Hypaconitine on M. separata Larvae
2.6. Monitoring the Developmental Duration of M. separata Treated with Hypaconitine
2.7. Analysis of the Effect of Hypaconitine on the Detoxification Enzymes of M. separata
2.8. Statistical Analysis
3. Results
3.1. Insecticidal Activity of Hypaconitine Against M. separata Larvae
3.2. Antifeedant Activity of Hypaconitine Against Third-Instar Larvae of M. separata
3.3. Growth Inhibitory Effect of Hypaconitine on Third-Instar Larvae of M. separata
3.4. Effect of Hypaconitine on the Developmental Duration of M. separata
3.5. Hypaconitine Impairs Pupation, Pupal Integrity, and Adult Emergence in M. separata
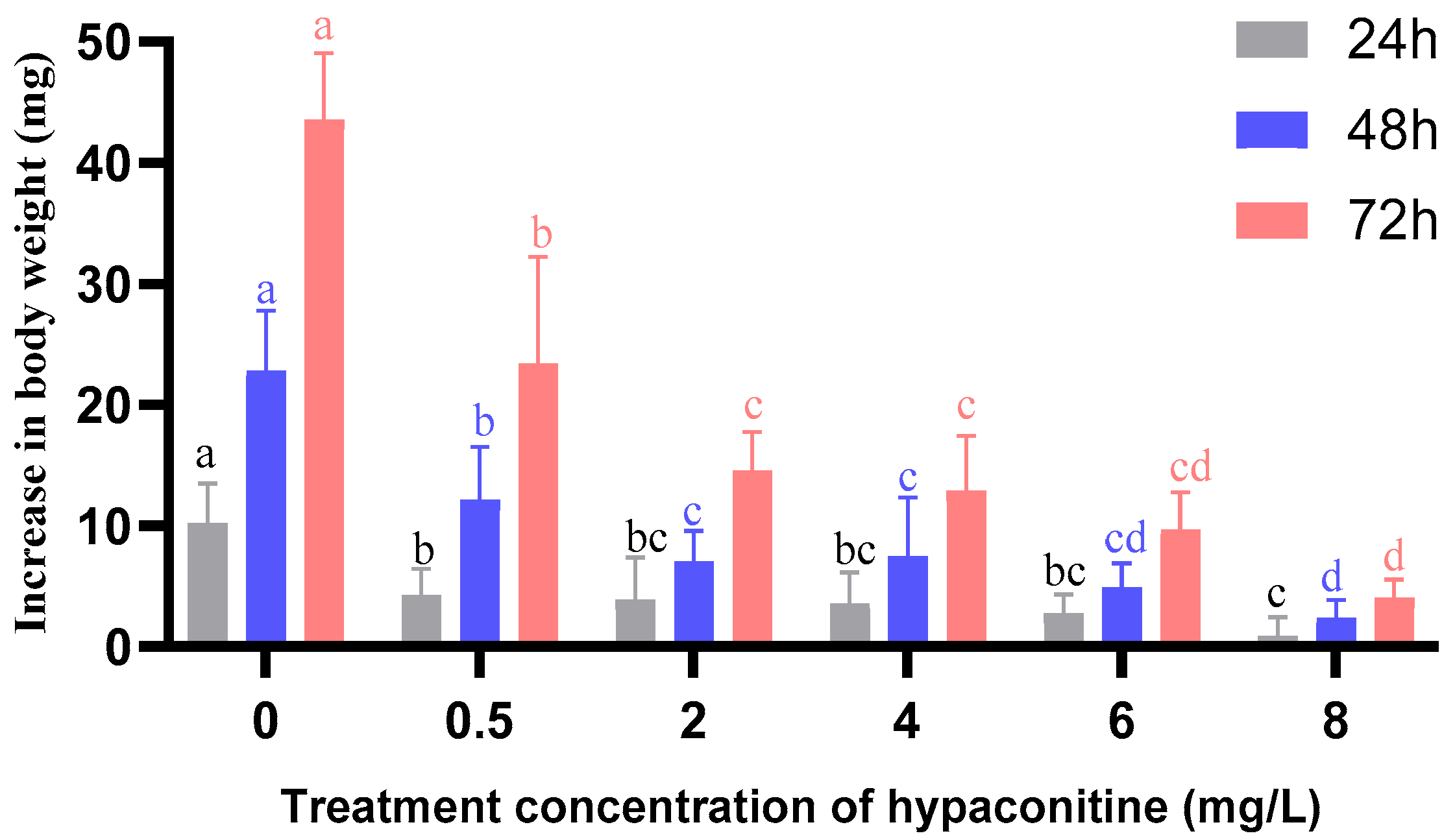
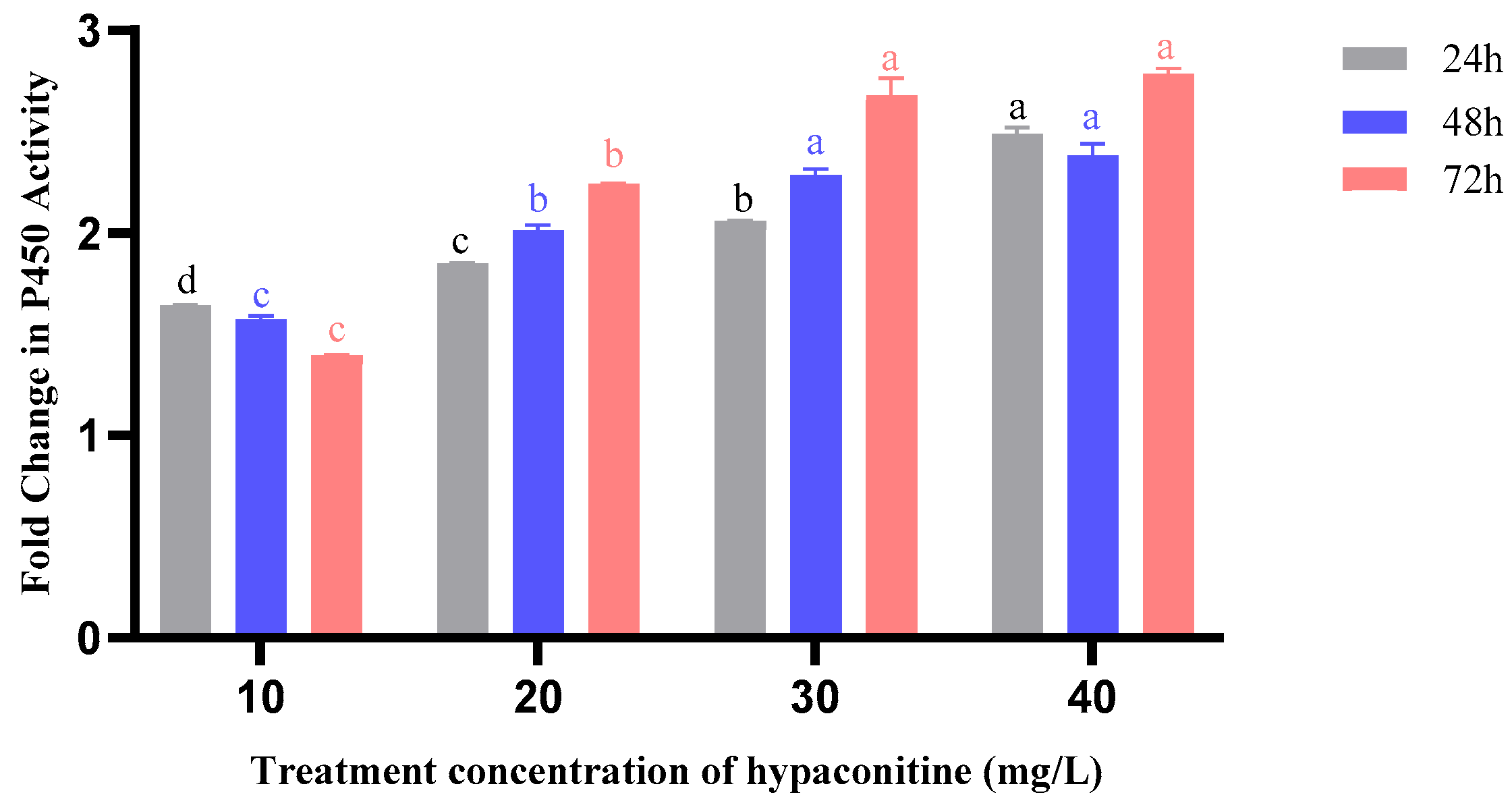
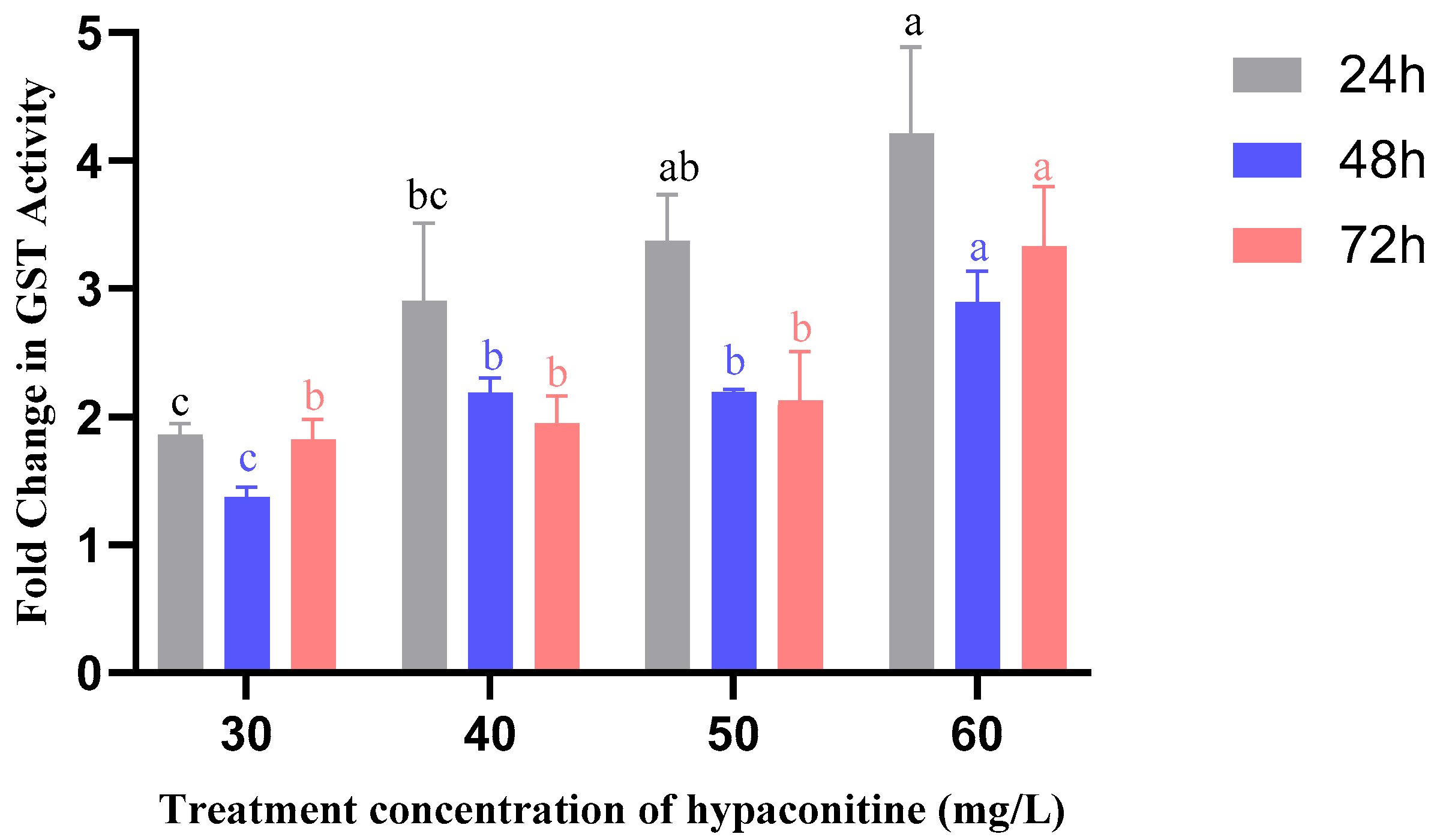
3.6. Hypaconitine Induces Time- and Concentration-Dependent Activation of Detoxification Enzymes in Third-Instar M. separata Larvae
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CABI. Mythimna separata (Paddy Armyworm). In CABI Compendium 2020; CABI: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.-D.; Qi, X.-H.; Xu, Y.-W.; Hou, Y.-H.; Fan, Z.-Y.; Shen, H.-L.; Liu, D.; Shi, X.-K.; Li, S.-M.; et al. The effects of climate warming on the migratory status of early summer populations of Mythimna separata (Walker) moths: A case-study of enhanced corn damage in central-northern China, 1980–2016. Ecol. Evol. 2019, 9, 12332–12338. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-P.; Zhang, Q.-W.; Ye, Z.-H.; Luo, L.-Z. The role of nectar plants in severe outbreaks of armyworm Mythimna separata (Lepidoptera: Noctuidae) in China. Bull. Entomol. Res. 2006, 96, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Li, B.L.; Jiang, S.X.; Zhao, Y.W.; Xu, X.L.; Wu, J.X. Microsatellite-based analysis of genetic structure and gene flow of Mythimna separata (Walker) (Lepidoptera: Noctuidae) in China. Ecol. Evol. 2019, 9, 13426–13437. [Google Scholar] [CrossRef]
- Kim, K.-N.; Yun, C.-N.; Sin, U.-C.; Huang, Z.-J.; Huang, Q.-Y.; Lei, C.-L. Green light and light stress in moth: Influence on antioxidant enzymes in the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2018, 25, 35176–35183. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Yang, H.; Chen, Y.; Fan, D. Decreased carboxylesterase expression associated with increased susceptibility to insecticide in Mythimna separata. Arch. Insect Biochem. Physiol. 2021, 109, e21859. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Su, L.; Li, S.; Li, Y.-P.; Xu, X.-L.; Cheng, W.-N.; Wang, Y.; Wu, J.-X. Insecticide resistance of the field populations of oriental armyworm, Mythimna separata (Walker) in Shaanxi and Shanxi provinces of China. J. Integr. Agric. 2018, 17, 1556–1562. [Google Scholar] [CrossRef]
- Rasul, A.; Akhtar, Z.R.; Rehman, M.A.; Saif-Ur-Rehman; Mustafa, T.; Ali, A.; Sagheer, M.; Rehman, H.U.; Mukhtar, M.L. Field-evolved resistance, inheritance patterns of laboratory-selected strains of Oriental armyworm (Mythimna separata) to lambda-cyhalothrin, and cross-resistance to other insecticides. Pak. J. Agric. Sci. 2021, 58, 103–109. [Google Scholar] [CrossRef]
- Chen, L.L.; Lai, C.J.S.; Mao, L.Y.; Yin, B.W.; Tian, M.; Jin, B.L.; Wei, X.Y.; Chen, J.L.; Ge, H.; Zhao, X.; et al. Chemical constituents in different parts of seven species of Aconitum based on UHPLC-Q-TOF/MS. J. Pharm. Biomed. Anal. 2021, 193, 113713. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Cheng, J.; Shi, X.-Y.; Yang, L.-Y.; Xie, X.-D. Progress in antitumor activity of diterpenoid alkaloids in plants of Aconitum. China J. Chin. Mater. Medica 2023, 48, 3765–3773. [Google Scholar] [CrossRef]
- Wada, K.; Ohkoshi, E.; Zhao, Y.; Goto, M.; Morris-Natschke, S.L.; Lee, K.-H. Evaluation of Aconitum diterpenoid alkaloids as antiproliferative agents. Bioorganic Med. Chem. Lett. 2015, 25, 1525–1531. [Google Scholar] [CrossRef]
- Tai, C.J.; El-Shazly, M.; Wu, T.Y.; Lee, K.T.; Csupor, D.; Hohmann, J.; Chang, F.R.; Wu, Y.C. Clinical Aspects of Aconitum Preparations. Planta Medica 2015, 81, 1017–1028. [Google Scholar] [CrossRef]
- Liu, Z.L.; Cao, J.; Zhang, H.M.; Lin, L.L.; Liu, H.J.; Du, S.S.; Zhou, L.; Deng, Z.W. Feeding Deterrents from Aconitum episcopale Roots against the Red Flour Beetle, Tribolium castaneum. J. Agric. Food Chem. 2011, 59, 3701–3706. [Google Scholar] [CrossRef]
- Kumar, S.; Anmol; Sharma, U.; Reddy, S.G.E. Insecticidal potential of extracts, fractions, and molecules of Aconitum heterophyllum Wall ex. Royle against aphid Aphis craccivora Koch (Hemiptera: Aphididae). Pest Manag. Sci. 2022, 79, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fan, H.; Nie, A.; Yang, K.; Xing, H.; Gao, Z.; Yang, L.; Wang, Z.; Zhang, L. Aconitine: A review of its pharmacokinetics, pharmacology, toxicology and detoxification. J. Ethnopharmacol. 2022, 293, 115270. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y.K. Aconitum Alkaloid Poisoning Related to the Culinary Uses of Aconite Roots. Toxins 2014, 6, 2605–2611. [Google Scholar] [CrossRef]
- Yan, X.-E.; Liu, Y.; Li, Z. Research progress of plant-derived aconitine as insecticide. Scienceasia 2022, 48, 119–127. [Google Scholar] [CrossRef]
- Yuan, C.-L.; Wang, X.-L. Isolation of active substances and bioactivity of Aconitum sinomontanum Nakai. Nat. Prod. Res. 2012, 26, 2099–2102. [Google Scholar] [CrossRef]
- Xu, K.; Song, Z.; Liu, J.; Yang, L.; Sun, G.; Lei, L.; Huang, S.; Gao, F.; Chen, L.; Zhou, X. Compositions analysis and insecticidal activity of Aconitum polycarpum Chang ex W.T.Wang petroleum ether fractions and essential oils. J. Ethnopharmacol. 2023, 303, 115989. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, X.; Xu, K.; Sun, G.; Yang, L.; Huang, L.; Liu, J.; Yin, P.; Huang, S.; Gao, F.; et al. Design, synthesis and insecticidal activity and mechanism research of Chasmanthinine derivatives. Sci. Rep. 2022, 12, 15290. [Google Scholar] [CrossRef]
- Yang, M. Effect of Hypaconitine on Chitin Deacetylase 2 (MsCDA2) of Oriental Armyworm Mythimna separata (Walker). Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2023. [Google Scholar]
- Chen, N. Mechanism of Lethal Moulting Induced by Hypaconitine in the Mythimna separata (Walker). Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2024. [Google Scholar]
- Li, T. Synergistic Control of Metarhizium rileyi and Azadirachtin on Spodoptera frugiperda (Lepidoptera: Noctuidae). Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2024. [Google Scholar]
- Zhu, K. Study of Chemical Constituents of Aconitum Coreanum (Lévl.) Rapaics and the Structure Modification of Diterpenoid Alkaloids. Master’s. Thesis, Northwest Normal University, Lanzhou, China, 2007. [Google Scholar]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Zhang, C.-X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Rakotondravelo, M.L.; Anderson, T.D.; Charlton, R.E.; Zhu, K.Y. Sublethal Effects of Three Pesticides on Activities of Selected Target and Detoxification Enzymes in the Aquatic Midge, Chironomus tentans (Diptera: Chironomidae). Arch. Environ. Contam. Toxicol. 2006, 51, 360–366. [Google Scholar] [CrossRef]
- Yin, F.; Ma, W.; Li, D.; Zhang, X.; Zhang, J. Expression and kinetic analysis of carboxylesterase LmCesA1 from Locusta migratoria. Biotechnol. Lett. 2021, 43, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Zhang, J.; Zhang, X.; Starkey, S.R.; Zhu, K.Y. Identification and characterization of eleven glutathione S-transferase genes from the aquatic midge Chironomus tentans (Diptera: Chironomidae). Insect Biochem. Mol. Biol. 2009, 39, 745–754. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.; Gartner, F.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shen, J.; Su, L.; Nie, Z.; Shikano, I.; Liu, T.-X.; Chen, L. Fitness of Mythimna separata (Lepidoptera: Noctuidae) on Cultivated Wheat and a Weed, Wild Oat (Avena fatua), and Its Implications for Pest Management. Biology 2024, 13, 1037. [Google Scholar] [CrossRef]
- Cang, X.; Zhao, S.; Yang, X.; Yuan, H.; Liu, J.; Liu, D.; Yang, X.; Wu, K. Migration Monitoring and Route Analysis of the Oriental Armyworm Mythimna separata (Walker) in Northeast China. Agronomy 2023, 13, 172. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Zhang, T.; Bai, S.; Wang, Z.; He, K. Inheritance and Fitness Costs of Vip3Aa19 Resistance in Mythimna separata. Toxins 2022, 14, 388. [Google Scholar] [CrossRef]
- Liang, X.; Su, W.; Zhang, W.; Wang, S.; Wu, X.; Li, X.; Gao, W. An overview of the research progress on Aconitum carmichaelii Debx.:active compounds, pharmacology, toxicity, detoxification, and applications. J. Ethnopharmacol. 2025, 337, 118832. [Google Scholar] [CrossRef]
- Heckel, D.G. Insect Detoxification and Sequestration Strategies. In Annual Plant Reviews; John Wiley and Sons: Hoboken, NJ, USA, 2014; pp. 77–114. [Google Scholar]
- Gul, H.; Gadratagi, B.G.; Güncan, A.; Tyagi, S.; Ullah, F.; Desneux, N.; Liu, X. Fitness costs of resistance to insecticides in insects. Front. Physiol. 2023, 14, 1238111. [Google Scholar] [CrossRef]
- Gassmann, A.J. Chapter fourteen–Fitness costs of resistance and their potential application for insect resistance management. In Insect Resistance Management, 3rd ed.; Onstad, D.W., Knolhoff, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 465–491. [Google Scholar]
- Wang, J.; Zheng, L.; Huang, W.; Li, L.; Yuan, J.; Chen, L. Insecticidal Activities of Diterpene Alkaloids in Plants of the Genera Aconitum and Delphin. Toxins 2025, 17, 254. [Google Scholar] [CrossRef]
- Pu, J.; Chung, H. New and emerging mechanisms of insecticide resistance. Curr. Opin. Insect Sci. 2024, 63, 101184. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-S.; Zhang, W.; Peng, Y.; Zhao, F.; Chang, X.-Q.; Xing, K.; Zhu, L.; Ma, G.; Yang, H.-P.; Rudolf, V.H.W. Climate warming promotes pesticide resistance through expanding overwintering range of a global pest. Nat. Commun. 2021, 12, 5351. [Google Scholar] [CrossRef] [PubMed]

| Instar | Time (h) | Regression Equation of Toxicity | R | LC50 (mg/L) | 95% Confidence Limits (mg/L) | χ2 (df = 3) | p-Value |
|---|---|---|---|---|---|---|---|
| 2nd | 48 h | y = (1.4138 ± 0.0562)x + (3.0802 ± 0.0708) | 0.9969 | 22.7968 | 20.6384~25.1809 | 8.4787 | 0.0755 |
| 72 h | y = (1.3815 ± 0.0787)x + (3.2325 ± 0.0992) | 0.9936 | 19.0300 | 16.6045~21.8099 | 8.5522 | 0.0733 | |
| 3rd | 48 h | y = (2.5905 ± 0.2867)x + (1.3282 ± 0.4449) | 0.9821 | 26.1475 | 22.0319~31.0320 | 6.7459 | 0.0805 |
| 72 h | y = (2.4347 ± 0.3107)x + (1.6071 ± 0.4821) | 0.9764 | 24.7491 | 20.2250~30.2852 | 6.8356 | 0.0773 |
| Concentration (mg/L) | 24 h Antifeedant Rate (%) | 48 h Antifeedant Rate (%) | 72 h Antifeedant Rate (%) |
|---|---|---|---|
| 5 | 10.40 ± 1.28(e) | 13.64 ± 1.33 (f) | 21.45 ± 0.38 (f) |
| 10 | 25.89 ± 1.07 (d) | 28.22 ± 1.29 (e) | 31.34 ± 1.01 (e) |
| 15 | 29.17 ± 0.42 (c) | 35.56 ± 0.78 (d) | 44.56 ± 1.18 (d) |
| 20 | 32.78 ± 0.29 (b) | 38.80 ± 0.63 (c) | 49.76 ± 1.35 (c) |
| 25 | 34.56 ± 0.68 (b) | 43.84 ± 0.46 (b) | 63.52 ± 0.23 (b) |
| 30 | 39.27 ± 0.44 (a) | 53.38 ± 0.89 (a) | 72.99 ± 0.59 (a) |
| Exposure Time | Regression Equation | R | AFC50 (mg/L) | 95% Confidence Limits (mg/L) | χ2 (df = 3) | p-Value |
|---|---|---|---|---|---|---|
| 24 h | y = (1.1727 ± 0.1590)x + (3.0269 ± 0.1914) | 0.9652 | 48.1443 | 33.9416~68.2901 | 9.3267 | 0.0534 |
| 48 h | y = (1.3962 ± 0.1056)x + (2.9596 ± 0.1272) | 0.9888 | 28.9362 | 25.3421~33.0400 | 9.2142 | 0.0560 |
| 72 h | y = (1.7457 ± 0.2168)x + (2.8715 ± 0.2611) | 0.9705 | 16.5683 | 14.2700~19.2368 | 9.0998 | 0.0587 |
| Concentration (mg/L) | 3rd Instar Duration (d) | 4th Instar Duration (d) | 5th Instar Duration (d) | 6th Instar Duration (d) | Larval Duration (d) | Prepupal Duration (d) | Pupal Duration (d) | Adult Duration (d) |
|---|---|---|---|---|---|---|---|---|
| 0 | 4.04 ± 0.06 (b) | 4.44 ± 0.49 (a) | 6.03 ± 0.95 (a) | 6.24 ± 0.20 (a) | 20.79 ± 0.69 (c) | 1.81 ± 0.33 (b) | 10.05 ± 0.83 (b) | 11.71 ± 0.90 (a) |
| 1 | 4.14 ± 0.13 (b) | 4.78 ± 0.25 (a) | 5.94 ± 0.34 (a) | 6.28 ± 0.54 (a) | 21.10 ± 0.72 (bc) | 2.44 ± 0.19 (ab) | 10.67 ± 0.31 (ab) | 10.00 ± 0.20 (a) |
| 2.5 | 4.12 ± 0.11 (b) | 4.68 ± 0.39 (a) | 6.00 ± 0.75 (a) | 6.32 ± 0.16 (a) | 21.13 ± 0.58 (bc) | 2.46 ± 0.47 (ab) | 10.89 ± 0.65 (ab) | 10.39 ± 0.35 (a) |
| 5 | 4.58 ± 0.18 (ab) | 5.15 ± 0.51 (a) | 5.95 ± 0.33 (a) | 6.92 ± 0.25 (a) | 22.60 ± 0.69 (ab) | 2.65 ± 0.09 (a) | 11.67 ± 0.67 (a) | 10.39 ± 1.08 (a) |
| 10 | 5.02 ± 0.53 (a) | 5.45 ± 0.32 (a) | 5.88 ± 0.23 (a) | 7.39 ± 0.82 (a) | 23.74 ± 0.63 (a) | 2.85 ± 0.14 (a) | 12.25 ± 0.55 (a) | 7.92 ± 0.66 (b) |
| Concentration (mg/L) | Pupation Rate (%) | Pupal Deformity Rate (%) | Average Pupal Weight (g) | Eclosion Rate (%) |
|---|---|---|---|---|
| 0 | 85.93 ± 0.07 (a) | 0.00 (e) | 0.4450 ± 0.02 (a) | 100.00 (a) |
| 1 | 64.45 ± 0.04 (c) | 5.56 ± 0.10 (d) | 0.4318 ± 0.02 (a) | 83.33 (b) |
| 2.5 | 64.88 ± 0.09 (b) | 6.67 ± 0.12 (c) | 0.4065 ± 0.03 (a) | 79.44 ± 0.04 (c) |
| 5 | 56.48 ± 0.10 (d) | 7.14 ± 0.12 (b) | 0.3831 ± 0.03 (a) | 65.00 ± 0.09 (d) |
| 10 | 55.22 ± 0.11 (e) | 19.45 ± 0.05 (a) | 0.3624 ± 0.04 (a) | 63.89 ± 0.13 (e) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xing, J.; Yang, M.; Chen, N.; Liang, Y. Toxicity of Hypaconitine from Aconitum coreanum (H. Lév.) Rapaics Against the Oriental Armyworm, Mythimna separata (Walker). Insects 2025, 16, 1080. https://doi.org/10.3390/insects16111080
Li X, Xing J, Yang M, Chen N, Liang Y. Toxicity of Hypaconitine from Aconitum coreanum (H. Lév.) Rapaics Against the Oriental Armyworm, Mythimna separata (Walker). Insects. 2025; 16(11):1080. https://doi.org/10.3390/insects16111080
Chicago/Turabian StyleLi, Xiuwei, Jiaqi Xing, Meng Yang, Naiwei Chen, and Yaping Liang. 2025. "Toxicity of Hypaconitine from Aconitum coreanum (H. Lév.) Rapaics Against the Oriental Armyworm, Mythimna separata (Walker)" Insects 16, no. 11: 1080. https://doi.org/10.3390/insects16111080
APA StyleLi, X., Xing, J., Yang, M., Chen, N., & Liang, Y. (2025). Toxicity of Hypaconitine from Aconitum coreanum (H. Lév.) Rapaics Against the Oriental Armyworm, Mythimna separata (Walker). Insects, 16(11), 1080. https://doi.org/10.3390/insects16111080






