Chemical Contaminants in Cerumen Samples from Ecuadorian Stingless Bees: Reporting Glyphosate, Aminomethylphosphonic Acid, and the Presence of Metals and Metalloids
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
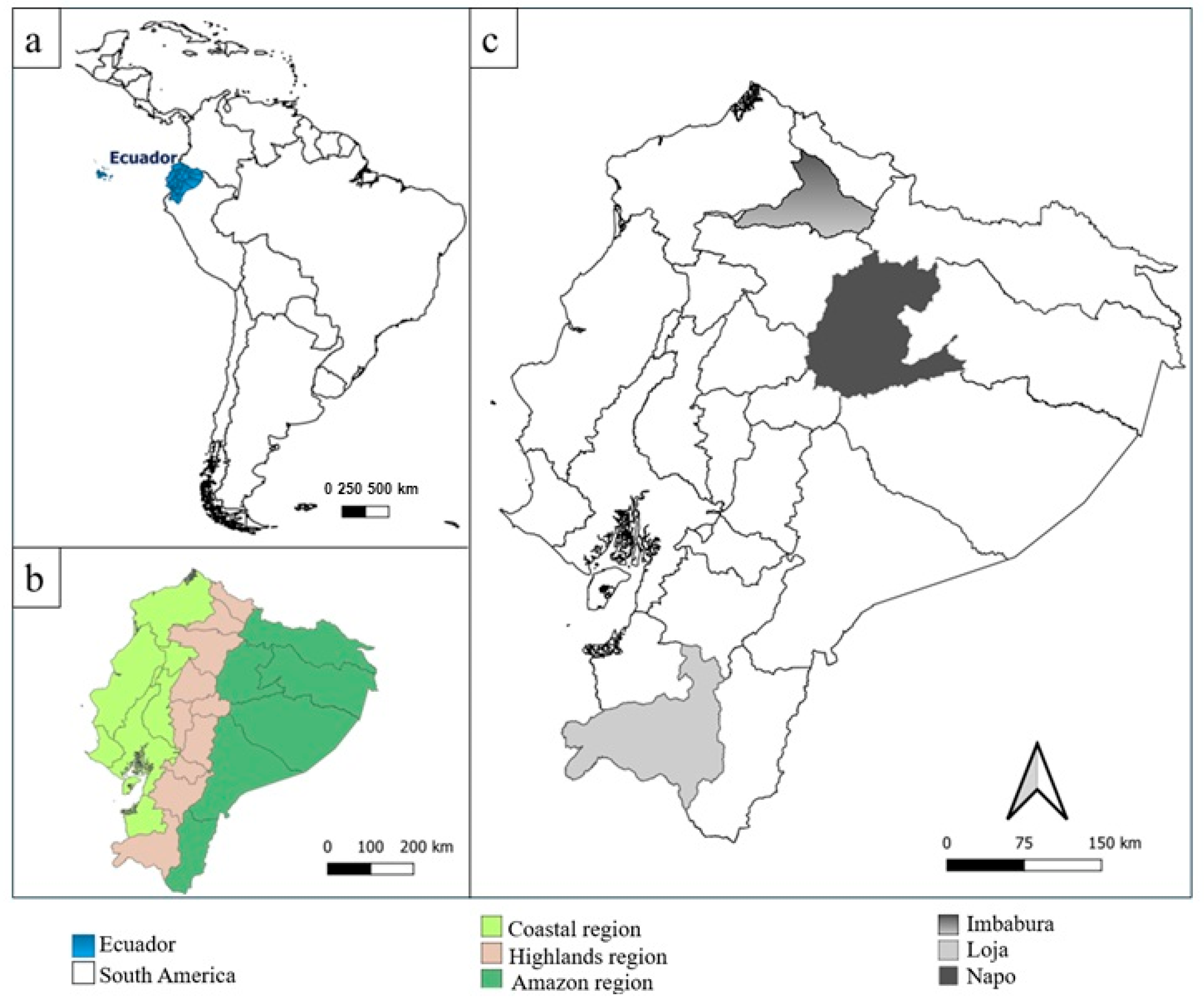
2.1. Sample Geographical Description
2.2. Chemical Contaminants Selected for Analysis
2.3. Chemical Extraction and Detection Process
2.3.1. Sample Preparation for Multi-Residue Analysis
2.3.2. Sample Preparation for Glyphosate (GLY) and AMPA Detection
2.3.3. Sample Preparation for Metals and Metalloid Trace Detection
2.4. Statistical Analysis
2.5. Risk Assessment of Glyphosate Exposure in Stingless Bees
2.6. Glyphosate Human Exposure Risk Assessment
3. Results
3.1. Metal and/Metalloids Trace Detection
3.2. Glyphosate (GLY) and AMPA Detection
3.3. Risk Assessment of the Presence of Agrochemicals and on the Health of Stingless Bees and Humans
4. Discussion
4.1. Glyphosate (GLY) and AMPA in Stingless Bees’ Cerumen
4.2. Metals, Metalloids, and Micronutrient Elements in Cerumen
4.3. Absence of Several Other Pesticides in Stingless Bee Cerumen
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


Appendix B
Appendix B.1. Chromatographic Parameters
| Time (min) | % Eluent A | % Eluent B |
|---|---|---|
| 0 | 80 | 20 |
| 0.1 | 80 | 20 |
| 1 | 50 | 50 |
| 9 | 20 | 80 |
| 12 | 0 | 100 |
| 13 | 0 | 100 |
| 13.5 | 80 | 20 |
| 15 | 80 | 20 |
Appendix B.2. Detection Parameters
| Group ID | Compound ID | Q1 Mass (Da) | Q3 Mass (Da) | Dwell Time (ms) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Positive Ion Electrospray Ionization | |||||||
| Acetamiprid | acetamiprid_1 | 223.2 | 126 | 20 | 10 | 27 | 15 |
| Acetamiprid | acetamiprid_2 | 223.2 | 90.1 | 20 | 10 | 45 | 15 |
| Benomyl | benomyl_1 | 291 | 192 | 20 | 10 | 17 | 10 |
| Benomyl | benomyl_2 | 291 | 160 | 20 | 10 | 39 | 8 |
| Carbendazim | carbendazim_1 | 192.1 | 160.1 | 20 | 10 | 25 | 15 |
| Carbendazim | carbendazim_2 | 192.1 | 132.1 | 20 | 10 | 41 | 15 |
| Methomyl | methomyl_1 | 163 | 88 | 20 | 10 | 13 | 14 |
| Methomyl | methomyl_2 | 163 | 106.1 | 20 | 10 | 15 | 8 |
| Flonicamid | flonicamid_1 | 230.1 | 203.1 | 20 | 10 | 25 | 12 |
| Flonicamid | flonicamid_2 | 230.1 | 174.05 | 20 | 10 | 27 | 8 |
| Thiacloprid | thiacloprid_1 | 253 | 126 | 20 | 10 | 29 | 15 |
| Thiacloprid | thiacloprid_2 | 253 | 186 | 20 | 10 | 19 | 15 |
| Thiamethoxam | thiamethoxam_1 | 292 | 211 | 20 | 10 | 17 | 10 |
| Thiamethoxam | thiamethoxam_2 | 292 | 181 | 20 | 10 | 33 | 8 |
| Malaoxon | malaoxon_1 | 315 | 127.1 | 20 | 10 | 17 | 15 |
| Malaoxon | malaoxon_2 | 315 | 99.2 | 20 | 10 | 31 | 15 |
| Malathion | malathion_1 | 331 | 127 | 20 | 10 | 17 | 29 |
| Malathion | malathion_2 | 331 | 284.9 | 20 | 10 | 11 | 16 |
| Chlorantraniliprole | chlorantraniliprole_1 | 484 | 453 | 20 | 10 | 23 | 12 |
| Chlorantraniliprole | chlorantraniliprole_2 | 484 | 286 | 20 | 10 | 19 | 18 |
| Trichlorfon | trichlorfon_1 | 257 | 108.9 | 20 | 10 | 27 | 18 |
| Trichlorfon | trichlorfon_2 | 257 | 220.8 | 20 | 10 | 17 | 14 |
| Pyrethrin I | pyrethrin I_1 | 329.179 | 161.2 | 20 | 10 | 15 | 14 |
| Pyrethrin I | pyrethrin I_2 | 329.179 | 143.2 | 20 | 10 | 25 | 10 |
| Pyrethrin I | pyrethrin I_3 | 329.179 | 133.1 | 20 | 10 | 25 | 14 |
| Imidacloprid | imidacloprid_1 | 256.007 | 209.1 | 20 | 10 | 23 | 12 |
| Imidacloprid | imidacloprid_2 | 256.007 | 175.2 | 20 | 10 | 29 | 10 |
| Diafenthiuron | diafenthiuron_1 | 385.238 | 329.148 | 20 | 10 | 26 | 7 |
| Diafenthiuron | diafenthiuron_2 | 385.238 | 287.05 | 20 | 10 | 36 | 6 |
| Diafenthiuron | diafenthiuron_3 | 385.238 | 262.045 | 20 | 10 | 64 | 8 |
| Diafenthiuron | diafenthiuron_6 | 385.2 | 278.2 | 20 | 10 | 45 | 8 |
| Negative Ion Electrospray Ionization | |||||||
| Clothianidin | clothianidin_1 | 247.873 | 57.9 | 20 | −10 | −16 | −9 |
| Clothianidin | clothianidin_2 | 249.966 | 58.1 | 20 | −10 | −16 | −7 |
Appendix C
Appendix C.1. Chromatographic Parameters
| Carrier Gas | Helium |
|---|---|
| Constant flow | 1 mL/min |
| Oven temperature | 60 °C |
| Injection mode | Solvent vent |
| Injection volume | 1 µL |
| Temperature (°C) | Ramp (°C/min) | Hold (min) | Total (min) |
|---|---|---|---|
| 45 | - | 0.02 | 0.02 |
| 325 | 600 | 5 | 18.497 |
| Temperature (°C) | Ramp (°C/min) | Hold (min) | Total (min) |
|---|---|---|---|
| 45 | - | 1.0 | 1.0 |
| 170 | 45.5 | 0.0 | 3.7473 |
| 310 | 10 | 0.75 | 18.497 |
| 310 (backflush) | 2.0 (backflush) | 2.0 (backflush) | 20.497 (backflush) |
Appendix C.2. Detection Parameters
| Molecule | N° Transition | Precursor Ion (m/z) | Product Ion (m/z) | CE (V) |
|---|---|---|---|---|
| Diazinon | 2 | 199.1 | 135.1 | 10 |
| Diazinon | 1 | 137.1 | 84 | 10 |
| Cypermethrine | 2 | 164.9 | 127 | 5 |
| Cypermethrine | 1 | 163 | 127 | 5 |
| Deltamethrine | 1 | 252.9 | 174 | 5 |
| Deltamethrine | 2 | 250.7 | 172 | 5 |
Appendix C.3. Validation Data
| Molecule | LOQ (µg/kg) | Mean Recovery (%) | RSD (%) |
|---|---|---|---|
| Acetamiprid | 10 | 75 | 8 |
| Benomyl | 10 | - | - |
| Carbendazim | 10 | 64 | 14 |
| Chlorantraniliprole | 10 | 67 | 7 |
| Clothianidin | 10 | 65 | 5 |
| Cypermethrin | 20 | 123 | 10 |
| Deltamethrin | 10 | 73 | 14 |
| Diafenthiuron | 50 | - | - |
| Diazinon | 10 | 65 | 17 |
| Flonicamid | 20 | 63 | 3 |
| Imidacloprid | 50 | 59 | 12 |
| Malaoxon | 20 | 76 | 7 |
| Malathion | 10 | 70 | 4 |
| Methomyl | 10 | 64 | 5 |
| Pyrethrin I | 50 | 63 | 13 |
| Thiacloprid | 20 | 69 | 5 |
| Thiamethoxam | 10 | 56 | 8 |
| Trichlorfon | 10 | 88 | 16 |
Appendix D
Appendix D.1. Chromatographic Parameters
| Eluent A | 0.1 mol/L ammonium bicarbonate in ultrapure water/ammonium hydroxide (100/0.1; v/v) |
| Eluent B | Ultrapure water/formic acid (100/0.02; v/v) |
| Flow rate | 0.7 mL/min |
| Oven temperature | 40 °C |
| Injection volume | 40 µL |
Appendix D.2. Gradient
| Time (min) | % Eluent A | % Eluent B |
|---|---|---|
| 0 | 10 | 90 |
| 0.5 | 10 | 90 |
| 0.6 | 20 | 80 |
| 2 | 20 | 80 |
| 7 | 50 | 50 |
| 10 | 50 | 50 |
| 10.5 | 100 | 0 |
| 11.5 | 100 | 0 |
| 11.6 | 10 | 90 |
| 14 | 10 | 90 |
Appendix D.3. Detection Parameters
| Q1 | Q3 | Dwell Time | Pesticide | DP | CE | CXP |
|---|---|---|---|---|---|---|
| 110 | 63 | 80 | AMPA_1 | −20 | −25 | −8.5 |
| 110 | 79 | 80 | AMPA_2 | −20 | −35 | −10 |
| 168 | 63 | 40 | glyphosate_1 | −25 | −28 | −6.7 |
| 168 | 81 | 40 | glyphosate_2 | −25 | −21 | −8 |
| 168 | 150 | 40 | glyphosate_3 | −25 | −14 | −7 |
Appendix D.4. Validation Data
| Molecule | LOQ (µg/kg) | Mean Recovery (%) | RSD (%) |
|---|---|---|---|
| AMPA | 10 | 105 | 8 |
| Glyphosate | 10 | 106 | 8 |
Appendix E
Appendix E.1. General Detection Parameters
| Nebulising gas | Argon at 1.08 L/min |
| Auxiliary gas | Argon at 0.90 L/min |
| Plasma gas | Argon at 15 L/min |
| Collision gas | Helium |
| ISTD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metalloids | Metals | |||||||||||||
| Sb | As | Sc | Cd | Cr | Cu | Sn | Hg | Ni | Pb | Se | Sc | Ir | Rh | |
| Isotope (Da) | 121 | 75 | 45 | 111 | 52 | 63 | 118 | 201 + 202 | 60 | 206,207,208 | 78 | 45 | 193 | 103 |
Appendix E.2. Validation Data
| LOQ (µg/kg) | Mean Recovery (%) | RSD (%) | |
|---|---|---|---|
| Metals | |||
| Cd | 8 | 102 | 5 |
| Cr | 20 | 101 | 3 |
| Cu | 125 | 113 | 8 |
| Hg | 20 | 102 | 3 |
| Ni | 20 | 104 | 5 |
| Pb | 20 | 101 | 5 |
| Se | 100 | 107 | 9 |
| Sn | 100 | 103 | 4 |
| Metalloids | |||
| Sb | 20 | 95 | 12 |
| As | 20 | 105 | 7 |
References
- Wittmann, D. Nest Architecture, Nest Site Preferences and Distribution of Plebeia Wittmanni (Moure & Camargo, 1989) in Rio Grande Do Sul, Brazil (Apidae: Meliponinae). Stud. Neotrop. Fauna Environ. 1989, 24, 17–23. [Google Scholar] [CrossRef]
- Camargo, J. Notas Sobre Habitos de Nidificacao de Scaura (Scaura) Latitarsis (Friese) (Hymenoptera, Apidae, Meliponinae). Bol. Mus. Para. Emilio Goeldi Zool. 1984, 1, 89–95. [Google Scholar]
- Roubik, D.W. Stingless Bee Nesting Biology. Apidologie 2006, 37, 124–143. [Google Scholar] [CrossRef]
- Wille, A.; Michener, C.D. The Nest Architecture of Stingless Bees with Special Reference to Those of Costa Rica (Hymenoptera, Apidae). Rev. Biol. Trop. 1973, 21. Available online: https://archivo.revistas.ucr.ac.cr/index.php/rbt/article/view/26200 (accessed on 19 October 2025).
- Cepeda-Aponte, O.I.; Imperatriz-Fonseca, V.L.; Velthuis, H.H.W. Lesser Wax Moth Achroia Grisella: First Report for Stingless Bees and New Capture Method. J. Apic. Res. 2002, 41, 107–108. [Google Scholar] [CrossRef]
- Drummond, P.M.; Bego, L.R.; Melo, G.A.R. Nest Architecture of The Stingless Bee Plebeia poecilochroa Moure & Camargo, 1993 And Related Considerations (Hymenoptera, Apidae, Meliponinae). Iheringia 1995, 79, 39–45. [Google Scholar]
- Dilworth, L.L.; Riley, C.K.; Stennett, D.K. Chapter 4—Plant Constituents: Carbohydrates, Oils, Resins, Balsams, and Plant Hormones. In Pharmacognosy, 2nd ed.; McCreath, S.B., Clement, Y.N., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 49–74. ISBN 978-0-443-18657-8. [Google Scholar]
- Shanahan, M.; Spivak, M. Resin Use by Stingless Bees: A Review. Insects 2021, 12, 719. [Google Scholar] [CrossRef]
- Leonhardt, S.D. Chemical Ecology of Stingless Bees. J. Chem. Ecol. 2017, 43, 385–402. [Google Scholar] [CrossRef]
- Wallace, H.M.; Lee, D.J. Resin-Foraging by Colonies of Trigona sapiens and T. hockingsi (Hymenoptera: Apidae, Meliponini) and Consequent Seed Dispersal of Corymbia torelliana (Myrtaceae). Apidologie 2010, 41, 428–435. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Heard, T.A.; Wallace, H. Differences in the Resource Intake of Two Sympatric Australian Stingless Bee Species. Apidologie 2014, 45, 514–527. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Blüthgen, N. A Sticky Affair: Resin Collection by Bornean Stingless Bees. Biotropica 2009, 41, 730–736. [Google Scholar] [CrossRef]
- Hilário, S.D.; Imperatriz-Fonseca, V.L.; Kleinert, A. Flight Activity and Colony Strength in the Stingless Bee Melipona bicolor bicolor (Apidae, Meliponinae). Rev. Bras. Biol. 2000, 60, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Biesmeijer, J.C.; Tóth, E. Individual Foraging, Activity Level and Longevity in the Stingless Bee Melipona beecheii in Costa Rica (Hymenoptera, Apidae, Meliponinae). Insectes Sociaux 1998, 45, 427–443. [Google Scholar] [CrossRef][Green Version]
- do Nascimento, D.L.; Nascimento, F.S. Extreme Effects of Season on the Foraging Activities and Colony Productivity of a Stingless Bee (Melipona asilvai Moure, 1971) in Northeast Brazil. Psyche A J. Entomol. 2012, 2012, 267361. [Google Scholar] [CrossRef]
- Silva, W. Pattern of the Daily Flight Activity in Two Colonies of Nannotrigona testaceicornis (Lepeletier, 1836) (Hymenoptera, Apidae) in Different Conditions in the Brazilian Semiarid Region. Sociobiology 2014, 61, 547–553. [Google Scholar] [CrossRef]
- de Freitas, P.V.D.X.; da Silva, I.E.; Faquinello, P.; Zanata, R.A.; Arnhold, E.; de Melo Silva-Neto, C. External Activity of the Stingless Bee Melipona fasciculata (Smith) Kept in the Brazilian Cerrado. J. Apic. Res. 2022, 61, 429–434. [Google Scholar] [CrossRef]
- do Nascimento, A.S.; Chambó, E.D.; de Jesus Oliveira, D.; Andrade, B.R.; Bonsucesso, J.S.; de Carvalho, C.A.L. Honey from Stingless Bee as Indicator of Contamination with Metals. Sociobiology 2018, 65, 727. [Google Scholar] [CrossRef]
- Yalamanchali, R. Lithium, an Emerging Environmental Contaminant, Is Mobile in the Soil-Plant System. Master’s Thesis, Lincoln University, Lincoln, New Zealand, 2012. [Google Scholar]
- Belzunces, L.P.; Tchamitchian, S.; Brunet, J.-L. Neural Effects of Insecticides in the Honey bee. Apidologie 2012, 43, 348–370. [Google Scholar] [CrossRef]
- Gekière, A.; Vanderplanck, M.; Michez, D. Trace Metals with Heavy Consequences on Bees: A Comprehensive Review. Sci. Total Environ. 2023, 895, 165084. [Google Scholar] [CrossRef]
- Gomes, I.N.; Gontijo, L.M.; Lima, M.A.P.; Zanuncio, J.S.; Resende, H.C. The Survival and Flight Capacity of Commercial Honeybees and Endangered Stingless Bees Are Impaired by Common Agrochemicals. Ecotoxicology 2023, 32, 937–947. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K.; Sanchez-Bayo, F.; Goka, K. Impacts of Pesticides on Honey bees. In Beekeeping and Bee Conservation—Advances in Research; IntechOpen: London, UK, 2016; ISBN 978-953-51-2412-2. [Google Scholar]
- Farder-Gomes, C.F.; de Oliveira, M.A.; Malaspina, O.; Nocelli, R.F.C. Exposure of the Stingless Bee Melipona scutellaris to Imidacloprid, Pyraclostrobin, and Glyphosate, Alone and in Combination, Impair Its Walking Activity and Fat Body Morphology and Physiology. Environ. Pollut. 2024, 348, 123783. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, M.; Jarau, S.; Barth, F.G. Stingless Bees (Meliponini): Senses and Behavior. J. Comp. Physiol. A 2016, 202, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, A.L.; de Souza Rosa-Fontana, A.; dos Santos, C.F.; Blochtein, B. Larvae of Stingless Bee Scaptotrigona bipunctata Exposed to Organophosphorus Pesticide Develop into Lighter, Smaller and Deformed Adult Workers. Environ. Pollut. 2021, 272, 116414. [Google Scholar] [CrossRef] [PubMed]
- Miotelo, L.; dos Reis, A.L.M.; Malaquias, J.B.; Malaspina, O.; Roat, T.C. Apis mellifera and Melipona scutellaris Exhibit Differential Sensitivity to Thiamethoxam. Environ. Pollut. 2021, 268, 115770. [Google Scholar] [CrossRef]
- Dennis, L.K.; Lynch, C.F.; Sandler, D.P.; Alavanja, M.C.R. Pesticide Use and Cutaneous Melanoma in Pesticide Applicators in the Agricultural Heath Study. Environ. Health Perspect. 2010, 118, 812–817. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public. Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Simeonov, L.I.; Macaev, F.Z.; Simeonova, B.G. Environmental Security Assessment and Management of Obsolete Pesticides in Southeast Europe; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-6461-3. [Google Scholar]
- Burgos-Aceves, M.A.; Banaee, M.; Vazzana, I.; Betancourt-Lozano, M.; González-Mille, D.J.; Aliko, V.; Faggio, C.; Ilizaliturri-Hernández, C.A. Effect of Emerging Pollutants on the Gut Microbiota of Freshwater Animals: Focusing on Microplastics and Pesticides. Sci. Total Environ. 2024, 948, 174809. [Google Scholar] [CrossRef]
- Raine, N.E.; Rundlöf, M. Pesticide Exposure and Effects on Non-Apis Bees. Annu. Rev. Entomol. 2024, 69, 551–576. [Google Scholar] [CrossRef]
- Tarish, M.; Ali, R.T.; Shan, M.; Amjad, Z.; Rui, Q.; Akher, S.A.; Al Mutery, A. Plant Tissues as Biomonitoring Tools for Environmental Contaminants. Int. J. Plant Biol. 2024, 15, 375–396. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy Metals and Living Systems: An Overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef]
- Ochoa, V.; Maestroni, B. Chapter 9—Pesticides in Water, Soil, and Sediments. In Integrated Analytical Approaches for Pesticide Management; Maestroni, B., Cannavan, A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 133–147. ISBN 978-0-12-816155-5. [Google Scholar]
- Ansari, I.; El-Kady, M.M.; El Din Mahmoud, A.; Arora, C.; Verma, A.; Rajarathinam, R.; Singh, P.; Verma, D.K.; Mittal, J. Persistent Pesticides: Accumulation, Health Risk Assessment, Management and Remediation: An Overview. Desalination Water Treat. 2024, 317, 100274. [Google Scholar] [CrossRef]
- Wei, X.; Pan, Y.; Zhang, Z.; Cui, J.; Yin, R.; Li, H.; Qin, J.; Li, A.J.; Qiu, R. Biomonitoring of Glyphosate and Aminomethylphosphonic Acid: Current Insights and Future Perspectives. J. Hazard. Mater. 2024, 463, 132814. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and Health Effects of the Herbicide Glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Maggi, F.; la Cecilia, D.; Tang, F.H.M.; McBratney, A. The Global Environmental Hazard of Glyphosate Use. Sci. Total Environ. 2020, 717, 137167. [Google Scholar] [CrossRef] [PubMed]
- Grandcoin, A.; Piel, S.; Baurès, E. AminoMethylPhosphonic Acid (AMPA) in Natural Waters: Its Sources, Behavior and Environmental Fate. Water Res. 2017, 117, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, A.V.; Zelenkova, N.F.; Vinokurova, N.G.; Ermakova, I.T.; Leontievsky, A.A. New Approaches to Identification and Activity Estimation of Glyphosate Degradation Enzymes. Biochemistry (Moscow) 2011, 76, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Venditti, S.; Kiesch, A.; Hansen, J. Fate of Glyphosate and Its Metabolite Aminomethylphosponic Acid (AMPA) from Point Source through Wastewater Sludge and Advanced Treatment. Chemosphere 2023, 340, 139843. [Google Scholar] [CrossRef]
- Reddy, K.N.; Rimando, A.M.; Duke, S.O.; Nandula, V.K. Aminomethylphosphonic Acid Accumulation in Plant Species Treated with Glyphosate. J. Agric. Food Chem. 2008, 56, 2125–2130. [Google Scholar] [CrossRef]
- Fréville, M.; Estienne, A.; Ramé, C.; Lefort, G.; Chahnamian, M.; Staub, C.; Venturi, E.; Lemarchand, J.; Maximin, E.; Hondelatte, A.; et al. Chronic Dietary Exposure to a Glyphosate-Based Herbicide Results in Total or Partial Reversibility of Plasma Oxidative Stress, Cecal Microbiota Abundance and Short-Chain Fatty Acid Composition in Broiler Hens. Front. Physiol. 2022, 13, 974688. [Google Scholar] [CrossRef]
- Drechsel, V.; Krais, S.; Peschke, K.; Ziegler, M.; Köhler, H.-R.; Triebskorn, R. Glyphosate- and Aminomethylphosphonic Acid (AMPA)-Induced Mortality and Residues in Juvenile Brown Trout (Salmo trutta f. fario) Exposed at Different Temperatures. Environ. Sci. Eur. 2024, 36, 30. [Google Scholar] [CrossRef]
- El Agrebi, N.; Tosi, S.; Wilmart, O.; Scippo, M.-L.; de Graaf, D.C.; Saegerman, C. Honeybee and Consumer’s Exposure and Risk Characterisation to Glyphosate-Based Herbicide (GBH) and Its Degradation Product (AMPA): Residues in Beebread, Wax, and Honey. Sci. Total Environ. 2020, 704, 135312. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace Elements in Agroecosystems and Impacts on the Environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of Phosphate Fertilizers in Heavy Metal Uptake and Detoxification of Toxic Metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kerur, S.S.; Bandekar, S.; Hanagadakar, M.S.; Nandi, S.S.; Ratnamala, G.M.; Hegde, P.G. Removal of Hexavalent Chromium-Industry Treated Water and Wastewater: A Review. Mater. Today Proc. 2021, 42, 1112–1121. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Binjamin, B.; Hasbullah, M.I.J.; Ador, K.; Gobilik, J.; Chin, C.F.S.; Lum, M.S.; Yaakub, N.M.; Benedick, S. Mineral and Heavy Metal Variations and Contaminations in Raw Honey of Stingless Bees, Heterotrigona itama, from Selected Geographical Areas of Origin in Malaysia. J. Nutr. Soc. Malays. 2024, 30, 403–415. [Google Scholar] [CrossRef]
- Salman, N.H.; Mok Sam, L.; Ador, K.; Binjamin, B.; Johny-Hasbulah, M.I.J.; Benedick, S. Linking Measure of the Tropical Stingless Bee (Apidae, Meliponini, and Heterotrigona itama) Honey Quality with Hives Distance to the Source of Heavy Metal Pollution in Urban and Industrial Areas in Sabah, Borneo. J. Toxicol. 2022, 2022, 4478082. [Google Scholar] [CrossRef]
- Hanapiah, N.A.M.; Salleh, S.N.A.S.; Johari, W.L.W.; Halimoon, N.; Adzahan, N.M.; Osman, N.H. Identification of Bioactive Compounds and Heavy Metal Concentrations in Propolis Ethanolic Extract Produced by Malaysian Stingless Bee. Biol. Trace Elem. Res. 2025. [Google Scholar] [CrossRef]
- Bonsucesso, J.S.; Gloaguen, T.V.; do Nascimento, A.S.; de Carvalho, C.A.L.; de S. Dias, F. Metals in Geopropolis from Beehive of Melipona scutellaris in Urban Environments. Sci. Total Environ. 2018, 634, 687–694. [Google Scholar] [CrossRef]
- Torres, B.S.S.; da Costa, G.C.; de França, V.F.; Souza, L.A.; Figueiredo, J.F.D.; Paim, A.P.S. Assessment of Essential and Potentially Toxic Elements in Beehive Products from Stingless Bees from the Northeast of Brazil: Determination and in Vitro Bioaccessibility Evaluation. Food Chem. 2025, 474, 143132. [Google Scholar] [CrossRef]
- Nowak, A.; Nowak, I. Review of Harmful Chemical Pollutants of Environmental Origin in Honey and Bee Products. Crit. Rev. Food Sci. Nutr. 2023, 63, 5094–5116. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.M.; Tran, L.; McKee, C.G.; Ortega Polo, R.; Newman, T.; Lansing, L.; Griffiths, J.S.; Bilodeau, G.J.; Rott, M.; Marta Guarna, M. Honey Bees as Biomonitors of Environmental Contaminants, Pathogens, and Climate Change. Ecol. Indic. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Bonilla, K.; Liria, J.; Rasmussen, C. Wing Phenotypic Diversity in Stingless Bees Genera (Apidae: Meliponini) from Ecuador Amazonia. Acta Biol. Colomb. 2024, 29, 112–118. [Google Scholar] [CrossRef]
- Guerrini, A.; Bruni, R.; Maietti, S.; Poli, F.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Scalvenzi, L.; Sacchetti, G. Ecuadorian Stingless Bee (Meliponinae) Honey: A Chemical and Functional Profile of an Ancient Health Product. Food Chem. 2009, 114, 1413–1420. [Google Scholar] [CrossRef]
- Ocaña-Cabrera, J.S.; Liria, J.; Vizuete, K.; Cholota-Iza, C.; Espinoza-Zurita, F.; Saegerman, C.; Martin-Solano, S.; Debut, A.; Ron-Román, J. Pollen Preferences of Stingless Bees in the Amazon Region and Southern Highlands of Ecuador by Scanning Electron Microscopy and Morphometry. PLoS ONE 2022, 17, e0272580. [Google Scholar] [CrossRef]
- Prado, I.S.; da Rocha, A.A.; Silva, L.A.; Gonzalez, V.C. Glyphosate-Based Formulation Affects Tetragonisca Angustula Worker’s Locomotion, Behavior and Biology. Ecotoxicol. Lond. Engl. 2023, 32, 513–524. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Guidance for Assessing Pesticide Risks to Bees. Off. Pestic. Programs USA Environ. Prot. Agency 2014, 59, 32–33. [Google Scholar]
- Obregón Hernández, F.; Arzaluz Gutiérrez, A. Influencia del cerumen en la propagación de la abeja sin aguijón Scaptotrigona mexicana Guérin (Hymenoptera: Apidae, Meliponinae). Folia Entomológica Mex. 2002, 41, 7–13. [Google Scholar]
- Duell, M.E.; Klok, C.J.; Roubik, D.W.; Harrison, J.F. Size-Dependent Scaling of Stingless Bee Flight Metabolism Reveals an Energetic Benefit to Small Body Size. Integr. Comp. Biol. 2022, 62, 1429–1438. [Google Scholar] [CrossRef]
- Paredes Bracho, A.J. Riqueza de Especies de Abejas Nativas Amazónicas Sin Aguijón de Los Géneros Melipona y tetragonisca (Hymenoptera: Apidae: Meliponini) y Usos de su Miel Según Los Pobladores de la Comunidad Etno-ecológica Pablo López de Oglán Alto, Cantón Arajuno-Provincia de Pastaza-Ecuador. Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2022. [Google Scholar]
- Vit, P.; Pedro, S.R.M.; Vergara, C.; Deliza, R. Ecuadorian Honey Types Described by Kichwa Community in Rio Chico, Pastaza Province, Ecuador Using Free-Choice Profiling. Rev. Bras. Farmacogn. 2017, 27, 384–387. [Google Scholar] [CrossRef]
- Vit, P.; Vargas, O.; Triny, L.; Vall, F.M. Meliponini Biodiversity and Medicinal Uses of Pot-Honey from El Oro Province in Ecuador. Emir. J. Food Agric. 2015, 27, 502–506. [Google Scholar] [CrossRef]
- Galli, F.S.; Mollari, M.; Tassinari, V.; Alimonti, C.; Ubaldi, A.; Cuva, C.; Marcoccia, D. Overview of Human Health Effects Related to Glyphosate Exposure. Front. Toxicol. 2024, 6, 1474792. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Álvarez, F.; Arena, M.; Auteri, D.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Crivellente, F.; Egsmose, M.; Fait, G.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Glyphosate. EFSA J. 2023, 21, e08164. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Mejía, K.; Rasmussen, C.; Romero, R. Traditional Knowledge of Stingless Bees (Hymenoptera: Apidae: Meliponini) in the Peruvian Amazon. Ethnobiol. Lett. 2023, 14, 1–9. [Google Scholar] [CrossRef]
- Costa-Neto, E.M. The Use of Insects in Folk Medicine in the State of Bahia, Northeastern Brazil, with Notes on Insects Reported Elsewhere in Brazilian Folk Medicine. Hum. Ecol. 2002, 30, 245–263. [Google Scholar] [CrossRef]
- Erwan, S.; Syamsuhaidi, D.K.P.; Muhsinin, M. Agussalim. Propolis Mixture Production and Foragers Daily Activity of Stingless Bee Tetragonula sp. in Bamboo and Box Hives. Livest. Res. Rural Dev. 2021, 33. Available online: https://www.lrrd.org/lrrd33/6/3382apis.html (accessed on 19 October 2025).
- Sangma, R.H.C.; Singh, H.K.; Chauhan, A. Nesting Structure of Stingless Bees, Lophotrigona canifrons Smith and Tetragonula iridipennis Smith (Hymenoptera: Apidae) in Natural Forests of Nagaland, India. Entomon 2022, 47, 183–188. [Google Scholar] [CrossRef]
- Vinueza-Veloz, A.F.; Tapia-Veloz, E.C.; Tapia-Veloz, G.; Nicolalde-Cifuentes, T.M.; Carpio-Arias, T.V.; Vinueza-Veloz, A.F.; Tapia-Veloz, E.C.; Tapia-Veloz, G.; Nicolalde-Cifuentes, T.M.; Carpio-Arias, T.V. Estado Nutricional de Los Adultos Ecuatorianos y Su Distribución Según Las Características Sociodemográficas. Estudio Transversal. Nutr. Hosp. 2023, 40, 102–108. [Google Scholar] [CrossRef]
- Checa, M.E.C.; Mendoza, W.L.A. Percentiles peso, talla y perímetro cefálico en recién nacidos a término, obtenidos por parto y cesárea, en el hospital Materno Infantil del Guasmo; 1 de enero al 31 de mayo de 2002. Medicina 2004, 9, 310–313. [Google Scholar]
- Ministério da Saúde. Regulamento Técnico MERCOSUL Sobre Limites Máximos de Contaminantes Inorgânicos Em Alimentos. Diário Oficial [Da] República Federativa Do Brasil. In Resolução RDC no 42, de 29 de agosto de 2013; Agência Nacional de Vigilância Sanitária—ANVISA: Brasília, Brazil, 2013; p. 33. [Google Scholar]
- de Camargo, R.C.R.; de Oliveira, K.L.; Berto, M.I. Mel de abelhas sem ferrão: Proposta de regulamentação. Braz. J. Food Technol. 2017, 20, e2016157. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) No 293/2013 of 20 March 2013 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Emamectin Benzoate, Etofenprox, Etoxazole, Flutriafol, Glyphosate, Phosmet, Pyraclostrobin, Spinosad and Spirotetramat in or on Certain Products Text with EEA Relevance; European Union: Brussels, Belgium, 2013; Volume 096. [Google Scholar]
- European Union. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance); European Union: Brussels, Belgium, 2023; Volume 119. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA); Nutrition and Food Safety (NFS); Standards & Scientific Advice on Food Nutrition (SSA). Evaluation of Certain Food Additives and Contaminants: Ninety-First Report of the Joint FAO/WHO Expert Committee on Food Additives. In Proceedings of the Ninety-first meeting of the Joint FAO/WHO Expert Committee on Food Additives, Virtual Meeting, 1–12 February 2021; WHO Technical Report Series, No. 1036. World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2022. ISBN 978-92-4-005458-5. [Google Scholar]
- Massaro, F.C.; Brooks, P.R.; Wallace, H.M.; Russell, F.D. Cerumen of Australian Stingless Bees (Tetragonula carbonaria): Gas Chromatography-Mass Spectrometry Fingerprints and Potential Anti-Inflammatory Properties. Naturwissenschaften 2011, 98, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, M.L.; Hettiarachchy, N.; Yu, X.; Howard, L.; Lee, S.-O. Penetration of Glyphosate into the Food Supply and the Incidental Impact on the Honey Supply and Bees. Food Control 2020, 109, 106859. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef]
- Shushkova, T.; Ermakova, I.; Leontievsky, A. Glyphosate Bioavailability in Soil. Biodegradation 2010, 21, 403–410. [Google Scholar] [CrossRef]
- Haney, R.L.; Senseman, S.A.; Hons, F.M.; Zuberer, D.A. Effect of Glyphosate on Soil Microbial Activity and Biomass. Weed Sci. 2000, 48, 89–93. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Sevakumaran, V.; Ariffin, F. Preliminary Study on Glyphosate-Degrading Bacteria Isolated from Agricultural Soil. Environ. Adv. 2023, 12, 100368. [Google Scholar] [CrossRef]
- Santos, A.C.C.; Borges, L.D.F.; Rocha, N.D.C.; de Carvalho Azevedo, V.A.; Bonetti, A.M.; dos Santos, A.R.; da Rocha Fernandes, G.; Dantas, R.C.C.; Ueira-Vieira, C. Bacteria, Yeasts, and Fungi Associated with Larval Food of Brazilian Native Stingless Bees. Sci. Rep. 2023, 13, 5147. [Google Scholar] [CrossRef]
- Arena, M.; Sgolastra, F. A Meta-Analysis Comparing the Sensitivity of Bees to Pesticides. Ecotoxicol. Lond. Engl. 2014, 23, 324–334. [Google Scholar] [CrossRef]
- Cham, K.O.; Nocelli, R.C.F.; Borges, L.O.; Viana-Silva, F.E.C.; Tonelli, C.A.M.; Malaspina, O.; Menezes, C.; Rosa-Fontana, A.S.; Blochtein, B.; Freitas, B.M.; et al. Pesticide Exposure Assessment Paradigm for Stingless Bees. Environ. Entomol. 2019, 48, 36–48. [Google Scholar] [CrossRef]
- Botina, L.L.; Barbosa, W.F.; Viana, T.A.; de Oliveira Faustino, A.; Martins, G.F. Physiological Responses of the Stingless bee Partamona helleri to Oral Exposure to Three Agrochemicals: Impact on Antioxidant Enzymes and Hemocyte Count. Environ. Sci. Pollut. Res. 2024, 31, 54648–54658. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Toledo, J.; Sánchez-Guillén, D. Efecto de la concentración de glifosato presente en cuerpos de agua cercanos a campos de soya transgénica sobre la abeja Apis mellifera y la abeja sin aguijón Tetragonisca angustula. Acta Zoológica Mex. 2014, 30, 408–413. [Google Scholar] [CrossRef]
- Nocelli, R.C.F.; Soares, S.M.M.; Monquero, P.A. Effects of Herbicides on the Survival of the Brazilian Native Bee Melipona scutellaris Latreille, 1811 (Hymenoptera: Apidae). Planta Daninha 2019, 37, e019220193. [Google Scholar] [CrossRef]
- Saurat, D.; Raffy, G.; Bonvallot, N.; Monfort, C.; Fardel, O.; Glorennec, P.; Chevrier, C.; Le Bot, B. Determination of Glyphosate and AMPA in Indoor Settled Dust by Hydrophilic Interaction Liquid Chromatography with Tandem Mass Spectrometry and Implications for Human Exposure. J. Hazard. Mater. 2023, 446, 130654. [Google Scholar] [CrossRef]
- Zarić, N.M.; Braeuer, S.; Goessler, W. Arsenic Speciation Analysis in Honey Bees for Environmental Monitoring. J. Hazard. Mater. 2022, 432, 128614. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Bermejo, F.J.O. Element Content of Propolis Collected from Different Areas of South Spain. Environ. Monit. Assess. 2013, 185, 6035–6047. [Google Scholar] [CrossRef]
- Maragou, N.C.; Pavlidis, G.; Karasali, H.; Hatjina, F. Determination of Arsenic in Honey, Propolis, Pollen, and Honey Bees by Microwave Digestion and Hydride Generation Flame Atomic Absorption. Anal. Lett. 2017, 50, 1831–1838. [Google Scholar] [CrossRef]
- Bastías, J.M.; Jambon, P.; Muñoz, O.; Manquián, N.; Bahamonde, P.; Neira, M. Honey as a Bioindicator of Arsenic Contamination Due to Volcanic and Mining Activities in Chile. Chil. J. Agric. Res. 2013, 73, 147–153. [Google Scholar] [CrossRef]
- Bundschuh, J.; Schneider, J.; Alam, M.A.; Niazi, N.K.; Herath, I.; Parvez, F.; Tomaszewska, B.; Guilherme, L.R.G.; Maity, J.P.; López, D.L.; et al. Seven Potential Sources of Arsenic Pollution in Latin America and Their Environmental and Health Impacts. Sci. Total Environ. 2021, 780, 146274. [Google Scholar] [CrossRef]
- Monchanin, C.; Drujont, E.; Le Roux, G.; Lösel, P.D.; Barron, A.B.; Devaud, J.-M.; Elger, A.; Lihoreau, M. Environmental Exposure to Metallic Pollution Impairs Honey Bee Brain Development and Cognition. J. Hazard. Mater. 2024, 465, 133218. [Google Scholar] [CrossRef]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human Health Effects from Chronic Arsenic Poisoning—A Review. J. Environ. Sci. Health Part A 2006, 41, 2399–2428. [Google Scholar] [CrossRef] [PubMed]
- Claeys, W.L.; De Voghel, S.; Schmit, J.-F.; Vromman, V.; Pussemier, L. Exposure Assessment of the Belgian Population to Pesticide Residues through Fruit and Vegetable Consumption. Food Addit. Contam. Part A 2008, 25, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Argüello, D.; Chavez, E.; Lauryssen, F.; Vanderschueren, R.; Smolders, E.; Montalvo, D. Soil Properties and Agronomic Factors Affecting Cadmium Concentrations in Cacao Beans: A Nationwide Survey in Ecuador. Sci. Total Environ. 2019, 649, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Barraza, F.; Schreck, E.; Lévêque, T.; Uzu, G.; López, F.; Ruales, J.; Prunier, J.; Marquet, A.; Maurice, L. Cadmium Bioaccumulation and Gastric Bioaccessibility in Cacao: A Field Study in Areas Impacted by Oil Activities in Ecuador. Environ. Pollut. 2017, 229, 950–963. [Google Scholar] [CrossRef]
- WHO. Exposure to Lead: A Major Public Health Concern. Preventing Disease through Healthy Environments; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-007813-0. [Google Scholar]
- Hrubá, F.; Strömberg, U.; Černá, M.; Chen, C.; Harari, F.; Harari, R.; Horvat, M.; Koppová, K.; Kos, A.; Krsková, A.; et al. Blood Cadmium, Mercury, and Lead in Children: An International Comparison of Cities in Six European Countries, and China, Ecuador, and Morocco. Environ. Int. 2012, 41, 29–34. [Google Scholar] [CrossRef]
- Rai, A.; Maurya, S.K.; Khare, P.; Srivastava, A.; Bandyopadhyay, S. Characterization of Developmental Neurotoxicity of As, Cd, and Pb Mixture: Synergistic Action of Metal Mixture in Glial and Neuronal Functions. Toxicol. Sci. 2010, 118, 586–601. [Google Scholar] [CrossRef]
- Gauthier, M.; Aras, P.; Jumarie, C.; Boily, M. Low Dietary Levels of Al, Pb and Cd May Affect the Non-Enzymatic Antioxidant Capacity in Caged Honey bees (Apis mellifera). Chemosphere 2016, 144, 848–854. [Google Scholar] [CrossRef]
- Sáenz, C.E.L.; de la Cruz, C.J.V. Contaminación por cadmio y plomo en miel altoandina del Perú: Un riesgo potencial para la salud. Innovaciencia 2025, 13, e4801. [Google Scholar] [CrossRef]
- Hernández-Medina, M.E.; Montiel Pimentel, J.V.; Castellanos, I.; Zuria, I.; Sánchez-Rojas, G.; Gaytán Oyarzun, J.C. Metal Concentration in Honeybees along an Urbanization Gradient in Central Mexico. Environ. Res. 2025, 264, 120199. [Google Scholar] [CrossRef]
- de Oliveira, D.F.; Braga, D.J.N.; Júnior, W.A.C.; Holanda, G.H.A.; Ronqui, L.; Parpinelli, R.S.; de Sousa-Filho, I.F.; de Azevedo, M.S.; de Almeida, R.; Bastos, W.R. Health Risk Due to the Presence of Trace Elements in Stingless bee Honey Consumed in the Amazon and Southern Brazil. J. Food Compos. Anal. 2025, 147, 108073. [Google Scholar] [CrossRef]
- Végh, R.; Csóka, M.; Sörös, C.; Sipos, L. Food Safety Hazards of Bee Pollen—A Review. Trends Food Sci. Technol. 2021, 114, 490–509. [Google Scholar] [CrossRef]
- Rojas Romero, J.E.; Rincón Ramírez, J.E.; Marín Leal, J.C.; Ortega Fuenmayor, P.C.; Buonocore Tovar, R.; Colina, M.; Brinolfo Montilla, J. Toxicidad y bioacumulación de Cromo (Cr+6) en la almeja Polymesoda solida del sistema estuarino Lago de Maracaibo. Bol. Cent. Investig. Biológicas 2015, 49, 5–25. [Google Scholar]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium Contamination and Effect on Environmental Health and Its Remediation: A Sustainable Approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, X.; Jiang, Z.; Li, Q.; Huang, P.; Zheng, C.; Liao, Q.; Yang, W. Reductive Materials for Remediation of Hexavalent Chromium Contaminated Soil—A Review. Sci. Total Environ. 2021, 773, 145654. [Google Scholar] [CrossRef]
- Liang, J.; Huang, X.; Yan, J.; Li, Y.; Zhao, Z.; Liu, Y.; Ye, J.; Wei, Y. A Review of the Formation of Cr(VI) via Cr(III) Oxidation in Soils and Groundwater. Sci. Total Environ. 2021, 774, 145762. [Google Scholar] [CrossRef]
- Montiel, J.; Marmolejo, Y.; Castellanos Sturemark, I.; Perez, F.; García, F.; Gaytán-Oyarzún, J.; Fonseca, M. Niveles de Cadmio, Cromo y Plomo En Abejas (Apis mellifera) y Sus Productos En Hidalgo, México. Rev. Iberoam. Cienc. 2020, 7, 57–68. [Google Scholar]
- Sgolastra, F.; Blasioli, S.; Renzi, T.; Tosi, S.; Medrzycki, P.; Molowny-Horas, R.; Porrini, C.; Braschi, I. Lethal Effects of Cr(III) Alone and in Combination with Propiconazole and Clothianidin in Honey Bees. Chemosphere 2018, 191, 365–372. [Google Scholar] [CrossRef]
- Hossini, H.; Shafie, B.; Niri, A.D.; Nazari, M.; Esfahlan, A.J.; Ahmadpour, M.; Nazmara, Z.; Ahmadimanesh, M.; Makhdoumi, P.; Mirzaei, N.; et al. A Comprehensive Review on Human Health Effects of Chromium: Insights on Induced Toxicity. Environ. Sci. Pollut. Res. 2022, 29, 70686–70705. [Google Scholar] [CrossRef]
- Romero-Estévez, D.; Yánez-Jácome, G.S.; Navarrete, H. Non-Essential Metal Contamination in Ecuadorian Agricultural Production: A Critical Review. J. Food Compos. Anal. 2023, 115, 104932. [Google Scholar] [CrossRef]
- Khan, U.A.; Kujala, K.; Nieminen, S.P.; Räisänen, M.L.; Ronkanen, A.-K. Arsenic, Antimony, and Nickel Leaching from Northern Peatlands Treating Mining Influenced Water in Cold Climate. Sci. Total Environ. 2019, 657, 1161–1172. [Google Scholar] [CrossRef]
- Haldar, A.K.; Sen, P.; Roy, S. Use of Antimony in the Treatment of Leishmaniasis: Current Status and Future Directions. Mol. Biol. Int. 2011, 2011, 571242. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Mbingwa, G.; Khanna, S.; Dalal, J.; Sankhyan, D.; Malik, A.; Badhwar, N. Environment and Health Hazards Due to Military Metal Pollution: A Review. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100857. [Google Scholar] [CrossRef]
- Clarke, D.; Morley, E.; Robert, D. The Bee, the Flower, and the Electric Field: Electric Ecology and Aerial Electroreception. J. Comp. Physiol. A 2017, 203, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.P.; Martins, G.F.; Oliveira, E.E.; Guedes, R.N.C. Agrochemical-Induced Stress in Stingless Bees: Peculiarities, Underlying Basis, and Challenges. J. Comp. Physiol. A 2016, 202, 733–747. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Baumann, A.-M.; Wallace, H.M.; Brooks, P.; Schmitt, T. The Chemistry of an Unusual Seed Dispersal Mutualism: Bees Use a Complex Set of Olfactory Cues to Find Their Partner. Anim. Behav. 2014, 98, 41–51. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Zeilhofer, S.; Blüthgen, N.; Schmitt, T. Stingless Bees Use Terpenes as Olfactory Cues to Find Resin Sources. Chem. Senses 2010, 35, 603–611. [Google Scholar] [CrossRef]
- Lourencetti, A.P.S.; Azevedo, P.; Miotelo, L.; Malaspina, O.; Nocelli, R.C.F. Surrogate Species in Pesticide Risk Assessments: Toxicological Data of Three Stingless bees species. Environ. Pollut. 2023, 318, 120842. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Vandame, R.; Castro-Chan, R.A.; Penilla-Navarro, R.P.; Gómez, J.; Sánchez, D. Organochlorine Pesticides in Honey and Pollen Samples from Managed Colonies of the Honey Bee Apis Mellifera Linnaeus and the Stingless Bee Scaptotrigona mexicana Guérin from Southern, Mexico. Insects 2018, 9, 54. [Google Scholar] [CrossRef]
- Biscassi, G.F.; Rabêlo, W.F.; Sardeli, R.; Rodrigues Garcia, G.R.; Brigante, J.; Daam, M.A.; José dos Santos Neto, Á.; Moscardi dos Santos, D.; Vieira, E.M. Residual Determination and Acute Toxicity of the Neonicotinoid Clothianidin in the Neotropical Stingless Bee Tetragonisca angustula Latreille, 1811 (Apidae: Meliponini). Chemosphere 2024, 349, 140878. [Google Scholar] [CrossRef]
- Carolina de Gouveia, M.D.E.; Oliveira, F.A.D.S.; Oloris, S.C.S.; da Silva, J.B.A.; Soto-Blanco, B. Pesticide Residues in Honey from Stingless Bee Melipona subnitida (Meliponini, Apidae). J. Apic. Sci. 2020, 64, 29–36. [Google Scholar] [CrossRef]
- Rondeau, S. Digging below the Surface: Hidden Risks for Ground-Nesting Bees. Science 2024, 386, 739. [Google Scholar] [CrossRef]
- Seide, V.E.; Bernardes, R.C.; Pereira, E.J.G.; Lima, M.A.P. Glyphosate Is Lethal and Cry Toxins Alter the Development of the Stingless Bee Melipona quadrifasciata. Environ. Pollut. 2018, 243, 1854–1860. [Google Scholar] [CrossRef]
- Ocaña-Cabrera, J.S.; Martin-Solano, S.; Saegerman, C. Development of Tools to Understand the Relationship between Good Management Practices and Nest Losses in Meliponiculture: A Pilot Study in Latin American Countries. Insects 2024, 15, 715. [Google Scholar] [CrossRef] [PubMed]
- Willis Chan, D.S.; Rondeau, S. Understanding and Comparing Relative Pesticide Risk among North American Wild Bees from Their Association with Agriculture. Sci. Total Environ. 2024, 951, 175378. [Google Scholar] [CrossRef] [PubMed]
| Ecuadorian Region | Province | Localities | N°. Pool | N°. Samples Within the Pool | Stingless Bee Genera * |
|---|---|---|---|---|---|
| Southern Highlands | Loja (mostly crop environment) | Naranjo, Huertas, Faique, Algarrobillo | 1 | 10 | Scaptotrigona (58%) Melipona (42%) |
| Caminuma, Panecillo | 2 | 5 | Scaptotrigona (81%) Melipona (19%) | ||
| Arenal | 3 | 7 | Scaptotrigona (74%) Nanotrigona (14%) Paratrigona (12%) | ||
| 4 | 3 | Scaptotrigona (65%) Melipona (35%) | |||
| Northern Highlands | Imbabura (crop area and secondary forest) | Intag | 5 | 2 | Partamona (50%) Trigona (50%) |
| 6 | 3 | Partamona (100%) | |||
| Low Amazon | Napo (urban environment-Archidona) (primary and secondary forest-Agua Santa) | Archidona | 7 | 2 | Melipona (50%) Nanotrigona (50%) |
| 8 | 1 | Melipona (100%) | |||
| Agua Santa | 9 | 4 | Tetragonisca (83%) Nanotrigona (17%) | ||
| 10 | 1 | Scaptotrigona (100%) | |||
| 11 | 1 | Melipona (100%) | |||
| 12 | 1 | Trigona (100%) | |||
| 13 | 1 | Scaptotrigona (100%) | |||
| 14 | 1 | Melipona (100%) |
| Name | Type | Group | * CAS Number | Main Crop Use in Ecuador |
|---|---|---|---|---|
| Acetamiprid | Insecticide | Neonicotinoid | 135410-20-7 | Vegetable, fruit, and ornamental crops |
| Carbendazim (benomyl) | Fungicide | Benzimidazole | 10605-21-7 | Roses, rice, bananas, coffee crops |
| Chlorantraniliprole | Insecticide | Ryanoids | 500008-45-7 | Corn crops |
| Clothianidin | Insecticide | Neonicotinoid | 210880-92-5 | Tomato, broccoli, roses |
| Cypermethrin | Insecticide | Pyrethroids | 52645-53-1 | Corn and broccoli crops |
| Deltamethrin | Herbicide, Acaricide | Pyrethroids | 52918-63-5 | Potatoes, grapes, and rose cultivation |
| Diafenthiuron | Herbicide | Sulfonylurea compound | 80060-09-9 | Tomatoes, beans, roses |
| Diazinon | Insecticide | Organophosphorus | 333-41-5 | Roses, rice, fruits |
| Flonicamid | Insecticide | Pyridine carboxamides | 158062-67-0 | Roses, tomatoes |
| Glyphosate + AMPA (its metabolite) | Herbicide and crop desiccant | Organophosphorus | 1071-83-6, 1066-51-9 | Weeds, perennial shrubs |
| Imidacloprid | Insecticide | Neonicotinoid | 138261-41-3 | Potatoes, corn, fruits, and vegetable crops |
| Malathion (malaoxon) | Insecticide | Organophosphorus | 121-75-5 | Rice and corn crops |
| Methomyl | Insecticide | Carbamates | 16752-77-5 | Rice and corn crops |
| Pyrethrin I | Insecticide | Pyrethrins | 8003-34-7 | Avocado, blueberry, potatoes |
| Thiacloprid | Insecticide | Neonicotinoid | 111988-49-9 | Roses crops |
| Thiamethoxam | Insecticide | Neonicotinoid | 153719-23-4 | Potatoes, African palm, and cocoa crops |
| Trichlorfon | Insecticide, Anthelmintic | Organophosphorus | 52-68-6 | Human and animal drugs |
| Stingless Bee Species | Glyphosate (GLY) Concentration (LD50) | Time of Exposure Until 100% of Death | Type of Exposure | Stingless Bee Stage | Reference |
|---|---|---|---|---|---|
| Tetragonisca angustula | 0.015 µg a.i./bee (95% CI: 0.005–0.04) (GLY mixed with honey/water) | 48 h | Orally | Adult bees (foragers) | [61] |
| Highlands | Amazon | Basic Sample Statistics | Permitted Levels | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pool | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Av ± SD | Min | Max | No | % | Brazil. Legis. for SB | EU. Guid. for HB | WHO for Humans | |
| ChC | |||||||||||||||||||||||
| Sb | 0.04 | 0.063 | 0.063 | 0.038 | 0.028 | 0.046 ± 0.016 | 0.028 | 0.063 | 5 | 36 | |||||||||||||
| As | 0.083 | 0.14 | 0.14 | 0.087 | 0.25 | 0.27 | 0.064 | 0.028 | 0.133 ± 0.087 | 0.028 | 0.27 | 8 | 57 | 0.30 | 1 | ||||||||
| Cd | 0.025 | 0.64 | 4.4 | 1.4 | 0.1 | 0.048 | 0.018 | 0.009 | 0.1 | 0.068 | 0.093 | 0.034 | 0.056 | 0.051 | 0.503 ± 1.184 | 0.009 | 4.4 | 14 | 100 | 0.10 | 0.008 | ||
| Cr | 0.34 | 1.7 | 0.74 | 0.32 | 7.1 | 6 | 0.11 | 0.25 | 3.3 | 0.29 | 0.66 | 0.22 | 0.15 | 0.21 | 1.528 ± 2.304 | 0.11 | 7.1 | 14 | 100 | 0.10 | 0.00013–0.0003. | ||
| Sn | 0.17 | 0.170 | 0.17 | 0.17 | 1 | 7 | |||||||||||||||||
| Ni | 0.13 | 0.52 | 0.63 | 0.3 | 1.8 | 1.4 | 0.093 | 0.08 | 0.95 | 0.13 | 0.37 | 0.71 | 0.48 | 0.19 | 0.556 ± 0.518 | 0.08 | 1.8 | 14 | 100 | 5 | 0.5 | ||
| Pb | 0.16 | 0.26 | 0.32 | 0.13 | 1.5 | 0.35 | 0.044 | 0.031 | 0.23 | 0.036 | 0.082 | 0.034 | 0.035 | 0.247 ± 0.393 | 0.031 | 1.5 | 13 | 93 | 0.30 | 0.10 | 0.05 | ||
| Se | 0.16 | 0.160 | 0.16 | 0.16 | 1 | 7 | |||||||||||||||||
| GLY | 0.02 | 0.014 | 0.15 | 0.2 | 0.096 ± 0.094 | 0.014 | 0.2 | 4 | 29 | 0.05 | 0.05 | 0.5 | |||||||||||
| AMPA | 0.028 | 0.028 | 0.028 | 0.028 | 1 | 7 | 0.2 | ||||||||||||||||
| Low Amazon— Northern Highlands | Low Amazon— Southern Highlands | Northern—Southern Highlands | |
|---|---|---|---|
| Glyphosate | 0.1797 | ||
| Antimony | 1 | 0.2754 | 0.7831 |
| Arsenic | 0.0411 a | 0.4544 | 0.4644 |
| Cadmium | 1 | 0.2237 | 1 |
| Chromium | 0.0378 b | 0.4297 | 0.6425 |
| Nickel | 0.0805 | 1 | 0.2344 |
| Lead | 0.0255 c | 0.1603 | 0.8981 |
| Variable | Estimated Coefficient | Standard Error | Z | p-Value | 95% Confidence | |
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| Glyphosate | −5.59 | 3.82 | −1.462 | 0.169 | −13.07 | 1.90 |
| Antimony | −25.79 | 7.97 | −3.36 | 0.005 a | −40.82 | −10.76 |
| Arsenic | −5.83 | 2.27 | −2.57 | 0.024 b | −10.28 | −1.39 |
| Cadmium | −0.44 | 0.18 | −2.42 | 0.033 c | −0.79 | −0.08 |
| Chromium | −0.06 | 0.11 | −0.52 | 0.616 | −0.28 | 0.16 |
| Nickel | −0.23 | 0.50 | −0.46 | 0.658 | −1.22 | 0.76 |
| Lead | −0.68 | 0.66 | −1.03 | 0.325 | −1.97 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocaña-Cabrera, J.S.; Ron-Román, J.; Martin-Solano, S.; Saegerman, C. Chemical Contaminants in Cerumen Samples from Ecuadorian Stingless Bees: Reporting Glyphosate, Aminomethylphosphonic Acid, and the Presence of Metals and Metalloids. Insects 2025, 16, 1079. https://doi.org/10.3390/insects16111079
Ocaña-Cabrera JS, Ron-Román J, Martin-Solano S, Saegerman C. Chemical Contaminants in Cerumen Samples from Ecuadorian Stingless Bees: Reporting Glyphosate, Aminomethylphosphonic Acid, and the Presence of Metals and Metalloids. Insects. 2025; 16(11):1079. https://doi.org/10.3390/insects16111079
Chicago/Turabian StyleOcaña-Cabrera, Joseline Sofía, Jorge Ron-Román, Sarah Martin-Solano, and Claude Saegerman. 2025. "Chemical Contaminants in Cerumen Samples from Ecuadorian Stingless Bees: Reporting Glyphosate, Aminomethylphosphonic Acid, and the Presence of Metals and Metalloids" Insects 16, no. 11: 1079. https://doi.org/10.3390/insects16111079
APA StyleOcaña-Cabrera, J. S., Ron-Román, J., Martin-Solano, S., & Saegerman, C. (2025). Chemical Contaminants in Cerumen Samples from Ecuadorian Stingless Bees: Reporting Glyphosate, Aminomethylphosphonic Acid, and the Presence of Metals and Metalloids. Insects, 16(11), 1079. https://doi.org/10.3390/insects16111079







