Diet-Driven Variations in Longevity and Fecundity of the Endangered Tiger Beetle Cicindela anchoralis (Coleoptera: Carabidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insect-Rearing Conditions
2.2. Prey Insects
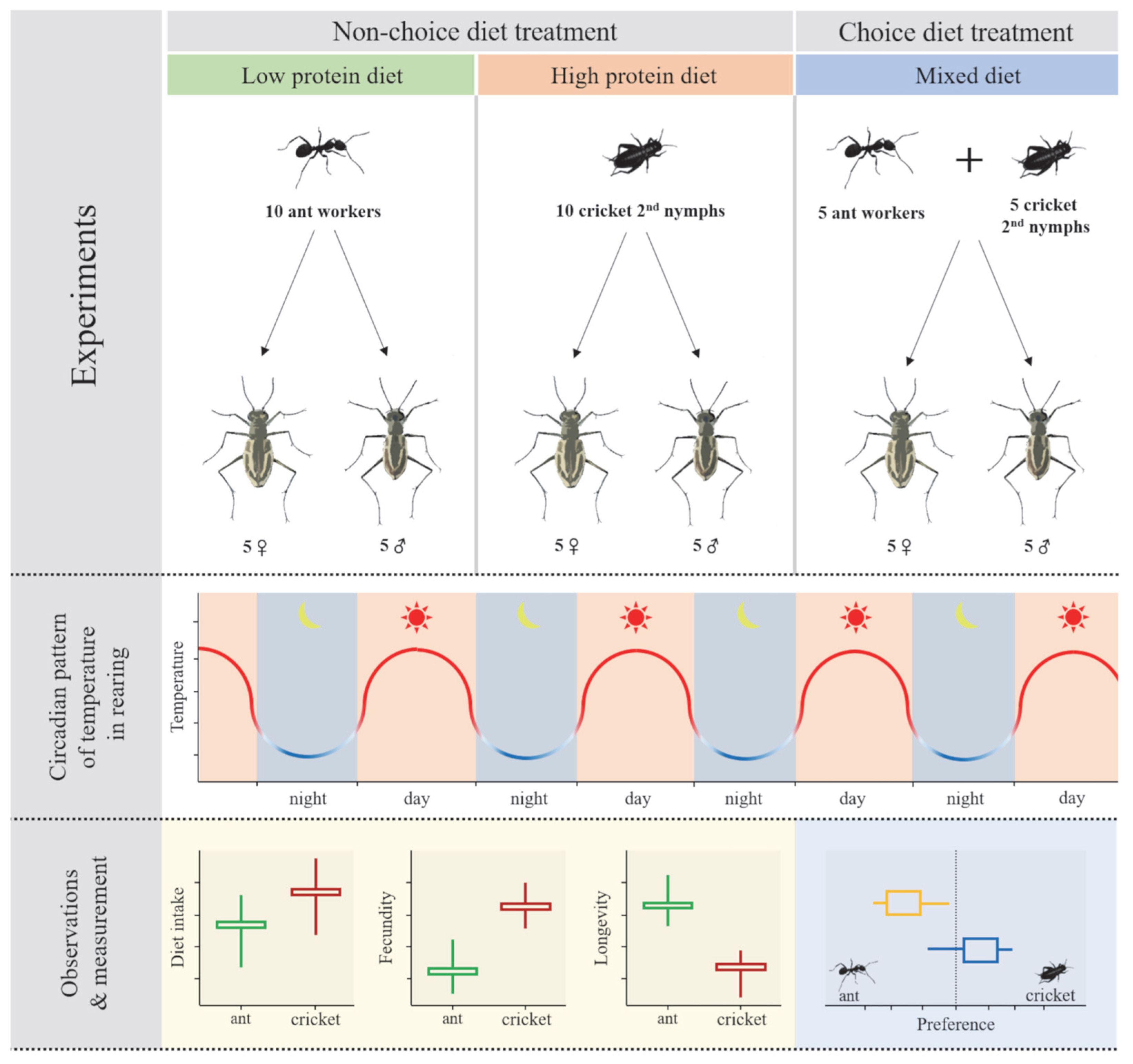
2.3. Experiments
2.3.1. Experiment 1: Daily Dietary Consumption by Sex Under Non-Choice Diet Treatment
2.3.2. Experiment 2: Daily Dietary Consumption by Sex Under Choice Diet Treatment
2.4. Measurement of Fecundity and Longevity
2.5. Data Analyses
3. Results
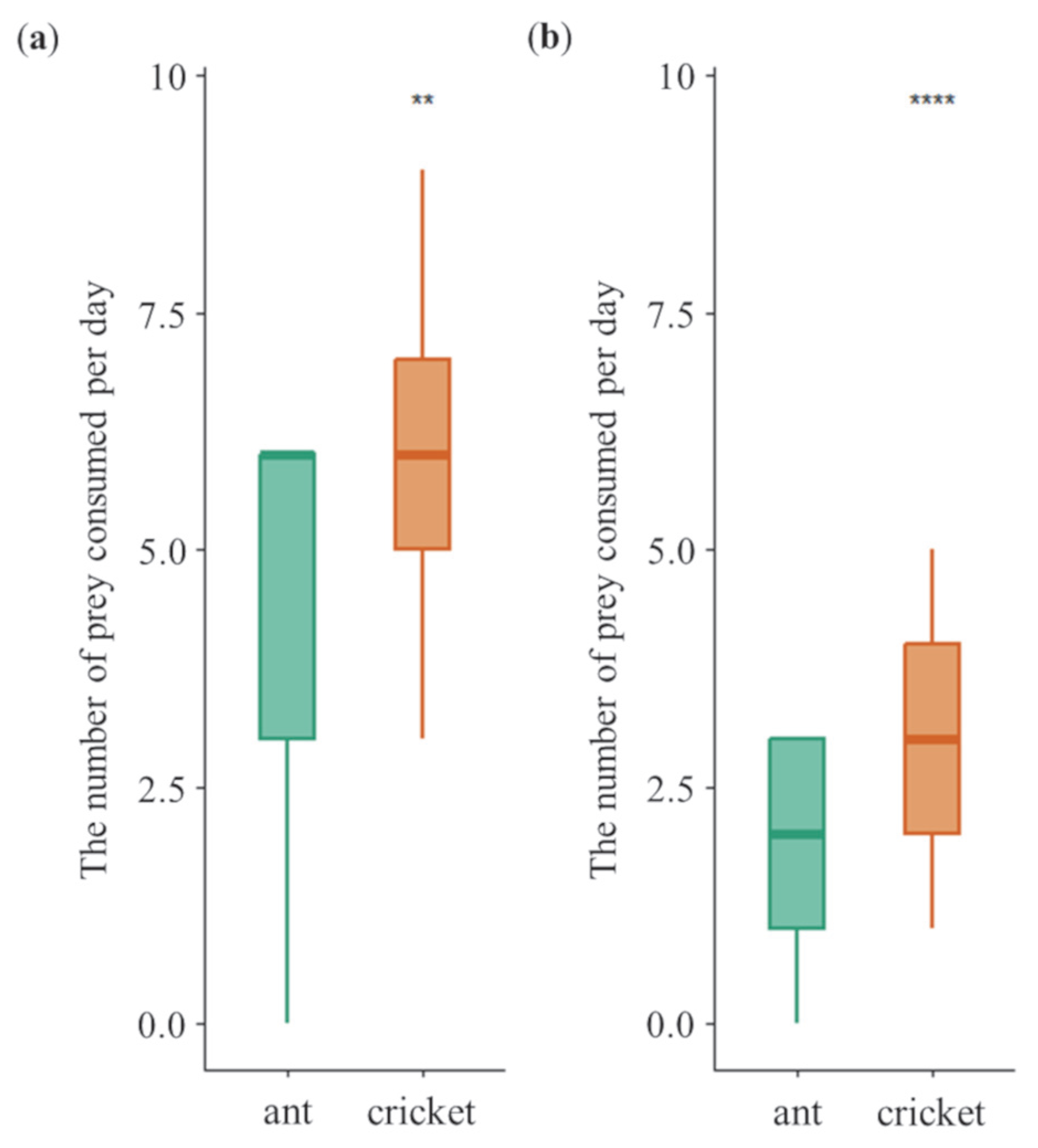
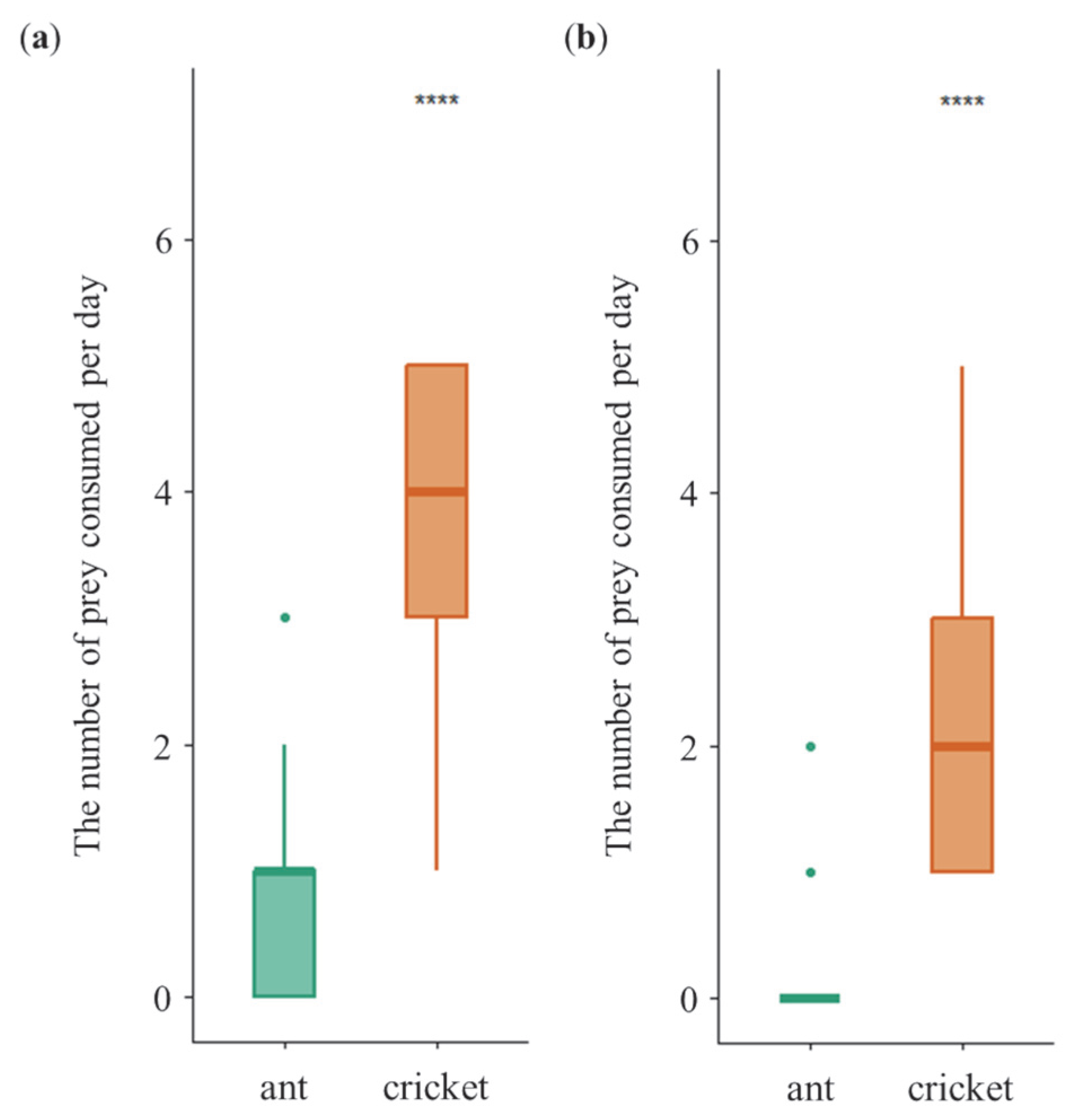
3.1. Daily Dietary Consumption Under Non-Choice Diet Treatment
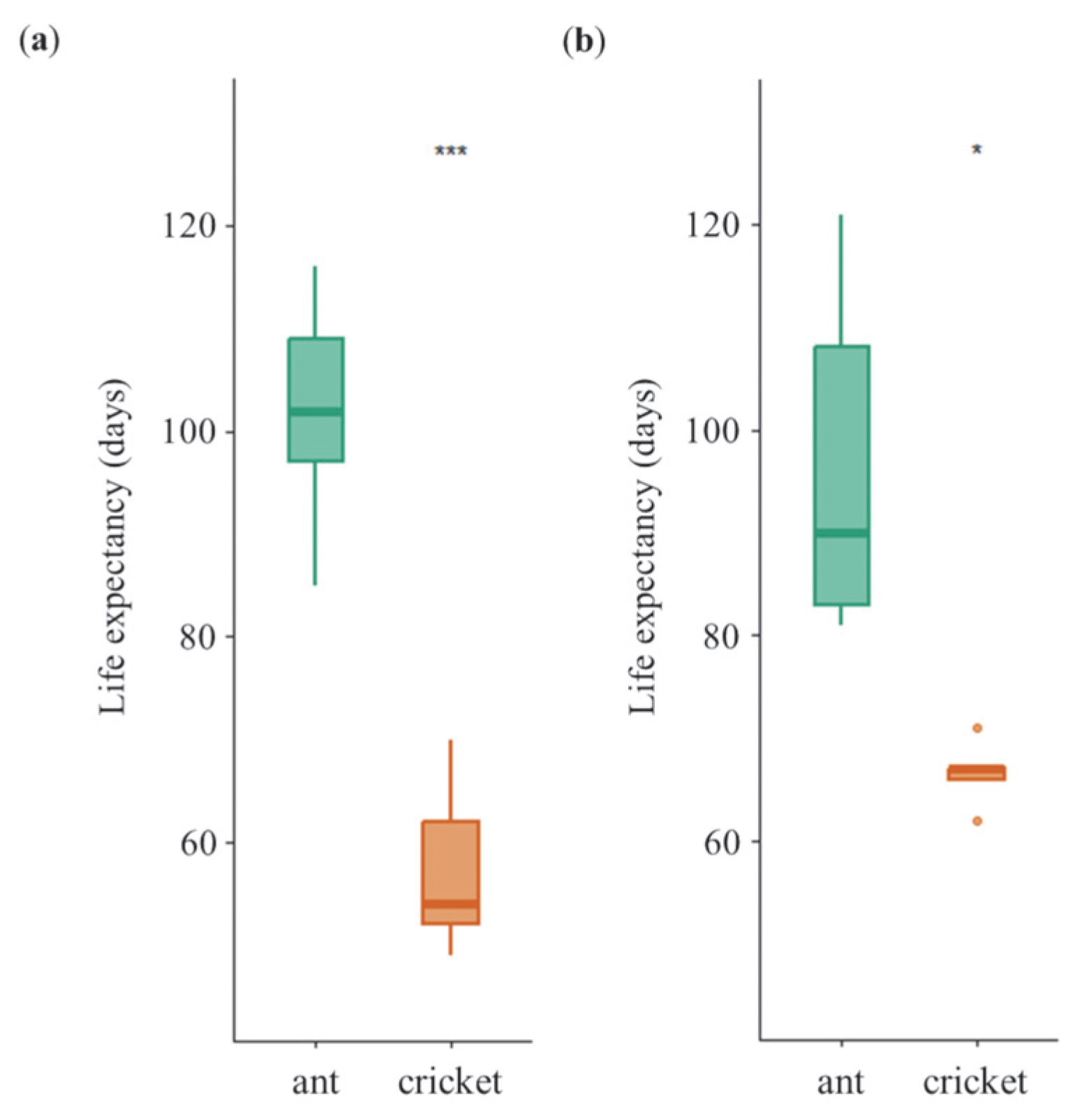
3.2. Longevity of Female and Male
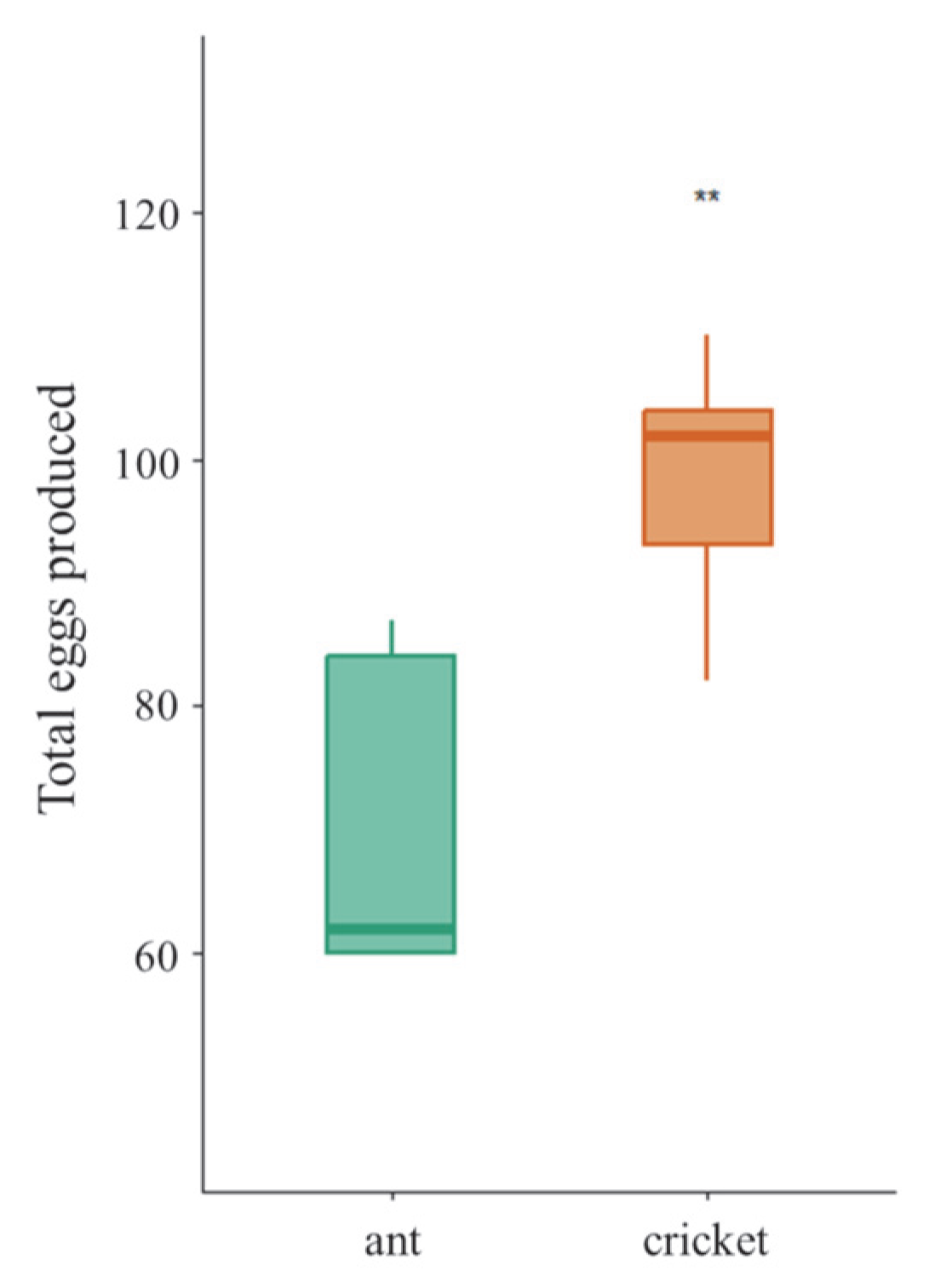
3.3. Fecundity of Female Tiger Beetle
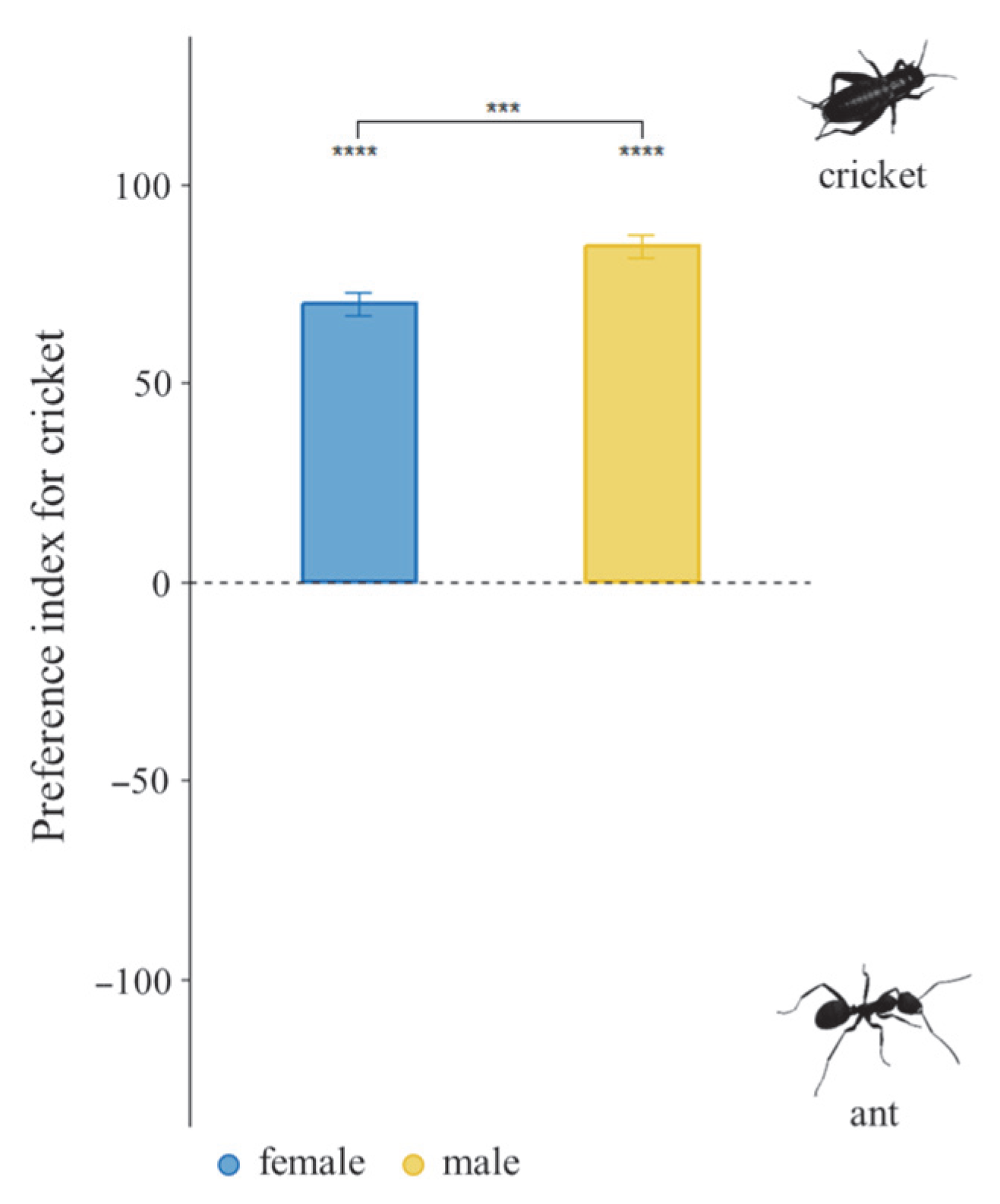
3.4. Dietary Preference Under Choice Diet Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weindruch, R.; Walford, R. Dietary restriction in mice beginning at 1 year of age: Effect on life-span and spontaneous cancer incidence. Science 1982, 215, 1415–1418. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span-from yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.; Cogger, V.C.; Mitchell, S.J.; Senior, A.; de Cabo, R.; Raubenheimer, D.; Simpson, S.J. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell. Mol. Life Sci. 2016, 73, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Roeder, K.A.; Behmer, S.T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 2014, 28, 1135–1143. [Google Scholar] [CrossRef]
- Arganda, S.; Bouchebti, S.; Bazazi, S.; Le-Hesran, S.; Puga, C.; Latil, G.; Simpson, S.J.; Dussutour, A. Parsing the life-shortening effects of dietary protein: Effects of individual amino acids. Proc. R. Soc. B 2017, 284, 20162052. [Google Scholar] [CrossRef] [PubMed]
- Sultanova, Z.; Ivimey-Cook, E.R.; Chapman, T.; Maklakov, A.A. Fitness benefits of dietary restriction. Proc. R. Soc. B 2021, 288, 20211787. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Walters, K.A.; Simanainen, U.K.; McMahon, A.C.; Ruohonen, K.; Ballard, J.W.O.; Raubenheimer, D.; Handelsman, D.J.; Le Couteur, D.G.; Simpson, S.J. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc. Natl. Acad. Sci. USA 2015, 112, 3481–3486. [Google Scholar] [CrossRef]
- May, C.M.; Doroszuk, A.; Zwaan, B.J. The effect of developmental nutrition on life span and fecundity depends on the adult reproductive environment in Drosophila melanogaster. Ecol. Evol. 2015, 5, 1156–1168. [Google Scholar] [CrossRef]
- Zajitschek, F.; Zajitschek, S.R.K.; Canton, C.; Georgolopoulos, G.; Friberg, U.; Maklakov, A.A. Evolution under dietary restriction increases male reproductive performance without survival cost. Proc. R. Soc. B 2016, 283, 20152726. [Google Scholar] [CrossRef]
- Rho, M.S.; Lee, K.P. Balanced intake of protein and carbohydrate maximizes lifetime reproductive success in the mealworm beetle, Tenebrio molitor (Coleoptera: Tenebrionidae). J. Insect Physiol. 2016, 91–92, 93–99. [Google Scholar] [CrossRef]
- Collins, D.H.; Prince, D.C.; Donelan, J.L.; Chapman, T.; Bourke, A.F.G. Developmental diet alters the fecundity–longevity relationship and age-related gene expression in Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2240–2250. [Google Scholar] [CrossRef]
- Rau, V.; Flatt, T.; Korb, J. The remoulding of dietary effects on the fecundity/longevity trade-off in a social insect. BMC Genom. 2023, 24, 244. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.J.K.; Traylor-Holzer, K.; Leus, K. IUCN guidelines for determining how ex situ management should be used in species conservation. Conserv. Lett. 2017, 10, 361–366. [Google Scholar] [CrossRef]
- Atkinson, J.; Bonser, S.P. “Active” and “passive” ecological restoration strategies in meta-analysis. Restor. Ecol. 2020, 28, 1032–1035. [Google Scholar] [CrossRef]
- Fardisi, M.; Mason, L.J.; Ileleji, K.E. Development and fecundity rate of Tribolium castaneum (Herbst) on distillers dried grains with solubles. J. Stored Prod. Res. 2013, 52, 74–77. [Google Scholar] [CrossRef]
- Pan, Q.; Ang, Y.; Shikano, I. Effects of adult diet on the longevity, fecundity and ovarian development of the rice leaffolder, Cnaphalocrocis medinalis. Physiol. Entomol. 2024, 49, 422–429. [Google Scholar] [CrossRef]
- Millar, J.G.; Paine, T.D.; Joyce, A.L.; Hanks, L.M. The effects of Eucalyptus pollen on longevity and fecundity of Eucalyptus longhorned borers (Coleoptera: Cerambycidae). J. Econ. Entomol. 2003, 96, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.H.; Wei, D.; Wei, D.D.; Yuan, G.R.; Wang, J.J. The effect of dietary restriction on longevity, fecundity, and antioxidant responses in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). J. Insect Physiol. 2013, 59, 1008–1016. [Google Scholar] [CrossRef]
- Su, S.; Zhang, X.; Jian, C.; Huang, B.; Peng, X.; Vreysen, M.J.B.; Chen, M. Effects of adult feeding treatments on longevity, fecundity, flight ability, and energy metabolism enzymes of Grapholita molesta Moths. Insects 2022, 13, 725. [Google Scholar] [CrossRef]
- Adler, M.I.; Bonduriansky, R. Why do the well-fed appear to die young? A new evolutionary hypothesis for the effect of dietary restriction on lifespan. Bioessays 2014, 36, 439–450. [Google Scholar] [CrossRef]
- Zajitschek, F.; Zajitschek, S.R.K.; Vasconcelos, A.C.O.; Bonduriansky, R. Dietary restriction fails to extend life in stressful environments. Funct. Ecol. 2023, 37, 2459–2470. [Google Scholar] [CrossRef]
- Chevrolat, L.A.A. Description of ten beetles from China, from the surroundings of Macao, and from an acquisition made at M. PARSUDAKI, naturalist dealer in Paris. Zool. Rev. Cuvierian Soc. 1845, 8, 95–99. [Google Scholar]
- Suh, M.H. Red Data Book of Republic of Korea Volume 8, Insecta II; National Institute of Biological Resources: Incheon, Republic of Korea, 2023; pp. 1–134. [Google Scholar]
- Kim, C.W. Distribution Atlas of Insects of Korea, Series 2, Coleoptera; Korea University Press: Seoul, Republic of Korea, 1978. [Google Scholar]
- Cha, D.; Jung, J.K. Captive propagation and observations of the endangered species Cicindela (Abroscelis) anchoralis (Coleoptera: Carabidae: Cicindelinae) in South Korea. Insect. Conserv. Divers. 2024, 18, 731–742. [Google Scholar] [CrossRef]
- Lee, K.P. Dietary protein:carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: A test using a chemically defined diet. J. Insect Physiol. 2015, 75, 12–19. [Google Scholar] [CrossRef]
- Schultz, T.D. Tiger beetles scavenging on dead vertebrates. Cicindela 1981, 13, 48. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Gutiérrez, Y.; Fresch, M.; Ott, D.; Brockmeyer, J.; Scherber, C. Diet composition and social environment determine food consumption, phenotype and fecundity in an omnivorous insect. R. Soc. Open Sci. 2020, 7, 200100. [Google Scholar] [CrossRef] [PubMed]
- Moeser, J.; Vidal, S. Nutritional resources used by the invasive maize pest Diabrotica virgifera virgifera in its new South-east-European distribution range. Entomol. Exp. Appl. 2005, 114, 55–63. [Google Scholar] [CrossRef]
- Mevi-Schütz, J.; Goverde, M.; Erhardt, A. Effects of fertilization and elevated CO2 on larval food and butterfly nectar amino acid preference in Coenonympha pamphilus L. Behav. Ecol. Sociobiol. 2003, 54, 36–43. [Google Scholar] [CrossRef]
- Yadav, P.; Patel, P.; Patel, A.K.; Chowdhury, R.; Upadhyay, A.; Kumar, B.; Kumar, D. Ageing and mating status affect food utilization efficiencies and assimilation of macronutrients in adults of Parthenium beetle, Zygogramma bicolorata Pallister. Physiol. Entomol. 2024, 49, 147–156. [Google Scholar] [CrossRef]
- Wheeler, D. The role of nourishment in oogenesis. Annu. Rev. Entomol. 1996, 41, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a Warming Climate: Insect Responses to Extreme High Temperatures. Annu. Rev. Entomol. 2021, 7, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Dreisig, H. Daily activity, thermoregulation and water loss in the tiger beetle Cicindela hybrid. Oecologia 1979, 44, 376–389. [Google Scholar] [CrossRef]
- Mautz, B.S.; Rode, N.O.; Bonduriansky, R.; Rundle, H.D. Comparing ageing and the effects of diet supplementation in wild vs. captive antler flies, Protopiophila litigata. J. Anim. Ecol. 2019, 88, 1913–1924. [Google Scholar] [CrossRef]
- Mair, W.; Sgrò, C.M.; Johnson, A.P.; Chapman, T.; Partridge, L. Lifespan extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exp. Gerontol. 2004, 39, 1011–1019. [Google Scholar] [CrossRef]
- Drewry, M.D.; Williams, J.M.; Hatle, J.D. Life-extending dietary restriction and ovariectomy result in similar feeding rates but different physiologic responses in grasshoppers. Exp. Gerontol. 2011, 46, 781–786. [Google Scholar] [CrossRef]
- Nakagawa, S.; Lagisz, M.; Hector, K.L.; Spencer, H.G. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell 2012, 11, 401–409. [Google Scholar] [CrossRef]
- Ellis, B.J.; Figueredo, A.J.; Brumbach, B.H.; Schlomer, G.L. Fundamental Dimensions of Environmental Risk. Hum. Nat. 2009, 20, 204–268. [Google Scholar] [CrossRef]
- Knisley, C.B.; Hill, J.M.; Scherer, A.M. Translocation of threatened tiger beetle Cicindela dorsalis dorsalis (Coleoptera: Cicindelidae) to Sandy Hook, New Jersey. Ann. Entomol. Soc. Am. 2005, 98, 552–557. [Google Scholar] [CrossRef]
- Bertinetti, C.; Samayoa, A.C.; Hwang, S.Y. Effects of feeding adults of Hermetia illucens (Diptera: Stratiomyidae) on longevity, oviposition, and egg hatchability: Insights into optimizing egg production. J. Insect. Sci. 2019, 19, 19. [Google Scholar] [CrossRef]
- Holtze, S.; Gorshkova, E.; Braude, S.; Cellerino, A.; Dammann, P.; Hildebrandt, T.B.; Hoeflich, A.; Hoffmann, S.; Koch, P.; Terzibasi Tozzini, E.; et al. Alternative Animal Models of Aging Research. Front. Mol. Biosci. 2021, 8, 660959. [Google Scholar] [CrossRef] [PubMed]
| Prey Insects | Body Size (mm) b | Body Weight (mg) c | Nutritional Contents a | P:C Ratio d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Energy Content (kcal/100 g) | Sodium (mg/100 g) | Carbohydrate (g/100 g) | Sugars (g/100 g) | Crude Fat (g/100 g) | Saturated Fat (g/100 g) | Crude Protein (g/100 g) | ||||
| Japanese wood ant | 5.5 ± 0.1 | 2.9 ± 0.4 | 136.66 | 98.40 | 6.22 | 1.12 | 3.34 | 0.39 | 20.43 | 3.28:1 |
| Two-spotted cricket | 6.3 ± 0.1 | 5.0 ± 0.4 | 123.18 | 102.88 | 3.03 | 0.13 | 4.18 | 0.90 | 18.36 | 6.06:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, D.; Lim, A.; Jung, J.-K. Diet-Driven Variations in Longevity and Fecundity of the Endangered Tiger Beetle Cicindela anchoralis (Coleoptera: Carabidae). Insects 2025, 16, 1066. https://doi.org/10.3390/insects16101066
Cha D, Lim A, Jung J-K. Diet-Driven Variations in Longevity and Fecundity of the Endangered Tiger Beetle Cicindela anchoralis (Coleoptera: Carabidae). Insects. 2025; 16(10):1066. https://doi.org/10.3390/insects16101066
Chicago/Turabian StyleCha, Deokjea, Anya Lim, and Jong-Kook Jung. 2025. "Diet-Driven Variations in Longevity and Fecundity of the Endangered Tiger Beetle Cicindela anchoralis (Coleoptera: Carabidae)" Insects 16, no. 10: 1066. https://doi.org/10.3390/insects16101066
APA StyleCha, D., Lim, A., & Jung, J.-K. (2025). Diet-Driven Variations in Longevity and Fecundity of the Endangered Tiger Beetle Cicindela anchoralis (Coleoptera: Carabidae). Insects, 16(10), 1066. https://doi.org/10.3390/insects16101066






