A DNA Barcode Inventory of Austrian Dragonfly and Damselfly (Insecta: Odonata) Species
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
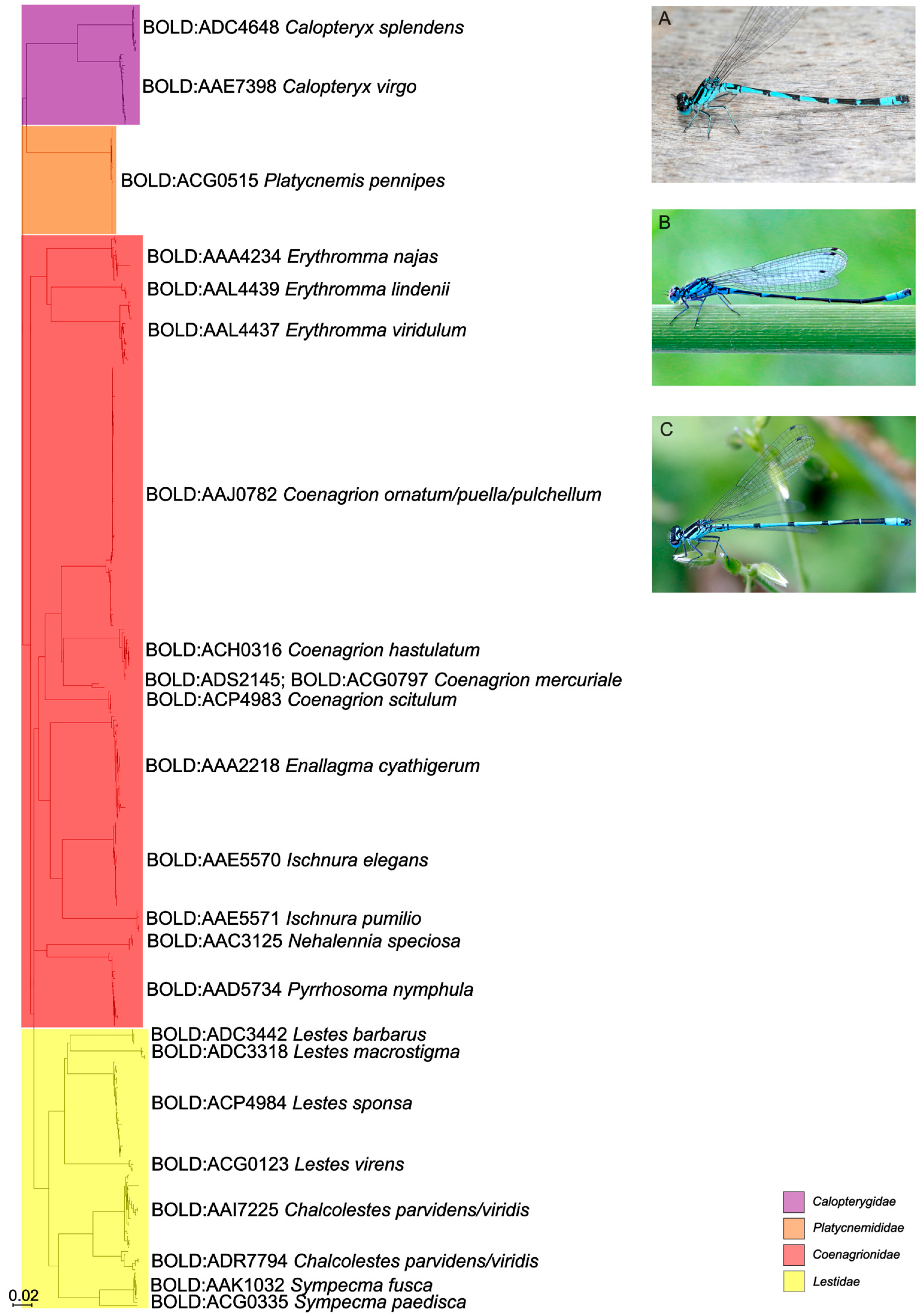
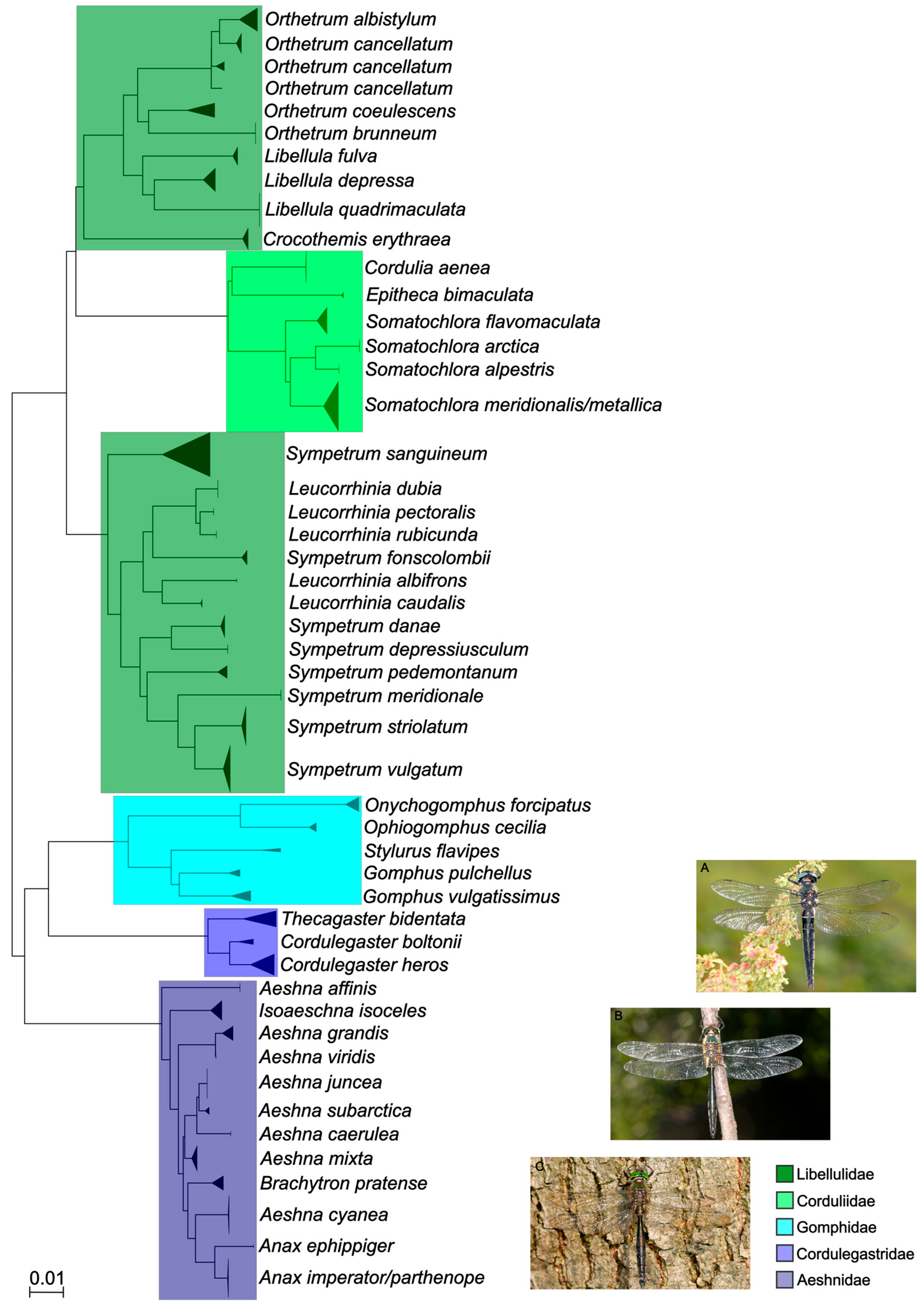
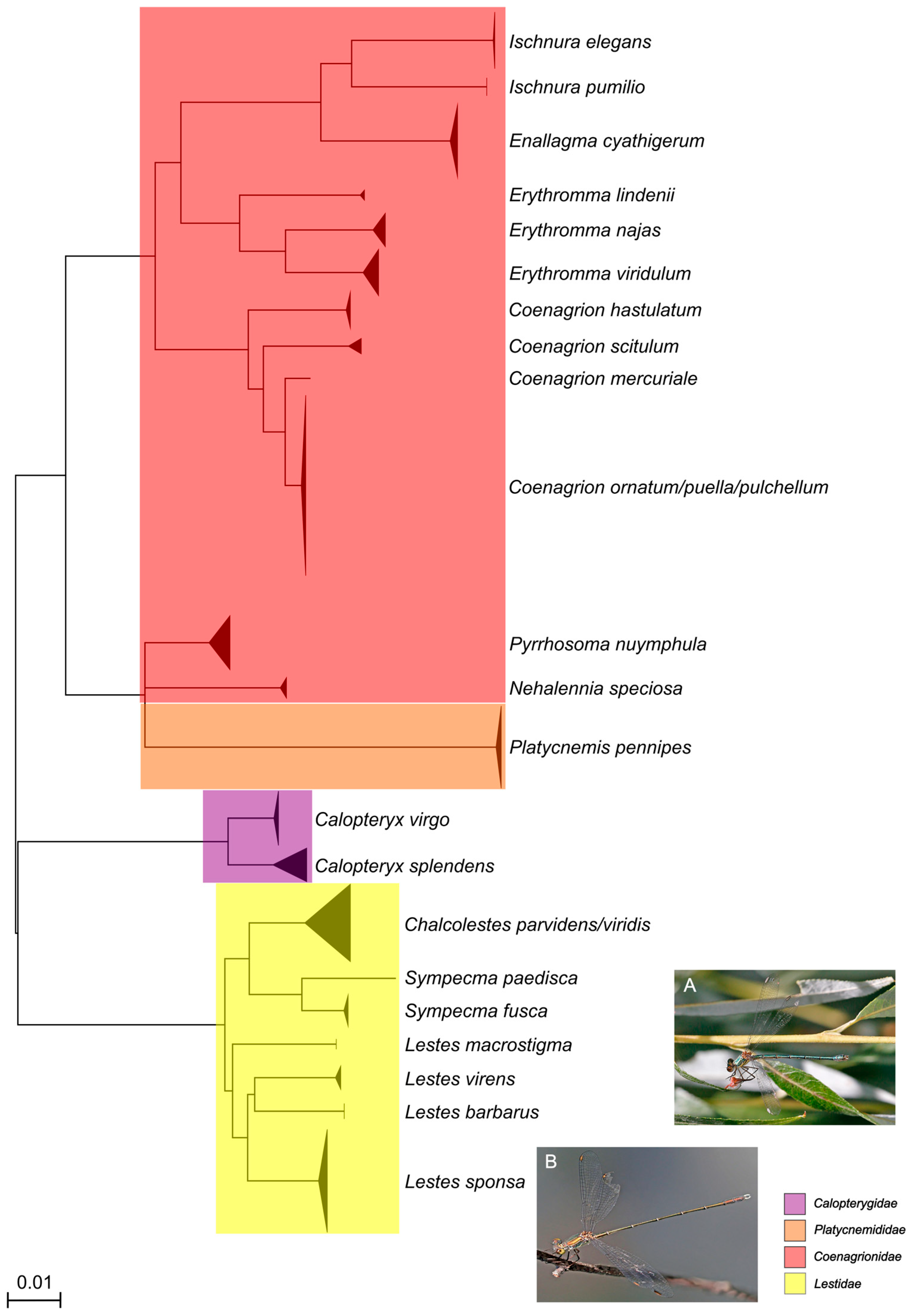
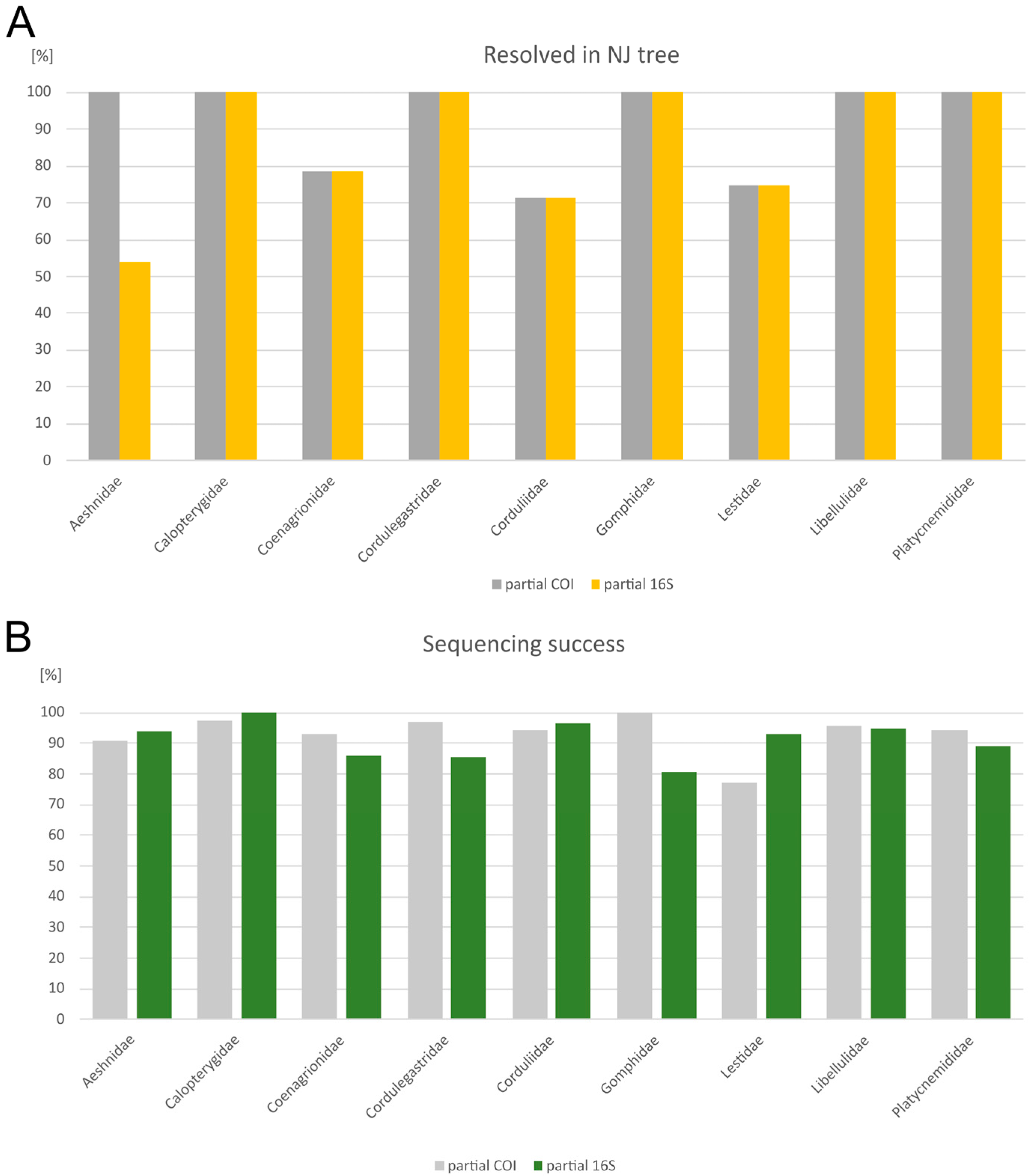
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahlén, G.; Kalkman, V.; Boudot, J.; Bernard, R.; Conze, K.; De Knijf, G.; Dyatlova, E.; Ferreira, S.; Jovic, M.; Ott, J.; et al. European Red List of Dragonflies; Publications Office of the European Union: Luxemburg, 2010. [Google Scholar] [CrossRef]
- Kalkman, V.; Boudot, J.; Bernard, R.; De Knijf, G.; Suhling, F.; Termaat, T. Diversity and conservation of European dragonflies and damselflies (Odonata). Hydrobiologia 2018, 811, 269–282. [Google Scholar] [CrossRef]
- Geiger, M.; Koblmüller, S.; Assandri, G.; Chovanec, A.; Ekrem, T.; Fischer, I.; Galimberti, A.; Grabowski, M.; Haring, E.; Hausmann, A.; et al. Coverage and quality of DNA barcode references for Central and Northern European Odonata. PeerJ 2021, 9, e11192. [Google Scholar] [CrossRef]
- De Marchi, G. Precopulatory reproductive isolation and wing colour dimorphism in Calopteryx splendens females in southern Italy (Zygoptera: Calopterygidae). Odonatologica 1990, 19, 243–250. [Google Scholar]
- Reinhardt, K.; Gerighausen, U. Oviposition site preference and egg parasitism in Sympecma paedisca (Odonata: Lestidae). Int. J. Odonatol. 2001, 4, 221–230. [Google Scholar] [CrossRef]
- Rivera, A.; Andrés, J.; Córdoba-Aguilar, A.; Utzeri, C. Postmating sexual selection: Allopatric evolution of sperm competition mechanisms and genital morphology in calopterygid damselflies (Insecta: Odonata). Evolution 2004, 58, 349–359. [Google Scholar] [CrossRef]
- Fincke, O.; Jödicke, R.; Paulson, D.; Schultz, T. The evolution and frequency of female color morphs in Holarctic Odonata: Why are male-like females typically the minority? Int. J. Odonatol. 2005, 8, 183–212. [Google Scholar] [CrossRef]
- Dijkstra, K.D.B.; Kalkman, V.J. Phylogeny, classification and taxonomy of European dragonflies and damselflies (Odonata): A review. Org. Divers. Evol. 2012, 12, 209–227. [Google Scholar] [CrossRef]
- Sparrow, D.J.; De Knijf, G.; Sparrow, R.L. Diversity, status and phenology of the dragonflies and damselflies of Cyprus (Insecta: Odonata). Diversity 2021, 13, 532. [Google Scholar] [CrossRef]
- European Parliament and Council. Directive 2000/60/EC of the European Parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Comm. 2000, L327, 1–73. [Google Scholar]
- Chovanec, A.; Schindler, M.; Waringer, J.; Wimmer, R. The dragonfly association index (Insecta: Odonata)—A tool for the type-specific assessment of lowland rivers. River Res. Appl. 2015, 31, 627–638. [Google Scholar] [CrossRef]
- Chovanec, A. Comparing and evaluating the dragonfly fauna (Odonata) of regulated and rehabilitated stretches of the fourth order metarhithron Gurtenbach (Upper Austria). Int. J. Odonatol. 2018, 21, 15–32. [Google Scholar] [CrossRef]
- Chovanec, A. The assessment of the dragonfly fauna (Insecta: Odonata) as a tool for the detailed typological characterisation of running waters. Acta ZooBot Austria 2022, 158, 129–147. [Google Scholar]
- Chovanec, A.; Waringer, J.; Holzinger, W.E.; Moog, O.; Janecek, B. Odonata. In Fauna Aquatica Austriaca, 3rd ed.; Moog, O., Hartmann, A., Eds.; BMLFUW: Vienna, Austria, 2017. [Google Scholar]
- Raab, R. Rote Liste der Libellen Österreichs. In Libellen Österreichs; Raab, R., Chovanec, A., Pennerstorfer, J., Eds.; Springer Nature: Berlin, Germany, 2006; pp. 325–344. [Google Scholar]
- De Knijf, G.; Billqvist, M.; van Grunsven, R.; Prunier, F.; Vinko, D.; Trottet, A.; Bellotto, V.; Clay, J.; Allen, D. European Red List of Dragonflies & Damselflies (Odonata): Measuring the Pulse of European Biodiversity; European Commission: Brussels, Belgium, 2024. [Google Scholar]
- Weekers, P.; De Jonckheere, J.; Dumont, H. Phylogenetic relationships inferred from ribosomal ITS sequences and biogeographic patterns in representatives of the genus Calopteryx (Insecta: Odonata) of the West Mediterraneans and adjacent West European zone. Mol. Phylogent. Evol. 2001, 20, 89–99. [Google Scholar] [CrossRef]
- Gyulavári, H.A.; Felföldi, T.; Benken, T.; Szabó, L.J.; Miskolczi, M.; Cserháti, C.; Horvai, V.; Márialigeti, K.; Dévai, G. Morphometric and molecular studies on the populations of the damselflies Chalcolestes viridis and C. parvidens (Odonata, Lestidae). Int. J. Odonatol. 2011, 14, 329–339. [Google Scholar] [CrossRef]
- Damm, S.; Schierwater, B.; Hadrys, H. An integrative approach to species discovery in odonates: From character-based DNA barcoding to ecology. Mol. Ecol. 2010, 19, 3881–3893. [Google Scholar] [CrossRef]
- Weigand, H.; Beermann, A.; Čiampor, F.; Costa, F.; Csabai, Z.; Duarte, S.; Geiger, M.F.; Grabowski, M.; Rimet, F.; Ruli, B.; et al. DNA barcode reference libraries for the monitoring of aquatic biota in Europe: Gap-analysis and recommendations for future work. Sci. Total Environ. 2019, 678, 499–524. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Pentinsaari, M.; Anderson, R.; Borowiec, L.; Bouchard, P.; Brunke, A.; Douglas, H.; Smith, A.B.T.; Hebert, P.D.N. DNA barcodes reveal 63 overlooked species of Canadian beetles (Insecta, Coleoptera). ZooKeys 2019, 894, 53–150. [Google Scholar] [CrossRef]
- Morinière, J.; Balke, M.; Doczkal, D.; Geiger, M.F.; Hardulak, L.A.; Haszprunar, G.; Hausmann, A.; Hendrich, L.; Regalado, L.; Rulik, B.; et al. A DNA barcode library for 5200 German flies and midges (Insecta: Diptera) and its implications for metabarcoding-based biomonitoring. Mol. Ecol. Resour. 2019, 19, 900–928. [Google Scholar] [CrossRef]
- Salvi, D.; Berrilli, E.; D’Alessandro, P.; Biondi, M. Sharpening the DNA barcoding tool through a posteriori taxonomic validation: The case of Longitarsus flea beetles (Coleoptera: Chrysomelidae). PLoS ONE 2020, 15, e0233573. [Google Scholar] [CrossRef]
- Zangl, L.; Glatzhofer, E.; Schmid, R.; Randolf, S.; Koblmüller, S. DNA barcoding of Austrian snow scorpionflies (Mecoptera, Boreidae) reveals potential cryptic diversity in Boreus westwoodi. PeerJ 2021, 9, e11424. [Google Scholar] [CrossRef]
- Schattanek-Wiesmair, B.; Huemer, P.; Wieser, C.; Stark, W.; Hausmann, A.; Koblmüller, S.; Sefc, K.M. A DNA barcode library of Austrian Geometridae (Lepidoptera) reveals high potential for DNA-based species identification. PLoS ONE 2024, 19, e0298025. [Google Scholar] [CrossRef]
- Galimberti, A.; Assandri, G.; Maggioni, D.; Ramazzotti, F.; Baroni, D.; Bazzi, G.; Chiandetti, I.; Corso, A.; Ferri, V.; Galuppi, M.; et al. Italian odonates in the Pandora’s box: A comprehensive DNA barcoding inventory shows taxonomic warnings at the Holarctic scale. Mol. Ecol. Resour. 2021, 21, 183–200. [Google Scholar] [CrossRef]
- Rewicz, T.; Móra, A.; Tończyk, G.; Szymczak, A.; Grabowski, M.; Calleja, E.J.; Pernecker, B.; Csabai, Z. First records raise questions: DNA barcoding of Odonata in the middle of the Mediterranean. Genome 2021, 64, 196–206. [Google Scholar] [CrossRef]
- Bergsten, J.; Bilton, D.T.; Fujisawa, T.; Elliott, M.; Monaghan, M.T.; Balke, M.; Hendrich, L.; Geijer, J.; Herrmann, J.; Foster, G.N.; et al. The effect of geographical scale of sampling on DNA barcoding. Syst. Biol. 2012, 61, 851–869. [Google Scholar] [CrossRef]
- Sittenthaler, M.; Fischer, I.; Chovanec, A.; Koblmüller, S.; Macek, O.; Sattmann, H.; Szucsich, N.; Zangl, L.; Haring, E. DNA barcoding of exuviae for species identification of Central European damselflies and dragonflies (Insecta: Odonata). J. Insect Conserv. 2023, 27, 435–450, Correction in J. Insect Conserv. 2023, 27, 451–453. [Google Scholar] [CrossRef]
- Yu, X. A description of the larva of Mesopodagrion tibetanum australe (Odonata: “Megapodagrionidae”). Int. J. Odonatol. 2016, 19, 275–282. [Google Scholar] [CrossRef]
- Schmidt, K.; Soluk, D.; Maestas, S.; Britten, H. Persistence and accumulation of environmental DNA from an endangered dragonfly. Sci. Rep. 2021, 11, 18987. [Google Scholar] [CrossRef] [PubMed]
- Hupało, K.; Schmidt, S.; Macher, T.; Weiss, M.; Leese, F. Fresh insights into Mediterranean biodiversity: Environmental DNA reveals spatio-temporal patterns of stream invertebrate communities on Sicily. Hydrobiologia 2022, 849, 155–173. [Google Scholar] [CrossRef]
- Deagle, B.E.; Jarman, S.N.; Coissac, E.; Pompanon, F.; Taberlet, P. DNA metabarcoding and the cytochrome c oxidase subunit I marker: Not a perfect match. Biol. Lett. 2014, 10, 20140562. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Boyer, F.; Valentini, A.; Bonin, A.; Meyer, A.; Dejean, T.; Gaboriaud, C.; Usseglio-Polatera, P.; Taberlet, P. Comparison of markers for the monitoring of freshwater benthic biodiversity through DNA metabarcoding. Mol. Ecol. 2021, 30, 3189–3202. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, C.S.; Guldberg Frøslev, T.; Bladt, J.; Bruhn Pedersen, L.; Colling Larsen, J.; Ejrnæs, R.; Fløgaard, C.; Hansen, A.J.; Heilmann-Clausen, J.; Dunn, R.R.; et al. Detecting flying insects using car nets and DNA metabarcoding. Biol. Lett. 2021, 17, 20200833. [Google Scholar] [CrossRef]
- Remmel, N.; Buchner, D.; Ess, J.; Hartung, V.; Leese, F.; Welti, E.A.R.; Sinclair, J.S.; Haase, P. DNA metabarcoding and morphological identification reveal similar richness, taxonomic composition and body size patterns among flying insect communities. Insect Conserv. Divers. 2024, 17, 449–463. [Google Scholar] [CrossRef]
- Buchner, D.; Sinclair, J.S.; Ayasse, M.; Beermann, A.J.; Buse, J.; Dziock, F.; Enss, J.; Frenzel, M.; Hörren, T.; Li, Y.; et al. Upscaling biodiversity monitoring: Metabarcoding estimtes 31,846 insect species from Malaise traps across Germany. Mol. Ecol. Resour. 2025, 25, e14023. [Google Scholar] [CrossRef]
- Allen, M.C.; Lockwood, J.L.; Kwait, R.; Vastano, A.R.; Peterson, D.; Tkacenko, L.A.; Kisurin, A.; Stringham, O.; Angle, J.; Jaffe, B. Using surface environmental DNA to assess arthropod biodiversity within a forested ecosystem. Environ. DNA 2023, 5, 1652–1666. [Google Scholar] [CrossRef]
- Jeunen, G.-F.; Lipinskaya, T.; Gadjuchenko, H.; Golovenchik, V.; Moroz, M.; Rizevsky, V.; Semenchenko, V.; Gemmell, N.J. Environmental DNA (eDNA) metabarcoding surveys show evidence of non-indigenous freshwater species invasion to new parts of Eastern Europe. Metabarcod. Metagenom. 2022, 6, 171–186. [Google Scholar] [CrossRef]
- Berry, T.E.; Coghlan, M.L.; Saunders, B.J.; Richardson, A.J.; Power, M.; Harvey, E.; Jarman, S.; Berry, O.; Davies, C.H.; Bunce, M. A 3-year plankton DNA metabarcoding survey reveals marine biodiversity patterns in Australian coastal waters. Divers. Distrib. 2023, 29, 862–878. [Google Scholar] [CrossRef]
- Korbel, K.L.; Hose, G.C.; Karwautz, C.; Greenfield, P.; Wang, H.; Chariton, A.A.; Griebler, C. Detection, movement and persistence of invertebrate eDNA in groundwater. Sci. Rep. 2024, 14, 17151. [Google Scholar] [CrossRef]
- Takenaka, M.; Hasebe, Y.; Yano, K.; Okamoto, S.; Tojo, K.; Seki, M.; Sekiguchi, S.; Jitsumasa, T.; Morohashi, N.; Handa, Y.; et al. Environmental DNA metabarcoding on aquatic insects: Comparing the primer sets of MtInsects-16S based on the mtDNA 16S and general marker based on the mtDNA COI region. Environ. DNA 2024, 6, e588. [Google Scholar] [CrossRef]
- Poyntz-Wright, I.P.; Harrison, X.A.; Pedersen, S.; Tyler, C. Effectiveness of eDNA for monitoring riverine macroinvertebrates. Sci. Total Environ. 2024, 941, 173621. [Google Scholar] [CrossRef]
- Haring, E.; Fischer, I.; Sittenthaler, M.; Wolf, P.; Chovanec, A.; Koblmüller, S.; Sattmann, H.; Beqiraj, S.; Pesic, V.; Zangl, L. Intraspecific genetic diversity in selected widespread dragonfly species (Insecta: Odonata). Acta ZooBot Austria 2020, 157, 239–256. [Google Scholar]
- Dijkstra, K.; Kalkman, V.; Dow, R.; Stokvis, F.; Van Tol, J. Redefining the damselfly families: A comprehensive molecular phylogeny of Zygoptera (Odonata). Syst. Entomol. 2014, 39, 68–96. [Google Scholar] [CrossRef]
- Richlen, M.; Barber, P. A technique for the rapid extraction of microalgal DNA from single live and preserved cells. Mol. Ecol. Notes 2005, 5, 688–691. [Google Scholar] [CrossRef]
- Koblmüller, S.; Resl, P.; Klar, N.; Bauer, H.; Zangl, L.; Hahn, C. DNA barcoding for species identification of moss-dwelling invertebrates: Performance of nanopore sequencing and coverage in reference database. Diversity 2024, 16, 196. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2023, 30, 2725–2729. [Google Scholar] [CrossRef]
- Cannings, S.; Cannings, R. The larva of Somatochlora sahlbergi Trybŏm, with notes on the species in the Yukon Territory, Canada (Anisoptera: Corduliidae). Odonatologica 1985, 14, 319–330. [Google Scholar]
- Cannings, S.; Cannings, R. Dragonflies (Odonata) of the Yukon. In Insects of the Yukon.; Dankes, H., Downes, J., Eds.; Biological Survey of Canada (Terrestrial Arthropods): Ottawa, ON, Canada, 1997; pp. 169–200. [Google Scholar]
- Kohli, M.; Sahlén, G.; Kuhn, W.; Ware, J. Extremely low genetic diversity in a circumpolar dragonfly species, Somatochlora sahlbergi (Insecta: Odonata: Anisoptera). Sci. Rep. 2018, 8, 15114. [Google Scholar] [CrossRef]
- Hernandez, J.; Cognato, A.I. Incomplete barriers to heterospecific mating among Somatochlora species (Odonata: Corduliidae) as revealed in multi-gene phylogenies. Cladistics 2024, 40, 598–617. [Google Scholar] [CrossRef]
- Mill, P.J. The Brilliant Emerald Somatochlora metallica (Vander Linden) and its close relative the Balkan Emerald S. meridionalis Nielson. J. Br. Dragonfly Soc. 2012, 28, 75–91. [Google Scholar]
- Goodman, A.; Abbott, J.; Breinholt, J.W.; Bybee, S.; Frandsen, P.B.; Guralnick, R.; Kalman, V.J.; Kohli, M.; Newton, L.; Ware, J.L. Systematics and biogeography of the Holarctic dragonfly genus Somatochlora (Anisoptera: Corduliidae). Syst. Entomol. 2025, 50, 585–610. [Google Scholar] [CrossRef]
- Dijkstra, K.-D.B. Libellen Europas: Der Bestimmungsführer; Haupt: Berne, Switzerland, 2014. [Google Scholar]
- Freeland, J.R.; Conrad, K.F. Genetic similarity within and among populations of variable and azure damselflies (Coenagrion pulchellum and C. puella). Hydrobiologia 2002, 479, 69–73. [Google Scholar] [CrossRef]
- Lowe, C.D.; Harvey, I.F.; Thompson, D.J.; Watts, P.C. Strong genetic divergence indicates that congeneric damselflies Coenagrion puella and C. pulchellum (Odonata: Zygoptera: Coenagrionidae) do not hybridise. Hydrobiologia 2008, 605, 55–63. [Google Scholar] [CrossRef]
- Miller, M.N.; Fincke, O.M. Mistakes in sexual recognition among sympatric Zygoptera vary with time of day and color morphism (Odonata: Coenagrionidae). Int. J. Odonatol. 2004, 7, 471–491. [Google Scholar] [CrossRef]
- Bilek, A. Ein Freiland-Hybrid der Gattung Agrion Leach (=Coenagrion Kirby) (Odonata, Agrionidae). NachrBl. Bayer. Entomol. 1963, 12, 56–58. [Google Scholar]
- Olias, M.; Weihrauch, F.; Bedjanič, M.; Hacet, N.; Marinov, M.; Salamun, A. Lestes parvidens and L. viridis in southeastern Europe: A chorological analysis (Odonata: Lestidae). Libellula 2007, 26, 243–272. [Google Scholar]
- Olias, M. Lestes parvidens am Südostrand Mitteleuropas: Erste Nachweise aus Österreich, der Slowakei, Ungarn und Rumänien (Odonata: Lestidae). Libellula 2005, 24, 155–161. [Google Scholar]
- Schneider, T.; Vierstraete, A.; Kosterin, O.E.; Ikemeyer, D.; Hu, F.-S.; Snegovaya, N.; Dumont, H. Molecular phylogeny of Holarctic Aeshnidae with a focus on the West Palaearctic and some remarks on its genera worldwide (Ashnidae, Odonata). Diversity 2023, 15, 950. [Google Scholar] [CrossRef]
- Bilek, A. Der erste Fall Hybridisation bei Libellen. Ein Anax-Hybrid (Odonata). Nachr. Bayer. Ent. 1955, 4, 115–117. [Google Scholar]
- Koroiva, R.; Kvist, S. Estimating the barcoding gap in a global dataset of cox1 sequences for Odonata: Close, but no cigar. Mitochondrial DNA Part A. 2018, 29, 765–771. [Google Scholar] [CrossRef]
- Drummond, A.J.; Newcomb, R.D.; Buckley, T.R.; Xie, D.; Dopheide, A.; Potter, B.C.; Heled, J.; Ross, H.A.; Tooman, L.; Grosser, S.; et al. Evaluating a multigene environmental DNA approach for biodiversity assessment. GigaScience 2015, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Schenekar, T.; Schletterer, M.; Lecaudey, L.A.; Weiss, S.J. Reference databases, primer choice, and assay sensitivity for environmental metabarcoding: Lessons learnt from a re-evaluation of an eDNA fish assessment in the Volga headwaters. River Res. Appl. 2020, 36, 1004–1013. [Google Scholar] [CrossRef]
- Schenekar, T. The current state of eDNA research in freshwater ecosystems: Are we shifting from the developmental phase to standard application in biomonitoring? Hydrobiologia 2023, 850, 1263–1282. [Google Scholar] [CrossRef]
- Othman, N.; Haris, H.; Fatin, Z.; Najmuddin, M.F.; Sariyati, N.H.; Md-Zain, B.M.; Abdul-Latiff, M.A.B. A review on environmental DNA (eDNA) metabarcoding markers for wildlife monitoring research. IOP Conf. Ser. Earth Environ. Sci. 2021, 736, 012054. [Google Scholar] [CrossRef]
- Andres, K.J.; Lodge, D.M.; Sethi, S.A.; Andrés, J. Detecting and analysing intraspecific genetic variation with eDNA: From population genetics to species abundance. Mol. Ecol. 2023, 32, 4118–4132. [Google Scholar] [CrossRef] [PubMed]
- Landmann, M.; Schlick-Steiner, B.C.; Steiner, F.M.; Landmann, A. Connectivity within isolation: Dispersal, population genetics, and conservation of the rarest European damselfly. Insect Conserv. Divers. 2021, 14, 800–813. [Google Scholar] [CrossRef]
- Laister, G. Die Libellenfauna der Linzer Donauauen—Entwicklung und aktuelle Situation. Ber. Für. Okol. Nat. Der Stadt. Linz. 2007, 1, 65–123. [Google Scholar]
- Gros, P. Die Libellenfauna des Mandlinger Moores (Gemeindegebiet Radstadt, Salzburg): Erster inneralpiner Nachweis der Großen Moosjungfer Leucorrhinia pectoralis (Charpentier, 1825) aus dem Bundesland Salzburg und erste Meldung der Glänzende Binsenjungfer Lestes dryas Kirby, 1890 aus dem Ennstal, Österreich (Odonata). Mitt. Haus Nat. 2010, 18, 29–34. [Google Scholar]
- Gros, P.; Kurz, M. Die Insektenfauna des Gemeindegebietes Neumarkt am Wallersee (Österreich, Salzburg): Eine bemerkenswerte Vielfalt mit hohem naturschutzfachlichem Wert. Sauteria 2013, 20, 107–125. [Google Scholar]
- Amann, P. Über das Vorkommen der Helm-Azurjungfer und anderer Libellen im Raum Dornbirn-Hohenems-Lustenau (Vorarlberg, Österreich). Ina.-Forsch. Online 2017, 36, 21. [Google Scholar]
- Heidemann, H. Ein neuer europäischer Fund von Coenagrion hylas (Trybom) (Zygoptera: Coenagrionidae). Odonatologica 1974, 3, 181–185. [Google Scholar]
- Lehmann, G. Coeangrion lunulatum (Charpentier, 1840) und andere Libellen an einem Torfstichweiher bei Bad Häring, Bezirk Kufstein (Tirol, Österreich). Ber. Nat.-Med. Ver. Innsbr. 1985, 72, 165–172. [Google Scholar]
- Termaat, T.; Ketelaar, R.; van Kleef, H.H.; Verberk, W.C.E.P.; van Grunsven, R.H.A.; WallisDeVries, M.F. Spearhead blues: How threats to the damselfly Coenagrion hastulatum changed over time. J. Insect Conserv. 2024, 28, 211–224. [Google Scholar] [CrossRef]
- Ott, J. Dragonflies and climatic change-recent trends in Germany and Europe. BioRisk 2010, 5, 253–286. [Google Scholar] [CrossRef]
- Calvão, L.B.; Juen, L.; de Oliveira Junior, J.M.B.; Batista, J.D.; De Marco Júnior, P. Land use modifies Odonata diversity in streams of the Brazilian Cerrado. J. Insect Conserv. 2018, 22, 675–685. [Google Scholar] [CrossRef]
- Hickling, R.; Roy, D.; Hill, J.; Thomas, C. A northward shift of range margins in British Odonata. Glob. Change Biol. 2005, 11, 502–506. [Google Scholar] [CrossRef]
- Olsen, K.; Svenning, J.-C.; Balslev, H. Niche breadth predicts geographical range size and northern range shift in European dragonfly species (Odonata). Diversity 2022, 14, 719. [Google Scholar] [CrossRef]
- Pélissié, M.; Johansson, F.; Hyseni, C. Pushed northward by climate change: Range shifts with a chance of co-occurrence reshuffling in the forecast for northern European odonates. Environ. Entomol. 2022, 51, 910–921. [Google Scholar] [CrossRef]
- Tang, D.H.Y.; Visconti, P. Biases of Odonata in Habitats Directive: Trends, trend drivers, and conservation status of European threatened Odonata. Insect Conserv. Diver. 2021, 14, 1–14. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence 5′-3′ | Gene | Tann [°C] | Primer Type | Reference |
|---|---|---|---|---|---|
| Tyr-Odo-F | CTCCTATATAGATTTACAGTCT | COI | 46–54 | PCR/seq. | [45] |
| Leu-Odo-R | CTTAAATCCATTGCACTTTTCTGCC | COI | 53–56 | PCR/seq. | [45] |
| CO1-Odo-F5 | TGCGACRATGRCTGTTTTC | COI | 47 | PCR/seq. | [45] |
| CO1-Odo-R6 | TGCACTTTTCTGCCACATTAAA | COI | 47–54 | PCR/seq. | [45] |
| ODO_LCO1490d | TTTCTACWAACCAYAAAGATATTGG | COI | 48–57 | PCR/seq. | [46] |
| ODO_HCO2198d | TAAACTTCWGGRTGTCCAAARAATCA | COI | 48–57 | PCR/seq. | [46] |
| CO1-Zyg-F1 | TTGGAGATGAYCAAATTTATAAYGT | COI | 57 | PCR/seq. | [45] |
| CO1-Odo-F3 | GATTCTTTGGACAYCCHGAAG | COI | 53 | PCR/seq. | [45] |
| CO1-Odo-R1 | TAATATGTGAAATTATWCCAA | COI | 46 | PCR/seq. | [45] |
| CO1-OdoCol-R1 | CCTCCAATTATRATAGGTATWACTA | COI | 57 | PCR/seq. | Present study |
| CO1-Odo-R8 | GTARTTTTTGATATCATTCRAT | COI | 60 | int. seq. primer | [45] |
| CO1-Lib-F1 | TTAACAGAYCGAAATATTAATAC | COI | 60 | int. seq. primer | [45] |
| CO1-Odo-F1 | GGWATAATTTCACATATTATTGC | COI | 60 | int. seq. primer | [45] |
| CO1-Odo-F4 | TATGCAATARTAGCHATTGG | COI | 60 | int. seq. primer | Present study |
| CO1-Sym-F1 | TTAACTGAYCGAAATATTAATACATC | COI | 60 | int. seq. primer | [45] |
| CO1-Lib-R1 | CCTARAATACCAATTGCTACTAT | COI | 60 | int. seq. primer | [45] |
| CO1-Odo-R3 | GTTTCCTTTTTACCTCTTTCTTG | COI | 60 | int. seq. primer | [45] |
| CO1-Odo-R4 | CCAATTGCTAYTATTGCRTA | COI | 60 | int. seq. primer | Present study |
| 16S-Odo-F1 | GGTCTGAACTCAGATCATGTAAG | 16S | 50–60 | PCR/seq. | Present study |
| 16S-Odo-R2 | CGCCTGTTTATCAAAAACATGTC | 16S | 50–60 | PCR/seq. | Present study |
| Species | BIN | N | Imax | DNN | Nearest Neighbor |
|---|---|---|---|---|---|
| Aeshnidae | |||||
| Aeshna affinis Vander Linden, 1820 | BOLD:AAJ5779 | 4 | 0.28 | 7.11 | A. mixta |
| Aeshna caerulea Strøm, 1783 | BOLD:AAA6531 | 3 | 0.00 | 5.99 | A. cyanea |
| Aeshna cyanea (Müller, 1764) | BOLD:ACI1053 | 22 | 0.74 | 5.71 | A. mixta |
| Aeshna grandis (Linnaeus, 1758) | BOLD:AAJ5811 | 8 | 0.75 | 3.15 | A. viridis |
| Aeshna juncea (Linnaeus, 1758) | BOLD:AAJ1281 | 20 | 1.55 | 3.50 | A. subarctica |
| Aeshna mixta Latreille, 1805 | BOLD:AAJ5810 | 14 | 0.94 | 5.71 | A. cyanea |
| Aeshna subarctica Walker, 1908 | BOLD:ABZ5296 | 4 | 1.03 | 3.50 | A. juncea |
| Aeshna viridis Eversmann, 1836 | BOLD:ADC2700 | 3 | 0.00 | 3.15 | A. grandis |
| Anax ephippiger (Burmeister, 1839) | BOLD:ACH7840 | 2 | 0.85 | 6.63 | A. imperator |
| Anax imperator Leach, 1815 | BOLD:ABX6596 | 15 | 0.89 | 0.98 | A. parthenope |
| Anax parthenope Selys, 1839 | BOLD:ABX6596 | 7 | 0.84 | 0.98 | A. imperator |
| Brachytron pratense (Müller, 1764) | BOLD:ACI1765 | 10 | 0.57 | 8.65 | A. mixta |
| Isoaeschna isoceles (Müller, 1767) | BOLD:ADC2941 | 11 | 1.67 | 7.88 | A. caerulea |
| Calopterygidae | |||||
| Calopteryx splendens (Harris, 1782) | BOLD:ADC4648 | 13 | 0.59 | 9.96 | C. virgo |
| Calopteryx virgo (Linnaeus, 1758) | BOLD:AAE7398 | 20 | 0.71 | 9.96 | C. splendens |
| Coenagrionidae | |||||
| Coenagrion hastulatum (Charpentier, 1825) | BOLD:ACH0316 | 15 | 1.39 | 10.15 | C. mercuriale |
| Coenagrion mercuriale (Charpentier, 1840) | BOLD:ADS2145 BOLD:ACG0797 | 2 | 2.10 | 7.91 | C. pulchellum |
| Coenagrion ornatum (Selys, 1850) | BOLD:AAJ0782 | 6 | 0.57 | 0.00 | C. puella |
| Coenagrion puella (Linnaeus, 1758) | BOLD:AAJ0782 | 42 | 0.71 | 0.00 | C. pulchellum |
| Coenagrion pulchellum (Vander Linden, 1825) | BOLD:AAJ0782 | 26 | 1.86 | 0.00 | C. puella |
| Coenagrion scitulum (Rambur, 1842) | BOLD:ACP4983 | 10 | 0.58 | 12.22 | C. mercuriale |
| Enallagma cyathigerum (Charpentier, 1840) | BOLD:AAA2218 | 30 | 1.33 | 13.50 | I. elegans |
| Erythromma lindenii (Selys, 1840) | BOLD:AAL4439 | 10 | 0.56 | 15.28 | E. viridulum |
| Erythromma najas (Hansemann, 1823) | BOLD:AAA4234 | 13 | 2.22 | 14.39 | E. viridulum |
| Erythromma viridulum (Charpentier, 1840) | BOLD:AAL4437 | 18 | 2.24 | 14.39 | E. najas |
| Ischnura elegans (Vander Linden, 1820) | BOLD:AAE5570 | 24 | 0.70 | 13.31 | I. pumilio |
| Ischnura pumilio (Charpentier, 1825) | BOLD:AAE5571 | 7 | 0.44 | 13.31 | I. elegans |
| Nehalennia speciosa (Charpentier, 1840) | BOLD:AAC3125 | 5 | 0.59 | 15.27 | P. nymphula |
| Pyrrhosoma nymphula (Sulzer, 1776) | BOLD:AAD5734 | 21 | 0.86 | 13.89 | L. albifrons |
| Cordulegastridae | |||||
| Cordulegaster boltonii (Donovan, 1807) | BOLD:AAJ5773 | 4 | 0.62 | 8.61 | C. heros |
| Cordulegaster heros Theischinger, 1979 | BOLD:ACQ4796 | 12 | 0.87 | 8.61 | C. boltonii |
| Thecagaster bidentata (Selys, 1843) | BOLD:AAJ5749 | 10 | 0.57 | 9.62 | C. boltonii |
| Corduliidae | |||||
| Cordulia aenea (Linnaeus, 1758) | BOLD:AAJ5771 | 19 | 0.70 | 10.0 | S. alpestris |
| Epitheca bimaculata (Charpentier, 1825) | BOLD:ACG0805 | 5 | 0.44 | 12.33 | A. alpestris |
| Somatochlora alpestris (Selys, 1840) | BOLD:ACP5227 | 5 | 0.72 | 4.25 | S. arctica |
| Somatochlora arctica (Zetterstedt, 1840) | BOLD:ACP7013 | 7 | 1.26 | 4.25 | S. alpestris |
| Somatochlora flavomaculata (Vander Linden, 1825) | BOLD:AEC6167 | 15 | 1.19 | 7.83 | S. alpestris |
| Somatochlora meridionalis Nielsen, 1935 | BOLD:ABW6681 | 4 | 0.68 | 0.00 | S. metallica |
| Somatochlora metallica (Vander Linden, 1825) | BOLD:ABW6681 | 22 | 1.34 | 0.00 | S. meridionalis |
| Gomphidae | |||||
| Gomphus pulchellus Selys, 1840 | BOLD:ADC4839 | 4 | 0.31 | 14.61 | O. cecilia |
| Gomphus vulgatissimus (Linnaeus, 1758) | BOLD:AAN0925 | 8 | 0.84 | 15.40 | G. pulchellus |
| Onychogomphus forcipatus (Linnaeus, 1758) | BOLD:AEM8698 | 9 | 2.84 | 10.96 | O. cecilia |
| Ophiogomphus cecilia (Fourcroy, 1785) | BOLD:ACP4340 | 8 | 0.42 | 10.96 | O. forcipatus |
| Stylurus flavipes (Charpentier, 1825) | BOLD:ADC2840 | 3 | 0.62 | 15.60 | O. forcipatus |
| Lestidae | |||||
| Chalcolestes parvidens (Vander Linden 1825) | BOLD:AAI7225 BOLD:ADR7794 | 4 | 9.59 | 0.00 | C. viridis |
| Chalcolestes viridis (Vander Linden 1825) | BOLD:AAI7225 BOLD:ADR7794 | 22 | 10.21 | 0.00 | C. parvidens |
| Chalcolestes parvidens x viridis | BOLD:ADR7794 | 1 | 0.00 | 0.74 | C. viridis |
| Lestes barbarus (Fabricius, 1798) | BOLD:ADC3442 | 5 | 0.28 | 11.07 | L. sponsa |
| Lestes macrostigma (Eversmann, 1836) | BOLD:ADC3318 | 4 | 0.46 | 12.83 | L. sponsa |
| Lestes sponsa (Hansemann, 1823) | BOLD:ACP4984 | 27 | 1.31 | 11.07 | L. barbarus |
| Lestes virens (Charpentier, 1825) | BOLD:ACG0123 | 4 | 0.69 | 12.37 | L. sponsa |
| Sympecma fusca (Vander Linden, 1820) | BOLD:AAK1032 | 9 | 0.74 | 7.73 | S. paedisca |
| Sympecma paedisca (Brauer, 1877) | BOLD:ACG0335 | 1 | 0.00 | 7.73 | S. fusca |
| Libellulidae | |||||
| Crocothemis erythraea (Brullé, 1832) | BOLD:AEX9418 | 13 | 1.45 | 13.23 | O. cancellatum |
| Leucorrhinia albifrons (Burmeister, 1839) | BOLD:ADR0815 | 2 | 0.00 | 9.88 | L. caudalis |
| Leucorrhinia caudalis (Charpentier, 1840) | BOLD:ADC4475 | 4 | 0.28 | 9.88 | L. albifrons |
| Leucorrhinia dubia (Vander Linden, 1825) | BOLD:AAJ2437 | 10 | 0.56 | 2.30 | L. rubicunda |
| Leucorrhinia pectoralis (Charpentier, 1825) | BOLD:ADC3719 | 4 | 0.00 | 4.55 | L. dubia |
| Leucorrhinia rubicunda (Linnaeus, 1758) | BOLD:ADC1709 | 4 | 0.28 | 2.30 | L. dubia |
| Libellula depressa Linnaeus, 1758 | BOLD:AAJ2758 | 13 | 0.56 | 12.06 | L. fulva |
| Libellula fulva Müller, 1764 | BOLD:ACP3530 | 11 | 0.71 | 12.06 | L. depressa |
| Libellula quadrimaculata Linnaeus, 1758 | BOLD:AEY0197 | 18 | 0.59 | 12.42 | O. coerulescens |
| Orthetrum albistylum (Selys, 1848) | BOLD:AED4117 | 13 | 0.28 | 5.33 | O. cancellatum |
| Orthetrum brunneum (Fonscolombe, 1837) | BOLD:AAK5997 | 12 | 0.74 | 9.95 | O. cancellatum |
| Orthetrum cancellatum (Linnaeus, 1758) | BOLD:AAK5996 | 18 | 2.37 | 5.33 | O. albistylum |
| Orthetrum coerulescens (Fabricius, 1798) | BOLD:AAI2353 | 8 | 2.11 | 10.0 | O. brunneum |
| Sympetrum danae (Sulzer, 1776) | BOLD:AAA3766 | 12 | 1.12 | 9.05 | S. depressiusculum |
| Sympetrum depressiusculum (Selys, 1841) | BOLD:ACQ1493 | 4 | 0.31 | 9.05 | S. danae |
| Sympetrum fonscolombii (Selys, 1840) | BOLD:AAI0218 | 5 | 0.85 | 14.34 | L. quadrimaculata |
| Sympetrum meridionale (Selys, 1841) | BOLD:AAD8296 | 6 | 3.17 | 9.31 | S. sanguineum |
| Sympetrum pedemontanum (Allioni, 1766) | BOLD:AAK1022 | 11 | 0.80 | 10.24 | S. vulgatum |
| Sympetrum sanguineum (Müller, 1764) | BOLD:AAB2237 | 25 | 1.44 | 9.31 | S. meridionale |
| Sympetrum striolatum (Charpentier, 1840) | BOLD:AAB2236 | 20 | 1.04 | 8.78 | S. vulgatum |
| Sympetrum vulgatum (Linnaeus, 1758) | BOLD:AAE2658 | 25 | 1.0 | 8.78 | S. striolatum |
| Platycnemididae | |||||
| Platycnemis pennipes (Pallas, 1771) | BOLD:ACG0515 | 30 | 0.44 | 16.91 | A. juncea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangl, L.; Fischer, I.; Sittenthaler, M.; Chovanec, A.; Gros, P.; Holzinger, W.; Kunz, G.; Lienhard, A.; Macek, O.; Mayerhofer, C.; et al. A DNA Barcode Inventory of Austrian Dragonfly and Damselfly (Insecta: Odonata) Species. Insects 2025, 16, 1056. https://doi.org/10.3390/insects16101056
Zangl L, Fischer I, Sittenthaler M, Chovanec A, Gros P, Holzinger W, Kunz G, Lienhard A, Macek O, Mayerhofer C, et al. A DNA Barcode Inventory of Austrian Dragonfly and Damselfly (Insecta: Odonata) Species. Insects. 2025; 16(10):1056. https://doi.org/10.3390/insects16101056
Chicago/Turabian StyleZangl, Lukas, Iris Fischer, Marcia Sittenthaler, Andreas Chovanec, Patrick Gros, Werner Holzinger, Gernot Kunz, Andrea Lienhard, Oliver Macek, Christoph Mayerhofer, and et al. 2025. "A DNA Barcode Inventory of Austrian Dragonfly and Damselfly (Insecta: Odonata) Species" Insects 16, no. 10: 1056. https://doi.org/10.3390/insects16101056
APA StyleZangl, L., Fischer, I., Sittenthaler, M., Chovanec, A., Gros, P., Holzinger, W., Kunz, G., Lienhard, A., Macek, O., Mayerhofer, C., Mladinić, M., Topić, M., Schäffer, S., Sefc, K. M., Sturmbauer, C., Haring, E., & Koblmüller, S. (2025). A DNA Barcode Inventory of Austrian Dragonfly and Damselfly (Insecta: Odonata) Species. Insects, 16(10), 1056. https://doi.org/10.3390/insects16101056







