The Defensin NldefB as a Potential Target for Brown Planthopper Control Based on the Combination of RNA Interference and Fungal Insect Pathogen
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Microbial Strains
2.2. RNA Extraction and cDNA Synthesis
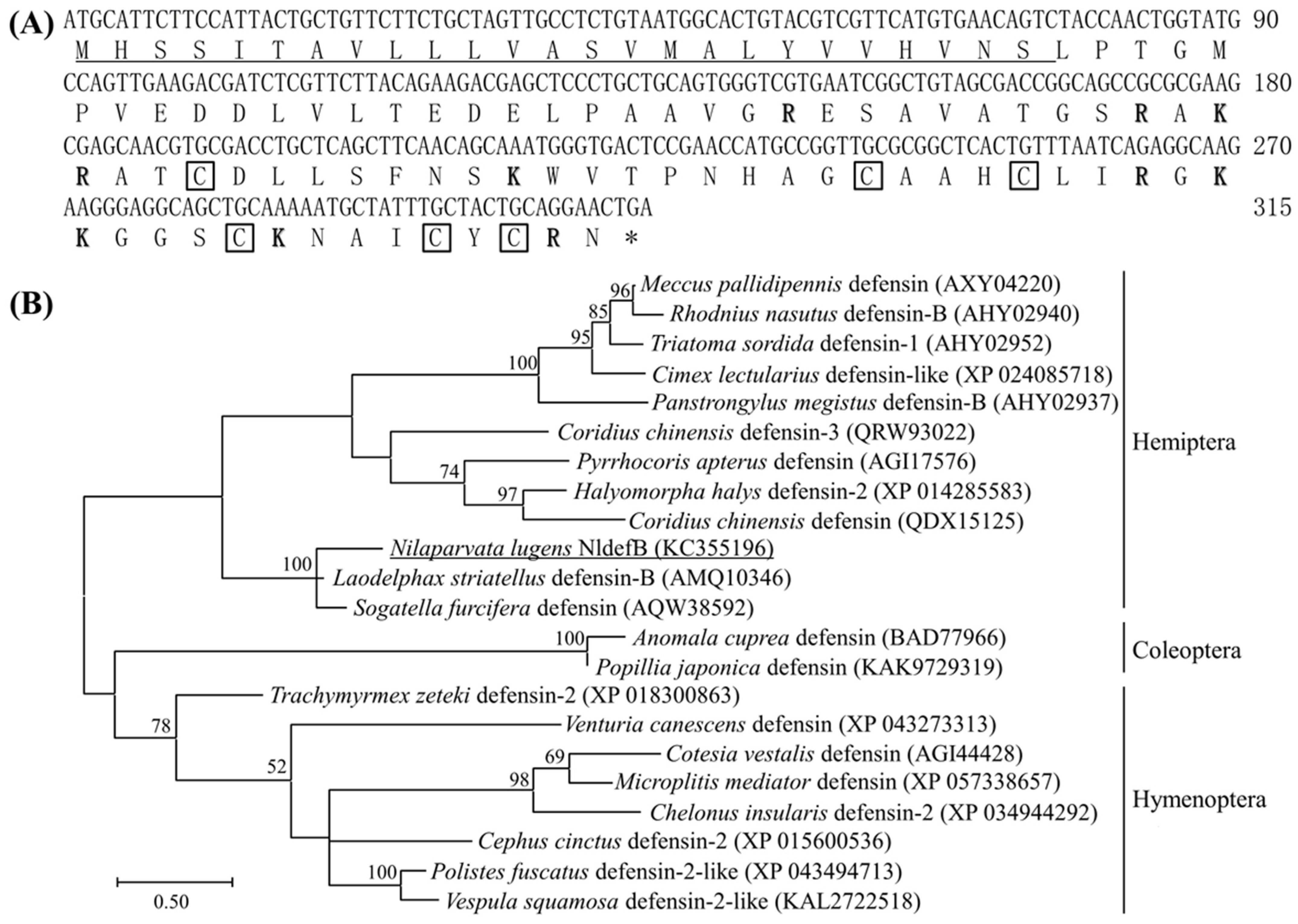
2.3. Gene Cloning and Structural Characterization of NldefB
2.4. Gene Expression Pattern Analysis of NldefB
2.5. Synthesis and Injection of Double-Stranded RNA (dsRNA)
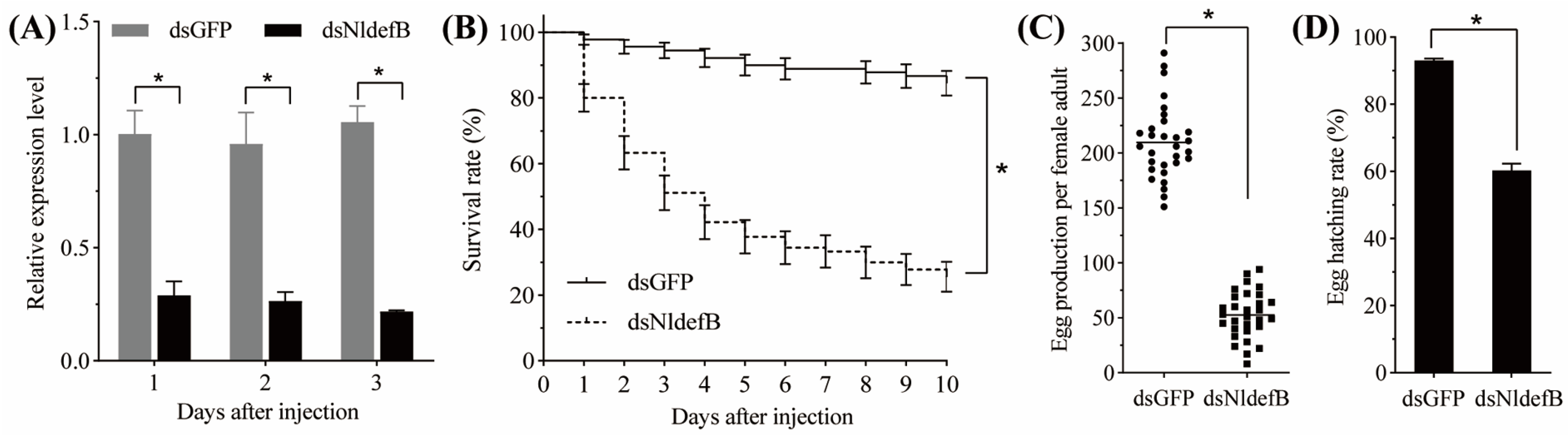
2.6. Analysis of Survival and Fecundity After NldefB Silencing
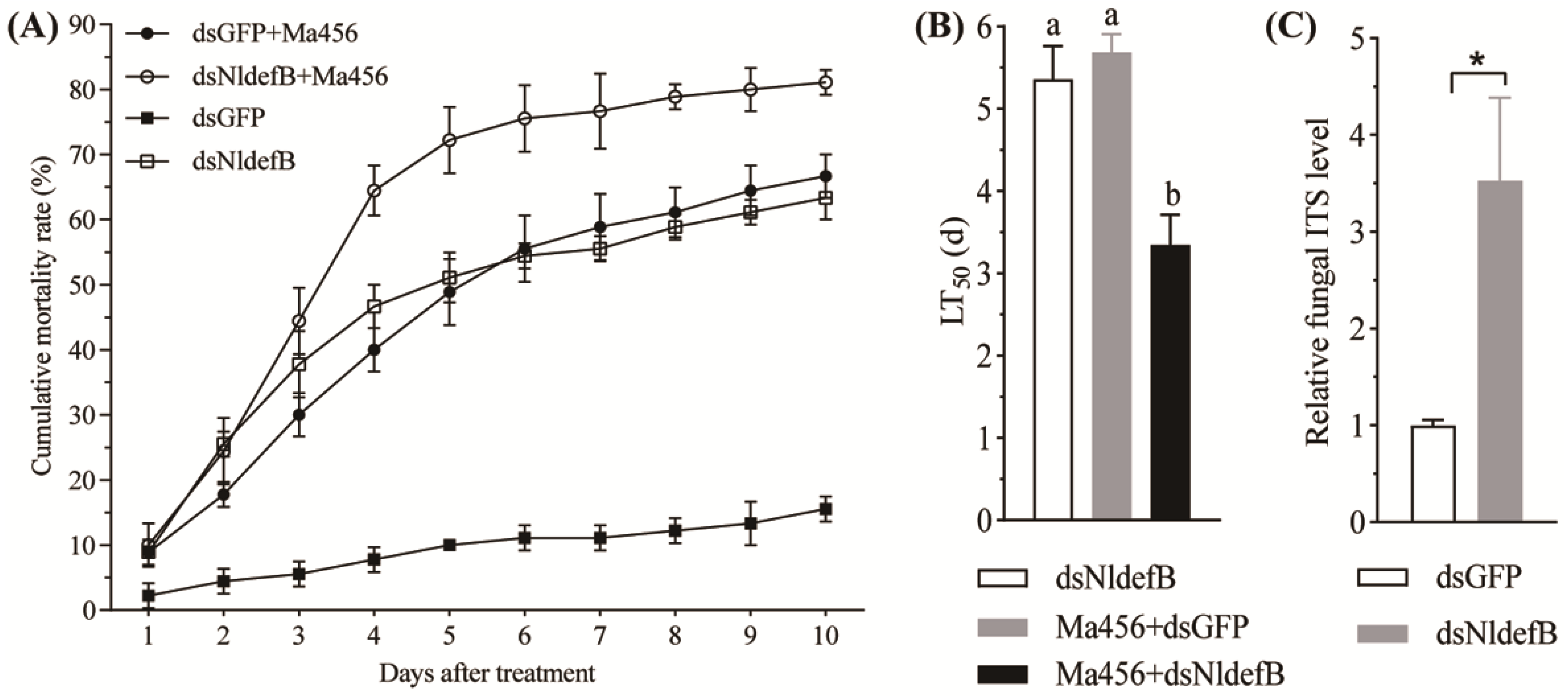
2.7. Analysis of Antifungal Defense After NldefB Silencing
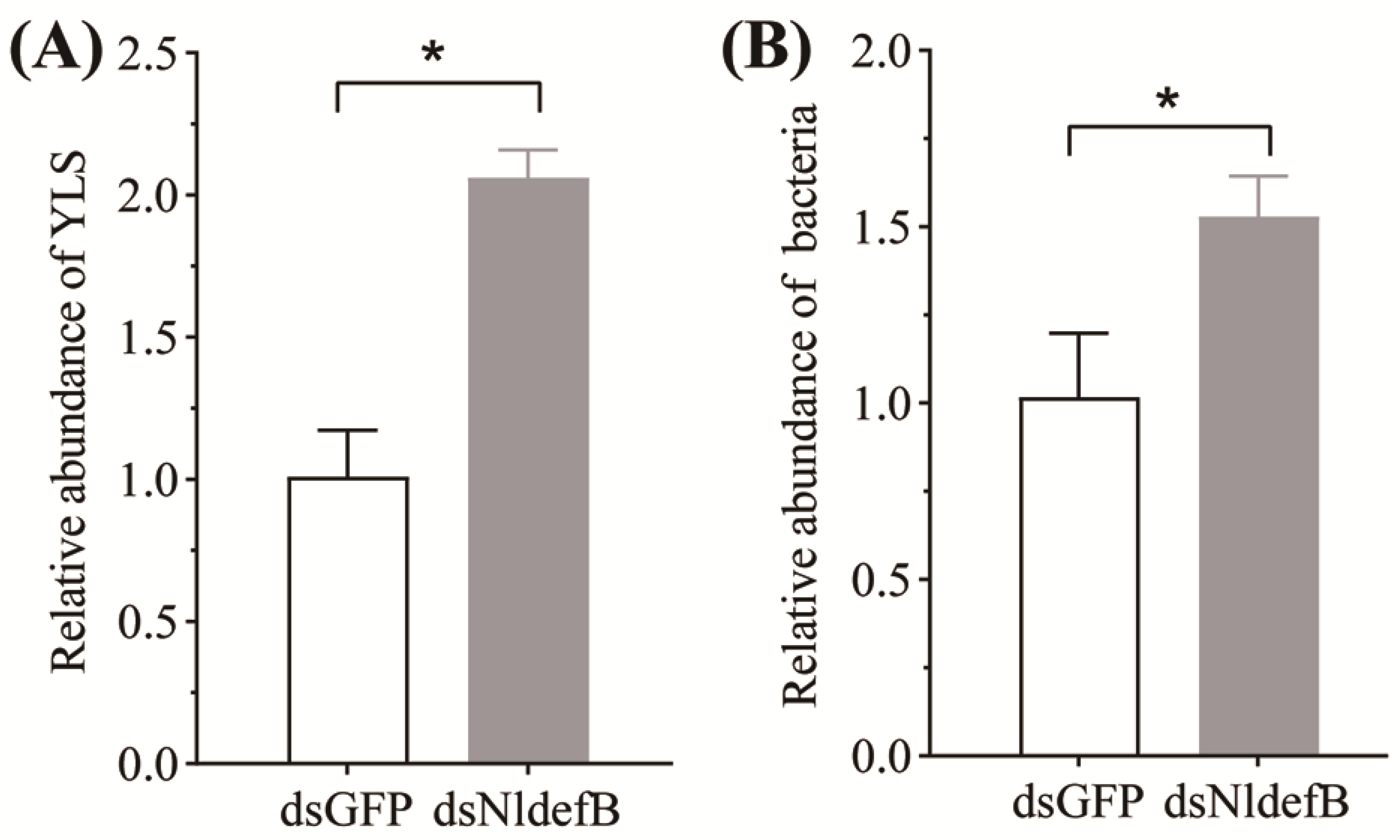
2.8. Analysis of Symbiont Load After NldefB Silencing
2.9. Statistical Analysis
3. Results
3.1. Identification and Characterization of NldefB
3.2. Expression Patterns of NldefB
3.3. NldefB Silencing Decreased BPH Survival and Fecundity
3.4. NldefB Silencing Reduced BPH Resistance to Fungal Infection
3.5. NldefB Silencing Boosted Fungal Proliferation in the Hemolymph of BPH
3.6. NldefB Silencing Enhanced Symbiont Load in BPH
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, L.; Luo, T.; Huang, D.; Wei, M.; Ma, Z.; Liu, C.; Qin, Y.; Zhou, X.; Lu, Y.; Li, R.; et al. Recent advances in molecular mechanism and breeding utilization of brown planthopper resistance genes in rice: An integrated review. Int. J. Mol. Sci. 2023, 24, 12061. [Google Scholar] [CrossRef]
- Shentu, X.; Wang, X.; Xiao, Y.; Yu, X. Effects of fungicide propiconazole on the yeast-like symbiotes in brown planthopper (BPH, Nilaparvata lugens Stål) and its role in controlling BPH infestation. Front. Physiol. 2019, 10, 89. [Google Scholar] [CrossRef]
- Tang, J.F.; Liu, X.Y.; Ding, Y.C.; Jiang, W.J.; Xie, J.Q. Evaluation of Metarhizium anisopliae for rice planthopper control and its synergy with selected insecticides. Crop Prot. 2019, 121, 132–138. [Google Scholar] [CrossRef]
- Lu, H.L.; St Leger, R.J. Insect immunity to entomopathogenic fungi. Adv. Genet. 2016, 94, 251–285. [Google Scholar] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect antimicrobial peptides, a mini review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Zhou, L.; Meng, G.; Zhu, L.; Ma, L.; Chen, K. Insect antimicrobial peptides as guardians of immunity and beyond: A review. Int. J. Mol. Sci. 2024, 25, 3835. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef]
- Adhikary, K.; Poget, S.F. Expanding the insect defensin landscape. Structure 2024, 32, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, R.S.; Silva Gunawardene, Y.I.; Tobe, S.S. Evolutionary selective trends of insect/mosquito antimicrobial defensin peptides containing cysteine-stabilized alpha/beta motifs. Peptides 2007, 28, 62–75. [Google Scholar] [CrossRef]
- Lambert, J.; Keppi, E.; Dimarcq, J.L.; Wicker, C.; Reichhart, J.M.; Dunbar, B.; Lepage, P.; Van Dorsselaer, A.; Hoffmann, J.; Fothergill, J.; et al. Insect immunity: Isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides. Proc. Natl. Acad. Sci. USA 1989, 86, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, A.N.; Rocher, J.; d’Alençon, E.; Bouton, M.; Landais, I.; Quesada-Moraga, E.; Vey, A.; Fournier, P.; Mita, K.; Devauchelle, G. Characterization and transcriptional profiles of three Spodoptera frugiperda genes encoding cysteine-rich peptides. A new class of defensin-like genes from lepidopteran insects? Gene 2003, 319, 43–53. [Google Scholar] [CrossRef]
- Klaudiny, J.; Albert, S.; Bachanová, K.; Kopernický, J.; Simúth, J. Two structurally different defensin genes, one of them encoding a novel defensin isoform, are expressed in honeybee Apis mellifera. Insect Biochem. Mol. Biol. 2005, 35, 11–22. [Google Scholar] [CrossRef]
- Wen, H.; Lan, X.; Cheng, T.; He, N.; Shiomi, K.; Kajiura, Z.; Zhou, Z.; Xia, Q.; Xiang, Z.; Nakagaki, M. Sequence structure and expression pattern of a novel anionic defensin-like gene from silkworm (Bombyx mori). Mol. Biol. Rep. 2009, 36, 711–716. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Qu, L.Y.; Zhao, D.; Chen, L.B.; Jin, H.Y.; Xu, L.M.; Cheng, J.A.; Zhang, C.X. The genome- and transcriptome-wide analysis of innate immunity in the brown planthopper, Nilaparvata lugens. BMC Genom. 2013, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Koehbach, J. Structure-activity relationships of insect defensins. Front. Chem. 2017, 5, 45. [Google Scholar] [CrossRef]
- Tonk, M.; Knorr, E.; Cabezas-Cruz, A.; Valdés, J.J.; Kollewe, C.; Vilcinskas, A. Tribolium castaneum defensins are primarily active against Gram-positive bacteria. J. Invertebr. Pathol. 2015, 132, 208–215. [Google Scholar] [CrossRef]
- Xu, X.X.; Zhang, Y.Q.; Freed, S.; Yu, J.; Gao, Y.F.; Wang, S.; Ouyang, L.N.; Ju, W.Y.; Jin, F.L. An anionic defensin from Plutella xylostella with potential activity against Bacillus thuringiensis. Bull. Entomol. Res. 2016, 106, 790–800. [Google Scholar] [CrossRef]
- Ilyasov, R.; Gaifullina, L.; Saltykova, E.; Poskryakov, A.; Nikolenko, A. Review of the expression of antimicrobial peptide defensin in honey bees Apis mellifera L. J. Apic. Sci. 2012, 56, 115–124. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P. Role of antimicrobial peptides in controlling symbiotic bacterial populations. Nat. Prod. Rep. 2018, 35, 336–356. [Google Scholar] [CrossRef]
- Onchuru, T.O.; Kaltenpoth, M. Established cotton stainer gut bacterial mutualists evade regulation by host antimicrobial peptides. Appl. Environ. Microbiol. 2019, 85, e00738-19. [Google Scholar] [CrossRef]
- Dong, Z.X.; Tang, Q.H.; Li, W.L.; Wang, Z.W.; Li, X.J.; Fu, C.M.; Li, D.; Qian, K.; Tian, W.L.; Guo, J. Honeybee (Apis mellifera) resistance to deltamethrin exposure by modulating the gut microbiota and improving immunity. Environ. Pollut. 2022, 314, 120340. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Hanson, M.A.; Kondo, S.; Erkosar, B.; Lemaitre, B. Drosophila antimicrobial peptides and lysozymes regulate gut microbiota composition and abundance. mBio 2021, 12, e0082421. [Google Scholar] [CrossRef]
- Gerardo, N.M.; Altincicek, B.; Anselme, C.; Atamian, H.; Barribeau, S.M.; De Vos, M.; Duncan, E.J.; Evans, J.D.; Gabaldón, T.; Ghanim, M. Immunity and other defenses in pea aphids, Acyrthosiphon pisum. Genome Biol. 2010, 11, R21. [Google Scholar] [CrossRef] [PubMed]
- Laughton, A.M.; Garcia, J.R.; Altincicek, B.; Strand, M.R.; Gerardo, N.M. Characterisation of immune responses in the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 2011, 57, 830–839. [Google Scholar] [CrossRef]
- Chen, W.; Hasegawa, D.K.; Kaur, N.; Kliot, A.; Pinheiro, P.V.; Luan, J.; Stensmyr, M.C.; Zheng, Y.; Liu, W.; Sun, H. The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biol. 2016, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.L.; Lan, C.P.; Yu, X.P.; Wang, Z.L. RNAi-mediated suppression of Toll-like receptor NlToll1 enhances Nilaparvata lugens susceptibility to entomopathogenic fungal infection. Biol. Control 2025, 205, 105771. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data processing system (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Arora, A.K.; Douglas, A.E. Hype or opportunity? Using microbial symbionts in novel strategies for insect pest control. J. Insect Physiol. 2017, 103, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Stączek, S.; Cytryńska, M.; Zdybicka-Barabas, A. Unraveling the role of antimicrobial peptides in insects. Int. J. Mol. Sci. 2023, 24, 5753. [Google Scholar] [CrossRef]
- Liu, K.; Hou, F.; Wahaab, A.; Kang, L.; Xie, F.; Ma, X.; Xia, Q.; Xiao, C.; Shao, D.; Li, B.; et al. Mosquito defensin facilitates Japanese encephalitis virus infection by downregulating the C6/36 cell-surface antiviral protein HSC70B. Vet. Microbiol. 2021, 253, 108971. [Google Scholar] [CrossRef] [PubMed]
- Emamifar, S.; Abolmaali, S.; Mohsen Sohrabi, S.; Mohammadi, M.; Shahmohammadi, M. Molecular characterization and evaluation of the antibacterial activity of a plant defensin peptide derived from a gene of oat (Avena sativa L.). Phytochemistry 2021, 181, 112586. [Google Scholar] [CrossRef]
- Kaneko, Y.; Tanaka, H.; Ishibashi, J.; Iwasaki, T.; Yamakawa, M. Gene expression of a novel defensin antimicrobial peptide in the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2008, 72, 2353–2361. [Google Scholar] [CrossRef]
- Lamberty, M.; Ades, S.; Uttenweiler-Joseph, S.; Brookhart, G.; Bushey, D.; Hoffmann, J.A.; Bulet, P. Insect immunity. Isolation from the lepidopteran Heliothis virescens of a novel insect defensin with potent antifungal activity. J. Biol. Chem. 1999, 274, 9320–9326. [Google Scholar] [CrossRef]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef]
- Christiaens, O.; Niu, J.; Nji Tizi Taning, C. RNAi in insects: A revolution in fundamental research and pest control applications. Insects 2020, 11, 415. [Google Scholar] [CrossRef]
- Wang, Z.L.; Pan, H.B.; Li, M.Y.; Wu, W.; Yu, X.P. Comprehensive insights into host-pathogen interaction between brown planthopper and a fungal entomopathogen by dual RNA sequencing. Pest Manag. Sci. 2021, 77, 4903–4914. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Pinto, C.E.; Coelho, R.R.; Leite, A.G.B.; Silveira, D.A.; de Souza, D.A.; Lopes, R.B.; Macedo, L.L.P.; Silva, M.C.M.; Ribeiro, T.P.; Morgante, C.V.; et al. Increasing Anthonomus grandis susceptibility to Metarhizium anisopliae through RNAi-induced AgraRelish knockdown: A perspective to combine biocontrol and biotechnology. Pest Manag. Sci. 2021, 77, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Zhang, Y.; Zhu, P.; Sun, L.; Xu, P.; Wang, T.; Luo, J.; Yu, J.; Xu, L. Enhanced toxicity of entomopathogenic fungi Beauveria bassiana with bacteria expressing immune suppressive dsRNA in a leaf beetle. Pestic. Biochem. Physiol. 2023, 193, 105431. [Google Scholar] [CrossRef]
- Silver, K.; Cooper, A.M.; Zhu, K.Y. Strategies for enhancing the efficiency of RNA interference in insects. Pest Manag. Sci. 2021, 77, 2645–2658. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, T.Z.; Zhu, H.F.; Pan, H.B.; Yu, X.P. Diversity and dynamics of microbial communities in brown planthopper at different developmental stages revealed by high-throughput amplicon sequencing. Insect Sci. 2020, 27, 883–894. [Google Scholar] [CrossRef]
- Cheng, D.J.; Hou, R.F. Histological observations on transovarial transmission of a yeast-like symbiote in Nilaparvata lugens Stål (Homoptera, Delphacidae). Tissue Cell 2001, 33, 273–279. [Google Scholar] [CrossRef]
- Wan, P.J.; Yang, L.; Wang, W.X.; Fan, J.M.; Fu, Q.; Li, G.Q. Constructing the major biosynthesis pathways for amino acids in the brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae), based on the transcriptome data. Insect Mol. Biol. 2014, 23, 152–164. [Google Scholar] [CrossRef]
- Fan, H.W.; Noda, H.; Xie, H.Q.; Suetsugu, Y.; Zhu, Q.H.; Zhang, C.X. Genomic analysis of an ascomycete fungus from the rice planthopper reveals how it adapts to an endosymbiotic lifestyle. Genome Biol. Evol. 2015, 7, 2623–2634. [Google Scholar] [CrossRef]
- Cai, T.; Nadal-Jimenez, P.; Gao, Y.; Arai, H.; Li, C.; Su, C.; King, K.C.; He, S.; Li, J.; Hurst, G.D.D.; et al. Insecticide susceptibility in a planthopper pest increases following inoculation with cultured Arsenophonus. ISME J. 2024, 18, wrae194. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.F.; Bing, X.L.; Zhao, D.S.; Guo, Y.; Xi, Z.; Hoffmann, A.A.; Zhang, K.J.; Huang, H.J.; Gong, J.T.; Zhang, X.; et al. Wolbachia supplement biotin and riboflavin to enhance reproduction in planthoppers. ISME J. 2020, 14, 676–687. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, C.-P.; Hu, Z.-G.; Yu, X.-P.; Wang, Z.-L. The Defensin NldefB as a Potential Target for Brown Planthopper Control Based on the Combination of RNA Interference and Fungal Insect Pathogen. Insects 2025, 16, 1041. https://doi.org/10.3390/insects16101041
Lan C-P, Hu Z-G, Yu X-P, Wang Z-L. The Defensin NldefB as a Potential Target for Brown Planthopper Control Based on the Combination of RNA Interference and Fungal Insect Pathogen. Insects. 2025; 16(10):1041. https://doi.org/10.3390/insects16101041
Chicago/Turabian StyleLan, Chen-Ping, Zhi-Guo Hu, Xiao-Ping Yu, and Zheng-Liang Wang. 2025. "The Defensin NldefB as a Potential Target for Brown Planthopper Control Based on the Combination of RNA Interference and Fungal Insect Pathogen" Insects 16, no. 10: 1041. https://doi.org/10.3390/insects16101041
APA StyleLan, C.-P., Hu, Z.-G., Yu, X.-P., & Wang, Z.-L. (2025). The Defensin NldefB as a Potential Target for Brown Planthopper Control Based on the Combination of RNA Interference and Fungal Insect Pathogen. Insects, 16(10), 1041. https://doi.org/10.3390/insects16101041





